Abstract
This review discusses known and speculated relationships between Alzheimer’s disease (AD) biochemical, molecular, and histologic phenomena. In the AD brain, various pathologies including neuritic plaques, neurofibrillary tangles, synaptic loss, oxidative stress, cell cycle re-entry, and mitochondrial changes have all been described. In an attempt to explain what exactly goes wrong in the AD brain various investigators have proposed different heuristic and hierarchical schemes. It is important to accurately define the AD pathology hierarchy because treatments targeting the true apex of its pathologic cascade arguably have the best chance of preventing, mitigating, or even curing this disease.
Keywords: aging, Alzheimer’s disease, amyloid, brain, oxidative stress, mitochondria
Introduction
Mark Smith was an indispensable member of the Alzheimer’s disease (AD) research community. A renegade to some and inspiration to others, Mark was not afraid to take an unpopular position if his data suggested that was the right position to take (Perry et al. 2000; Castellani et al. 2009). Data generated by Mark and his colleagues questioned the AD field’s prevailing dogma, and Mark attacked that dogma with both gusto and, somehow, charm. Mark certainly had a remarkable ability to disagree without being disagreeable. Even during heated arguments he seemed to be enjoying himself, which made him a joy to be around. These qualities endeared him to both allies and antagonists alike.
One of Mark’s insights was that in a disease such as AD in which neurons are dying, many phenomena would manifest but only one could constitute (to borrow a quote from Mark) “the head of the snake”. He further assumed downstream phenomena would, more often than not, represent adaptive responses (Lee et al. 2006). Preventing those adaptive downstream responses without fixing the upstream pathology that drives them would probably not help, and could conceivably hurt, AD patients. Those working to understand why AD subjects treated with semagacestat, a gamma secretase inhibitor, showed accelerated cognitive decline will certainly need to consider this possibility.
This review will discuss several different AD brain phenomena and the relationships that are known to exist between these phenomena. In doing so it will discuss pro and con arguments over which particular pathologic phenomenon constitutes the “head of the snake”.
Does Beta Amyloid Cause AD?
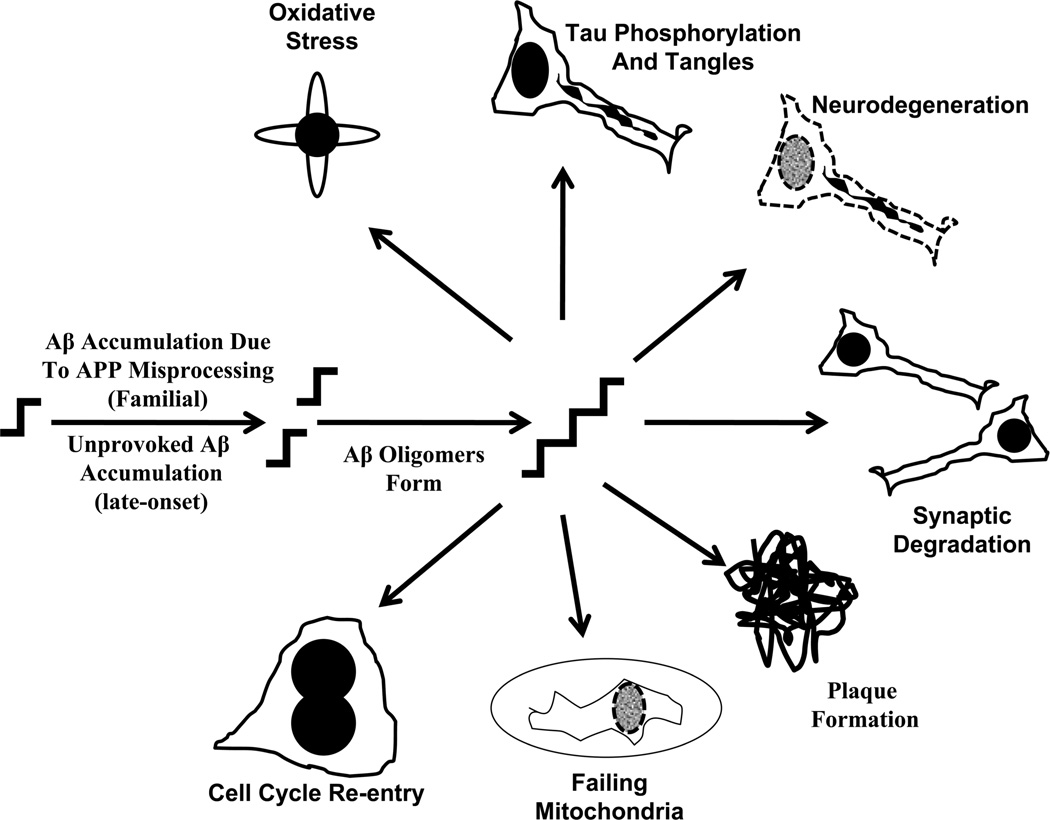
This is certainly the most popular current view. The idea that beta amyloid (Aβ) protein causes AD forms the basis of the “amyloid cascade hypothesis” (Hardy and Allsop 1991; Hardy and Higgins 1992; Hardy and Selkoe 2002). The amyloid cascade hypothesis proposes accumulation of Aβ, as either a consequence of increased production or decreased removal, incites all other downstream AD-associated phenomena and ultimately the disease itself (Figure 1). Mark was a fierce opponent of this hypothesis (Perry et al. 2000; Lee et al. 2006).
Figure 1. The amyloid cascade hypothesis.
According to this hypothesis, an unprovoked imbalance in either Aβ production or removal rates causes Aβ accumulation within particular cortical regions. Mutations in the APP gene or in presenilin genes presumably cause this imbalance in autosomal dominant, familial AD. Either polymorphisms or mutations in genes whose products directly influence Aβ production, removal, or handling cause the imbalance in non-autosomal dominant, late-onset AD. This crucial Aβ imbalance may ultimately reflect higher total Aβ or else an increased Aβ42/Aβ40 ratio. In any case, the Aβ42 monomer excess results in Aβ42 oligomer production. Aβ oligomers eventually form extracellular fibrils, the fibrils collect to form plaques, and the plaques themselves may be toxic. Extracellular oligomers also attack synapses and destroy neurons. Exactly how Aβ oligomers cause most of their other downstream consequences is not settled. Downstream phenomena, such as oxidative stress, tangle formation, mitochondrial failure, and cell cycle re-entry may arise due to Aβ intracellular effects, Aβ extracellular effects, or as a general indirect effect of cell damage.
The evolution of this hypothesis is discussed in other reviews but is worth briefly summarizing (Swerdlow 2007a). Alois Alzheimer’s initial presenile dementia patients, who were later said to have “Alzheimer’s disease” (Kraepelin 1910), were noted to have extracellular protein aggregations within their brains (Alzheimer 1907; Alzheimer et al. 1995). These extracellular protein aggregations, called plaques, were also known to collect within the brains of elderly humans as well as aging animals (Blocq and Marinesco 1892; Fischer 1907; Redlich 1898; Critchley 1929). These plaques were initially felt to represent a consequence, and not a cause, of either aging or (in the case of presenile dementia) whatever causes AD (Davis and Chisholm 1999). Over subsequent decades the main plaque constituent was shown to be an amyloid protein (Divry 1927). This means that following Congo red staining, exposure to polarized light generates a green birefringent fluorescence pattern.
Amyloid proteins generally contain β-sheet folded peptides (Sipe and Cohen 2000). Eventually the main β-sheet folded amyloid peptide within plaques was determined and this protein was named the beta amyloid, or Aβ, protein (Glenner and Wong 1984b). This Aβ protein was also shown to accumulate in the plaques of aging Down’s syndrome patients with trisomy 21 (Glenner and Wong 1984a; Masters et al. 1985). It turned out to be the case that Aβ derived from processing of a larger protein, the amyloid precursor protein (APP), which interestingly resided on chromosome 21 (Kang et al. 1987). In 1990, an APP gene mutation was found to cause the Dutch variant of a devastating autosomal dominant cerebral hemorrhage syndrome, hereditary cerebral hemorrhage with amyloidosis (HCHWA), in which carriers develop vascular wall amyloid aggregations that structurally resemble those which accumulate in AD brain cortex (Levy et al. 1990). The following year, an APP gene mutation was shown to segregate with disease in a family with autosomal dominant amyloid angiopathy, cortical plaques, and presenile dementia (Goate et al. 1991).
The amyloid cascade hypothesis soon followed (Hardy and Allsop 1991; Hardy and Higgins 1992). This hypothesis speculated the intact, mutated APP molecule itself did not directly initiate the essential AD cascade, but rather APP mutation increased Aβ to high levels and at high levels Aβ initiated the cascade. Studies performed over the next twenty years have concluded Aβ can be toxic when administered to cultured cells, that the 42 amino acid Aβ species (Aβ42) is more toxic than other physiologically relevant species, and oligomeric configurations of Aβ42 are more likely to initiate the cascade than fibrillar, protofibrillar, or monomeric forms (Yankner et al. 1989; Hardy and Selkoe 2002; Walsh and Selkoe 2007).
The amyloid cascade hypothesis was developed from studies of rare, autosomal dominant AD kindreds with APP mutations. It was extrapolated to also apply to the non-autosomal dominant pre-senile and also the senile AD variants that comprise over 99% of AD cases (Swerdlow 2007a). Sporadic pre-senile AD and senile AD patients, however, do not have demonstrable APP mutations and APP polymorphisms appear to have no-to-little impact on the disease (Guyant-Marechal et al. 2007). It has therefore been proposed variants in other genes that directly influence either APP processing or Aβ disposal cause Aβ to accumulate. Several genes found to associate with AD risk, including some genes implicated through large scale genome-wide association studies (GWAS), may indeed ultimately impact amyloidosis but how directly or indirectly these variants do in fact influence amyloidosis is unclear (Rogaeva et al. 2007; Jun et al. 2010; Castellano et al. 2011; Harold et al. 2009). Further, unless specific variants in the apolipoprotein E protein turn out to directly affect Aβ homeostasis (Castellano et al. 2011), genetically-determined variations in Aβ-handling appear to minimally affect AD risk. The obvious conceptual inference, which much of the AD research field has been reluctant to accept, is that although it is possible early onset, autosomal dominant and late-onset, non-autosomal dominant AD could ultimately share common final pathways they may not share a common etiology.
It is important to emphasize this conceptual approach does not claim amyloid protein cannot initiate and drive central neurodegeneration and cause dementia. There are few reasons to question whether amyloid protein accumulation initiates a toxic cascade in autosomal dominant AD arising from APP mutation. Amyloid accumulation also appears to initiate degeneration in other degenerative dementia syndromes, including familial British dementia (FBD) and familial Danish dementia (FDD) with amyloid angiopathy (Revesz et al. 2009; Mead et al. 2000; Holton et al. 2001; Holton et al. 2002). FBD and FDD arise from mutations in the BRI2 gene, which cause overproduction of a non-Aβ amyloid product. A key question that remains to be settled is whether it is more appropriate to group AD arising from APP mutation with other identified primary amyloidoses such as FBD and FDD than it is to group it with late-onset, sporadic AD, which could constitute a secondary amyloidosis.
Observational and experimental data have related and tied Aβ to other AD-associated phenomena. AD subject biomarker studies suggest Aβ changes temporally precede tau changes (Jack et al. 2010). In transgenic mice with concomitant production of Aβ and a mutant tau protein, Aβ perturbs tau homeostasis (Oddo et al. 2004). Transgenic mice that overproduce Aβ due to mutant APP overexpression develop synapse damage (Dong et al. 2007). Some of these mouse models also show an increase in the number of neurons that re-enter the cell cycle (Yang et al. 2006). Aβ can facilitate the production of reactive oxygen species (ROS) through reactions mediated by a particular methionine residue (Varadarajan et al. 1999). Inhibition of the electron transport chain (ETC) enzyme cytochrome oxidase (COX) is observed in isolated mitochondria or whole cells exposed to Aβ protein, and APP-mutant transgenic mice have reduced COX activity and other mitochondrial perturbations (Canevari et al. 1999; Casley et al. 2002; Crouch et al. 2005; Dragicevic et al. 2010; Du et al. 2010; Hauptmann et al. 2009; Pereira et al. 1998).
The AD and physiologic relevance of these experimental data, though, is unclear. It is abundantly clear that brain amyloidosis can occur in human brains for years, if not decades, without concomitant clinical symptoms or neurodegeneration (Sperling et al. 2011). Even if Aβ does constitute an upstream AD pathology, it is not sufficient by itself to cause full-blown AD. Supporters of the amyloid cascade hypothesis have responded to this by essentially changing the definition of AD so that changes in Aβ homeostasis are now the sole required diagnostic feature; the presence of a dementia or even a dementia prodrome syndrome is no longer required (Sperling et al. 2011).
Beyond this semantic nuance, though, the biggest problem with the amyloid cascade hypothesis is that for most AD patients why Aβ production or disposal changes in the first place is unknown. Because of this, it seems reasonable to consider that both APP processing and Aβ handling are highly regulated processes that are controlled by upstream physiologic events. If it turns out to be the case that a change in any of these upstream events alters Aβ accumulation in AD, then Aβ accumulation cannot constitute the primary cause.
Does Tau Cause AD?
Neurofibrillary tangles were the other hallmark feature described by Alois Alzheimer in his original presenile dementia clinical-pathological case reports (Alzheimer 1907; Alzheimer 1911). These intracellular formations were later shown to consist of phosphorylated tau protein (Grundke-Iqbal et al. 1986). In subsequent studies primarily consisting of late-onset AD subjects, tangle counts were shown to correlate better with cognitive status than plaque counts (Berg et al. 1993; Berg et al. 1998; Giannakopoulos et al. 2003; Nagy et al. 1996), and both hyperphosphorylated tau and tangles are found in the brains of middle-aged, non-demented individuals (Braak and Braak 1995; Arriagada et al. 1992; Hof et al. 1996). For reasons such as these some investigators considered whether tangles, or at least tau protein, might initiate AD.
Tau protein aggregations are in fact observed in several neurodegenerative diseases. To recognize this fact the “tauopathy” family of diseases was defined (Lee et al. 2001). In the tauopathies, aberrant tau physiology is presumed to either cause disease or, by contributing to neurodegeneration, mediate disease symptoms. The strongest evidence that tau can play an etiologic role in neurodegenerative diseases comes from genetic studies of families with autosomal dominant frontotemporal dementia syndromes in which tau gene mutations segregate with the disease (Seelaar et al. 2011). Neurofibrillary tangles are typically seen in these families.
Tau also appears to be etiologically relevant, although not causal, in two other related neurodegenerative diseases, progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) (Pittman et al. 2006; Sergeant et al. 1999). This is because polymorphisms in the tau gene influence disease risk; most PSP and CBD patients are homozygous for the H1 tau gene haplotype (Baker et al. 1999). Tangles are seen in the brains of PSP and CBD patients. Additionally, the H1 tau gene haplotype influences Parkinson’s disease (PD) risk (Elbaz et al. 2011). This is particularly interesting because PD brain histology studies do not typically emphasize tangles as being characteristic features.
Tau gene mutations have not been implicated in autosomal dominant AD families, and if tau gene polymorphic variation does contribute to AD that contribution is at most very small (Myers et al. 2005; Mukherjee et al. 2007). In AD biomarker studies, changes in cerebrospinal fluid (CSF) tau levels occur after changes in CSF Aβ (Jack et al. 2010). For reasons such as these, while many still suspect aberrant tau protein plays an important role in AD neurodegeneration cascades few seem to believe tau protein initiates those cascades.
Compared to Aβ, tau’s relationship to other AD brain phenomena has not been as extensively studied. Despite this, tau’s normal physiologic role is perhaps better understood than that of Aβ (Goedert et al. 2006; Goedert 1998; Lovestone and Reynolds 1997). Tau interacts with microtubules, constitutes part of the cytoskeleton, and in this capacity tau likely influences axonal transport (Adalbert et al. 2007). Malfunctioning axonal transport systems would predictably impair the delivery of mitochondria to synapses, as well as the return of degraded mitochondria to the cell body. This could lead to synaptic degradation and oxidative stress.
As is the case with Aβ, tau homeostasis is an exquisitely regulated process. For example, during embryonic development, while cells are rapidly dividing and migrating tau is highly phosphorylated (Lovestone and Reynolds 1997; Goedert 1998). Later on, tau in differentiated cells is found in its relatively unphosphorylated form. In various situations, such as in AD neurons or in tumor cells, tau phosphorylation occurs again. As noted by Mark Smith, AD neurons actually appear to re-enter the cell cycle, which could relate to why AD neurons also have phosphorylated tau (McShea et al. 1997; McShea et al. 2007; Zhu et al. 2004a). In essence, phosphorylated tau may therefore simply be a characteristic of dividing cells (Swerdlow and Khan 2004).
In the mouse brain, fasting induces tau phosphorylation (Yanagisawa et al. 1999) and tau phosphorylates during hibernation (Arendt et al. 2003; Hartig et al. 2007). This phosphorylation is apparently mediated through kinase activation. It is interesting that just as bioenergetic stress alters APP processing and promotes Aβ production, bioenergetic stress also promotes tau phosphorylation (Blass et al. 1990; Szabados et al. 2004). It therefore seems to be the case that cell bioenergetics constitutes an important upstream regulator of both Aβ production and tau phosphorylation.
Does Synaptic Loss Cause AD?
In AD brains, synaptic loss correlates better with cognitive decline than either Aβ plaques or tau tangles (Coleman and Yao 2003; DeKosky et al. 1996; Terry et al. 1991). Clinical-pathological surveys of subjects with AD therefore suggest synapse degradation could constitute a defining event. Despite this, few would place synaptic degradation at the apex of any potential AD pathophysiologic cascade. Synapse formation and maintenance are highly regulated processes, so synapse degradation would more likely represent a consequence of failed synaptic maintenance or induced synaptic damage than it would a primary event. In other words, failing neuron health in AD is probably not a function of synaptic loss; synaptic loss in AD is probably a function of failing neuron health.
If synapses are being externally attacked in the AD brain, then Aβ could represent a potential culprit (Walsh et al. 2002). If synapses are being destroyed from within, oxidative stress or perturbed mitochondria could be responsible. Failed axonal transport due to tau alterations, or bioenergetic stress due to mitochondrial failure, could also interfere with synapse maintenance.
Does Oxidative Stress Cause AD?
Mark Smith and colleagues demonstrated how pervasive oxidative stress is within the AD brain (Smith et al. 1994; Smith et al. 1995; Perry et al. 2002; Nunomura et al. 1999). This line of influential work represents one of Mark’s major contributions to the AD research field.
In this respect several seminal observations made by Mark Smith and colleagues are particularly relevant to the AD etiology debate. One observation is that oxidative stress in the AD brain is seen independent of plaques. In fact, oxidative stress levels are actually lower in plaque-containing areas than they are in plaque-free areas (Nunomura et al. 2001). One potential explanation for this is that Aβ production could actually represent a compensatory response to increased oxidative stress (Smith et al. 2002; Lee et al. 2006). This is one of the reasons why Mark predicted removing Aβ from the AD brain or preventing its formation might prove detrimental to AD patients. This further suggests oxidative stress temporally precedes Aβ production (Nunomura et al. 2001). Consistent with this view is the observation that oxidative stress activates the beta secretase (BACE) enzyme that helps process APP to Aβ (Tamagno et al. 2002).
The presence of oxidative stress in the AD brain, though, does not address why it developed or where it was produced. Oxidative stress is classically considered to represent a consequence of either ROS overproduction or underscavenging. Free radicals are eliminated by families of antioxidant enzymes that include the superoxide dismutases, glutathione peroxidase and reductase, catalase, and the thioredoxins. Free radical scavenging molecules, which are commonly referred to as “antioxidants”, also counter oxidative stress because they can accept and stabilize unstable electrons. Scant evidence, though, suggests critical free radical scavenging enzyme defects or antioxidant deficiencies occur in AD. For this reason, oxidative stress in AD is mostly suspected to result from radical overproduction.
ROS are produced through a variety of enzyme-mediated oxidase reactions; xanthine oxidase, monoamine oxidase, and NADPH oxidase catalyzed reactions are each associated with oxygen radical production (Bruce-Keller et al. 2010). Because nitric oxide is a radical molecule, nitric oxide synthase (NOS) produces reactive nitrogen species (RNS). Some ROS are also produced or modified through non-enzymatic reactions. In the Fenton reaction, hydrogen peroxide oxidizes ferrous iron (Fe2+) to produce ferric iron (Fe3+) and hydroxyl radicals. In the Haber-Weiss reaction, hydrogen peroxide reacts with superoxide to produce hydroxyl radical. Hydroxyl radical also results from the peroxynitrite reaction in which nitric oxide and superoxide react with each other. Arachidonic acid produced through phospholipase C or phospholipase A2-mediated phospholipid hydrolysis further promote hydroxyl radical production (Singh et al. 1981). Isoprostanes, which are used as a clinical biomarker of oxidative stress, are themselves a byproduct of arachidonic acid auto-oxidation.
The mitochondrial ETC is commonly cited as the main producer of intracellular oxidative stress (Shigenaga et al. 1994), and evidence suggests mitochondria also constitute the source of increased oxidative stress in AD brain (Nunomura et al. 1999). Mitochondrial ROS production increases when the mitochondrial membrane potential increases (Brand et al. 2004). In this case, mitochondrial ROS likely performs an important signaling function that informs the nucleus of the cell’s bioenergetic status (Finkel 2001, 2003; Petersen et al. 2007). ETC enzyme defects also cause mitochondrial ROS production, and in the setting of ETC enzyme defects mitochondrial ROS overproduction occurs in the presence of depolarized mitochondria (Barrett et al. 2004; Swerdlow 2011).
Mitochondrial dysfunction-oxidative stress relationships, though, can be reciprocal. Free radicals can inhibit ETC enzymes (Brown 1999; Riobo et al. 2001). Free radicals also oxidize mitochondrial DNA (mtDNA). During mtDNA replication, oxidized bases are misread and somatic mutations result (Minnick et al. 1994; Pavlov et al. 1994; Ozawa 1997).
If free radicals are a cause of AD mitochondrial dysfunction, and not a consequence of this, then where might these radicals arise from? One hypothesis is that Aβ itself acts as a pro-oxidant (Varadarajan et al. 1999). Data from Mark Smith and colleagues, though, is not consistent with this view because in the AD brain Aβ deposition anatomically correlates with reduced oxidative stress (Nunomura et al. 2001).
Does Cell Cycle Re-entry Cause AD?
Mark Smith was one of the first to report aberrant cell cycle activity in the AD brain (McShea et al. 1997; McShea et al. 2007; Zhu et al. 2004a; Nagy et al. 1998). Specifically, in the AD brain neurons appear to re-enter the cell cycle (Vincent et al. 1996; Arendt et al. 2000; Herrup and Arendt 2002; Mosch et al. 2007; Yang et al. 2001; Yang et al. 2003; Vincent et al. 1998). These cycling neurons proceed through the G1, S, and even the G2 phases of replication but cannot successfully replicate. G2-M arrest and degeneration result in neurons that make it that far, although abnormal entry into any phase probably reflects compromised neuron health.
Work conducted by Mark and colleagues further suggests that from a temporal perspective, AD brain cell cycle abnormalities are a relatively early pathologic manifestation. Because data from Mark’s lab also showed oxidative stress constitutes an early pathologic manifestation, a “two-hit hypothesis” was proposed in which mitotic perturbation was postulated to induce oxidative stress and oxidative stress was postulated to induce mitotic perturbation (Zhu et al. 2007; Zhu et al. 2004b; Zhu et al. 2001). According to this hypothesis, critical dysfunction and degeneration result when oxidative stress and cell cycle re-entry co-exist within a neuron. The two-hit hypothesis assumes amyloidosis and tau changes may or may not facilitate neuron loss, but are unlikely to initiate the critical pathologic cascades. Other investigators have also proposed aberrant cell cycle phenomena directly contribute to AD pathogenesis (Herrup 2010).
Do Mitochondria Cause AD?
Mitochondria are abnormal in AD patients and increasingly mitochondria are considered to play an important role in AD (Swerdlow 2009; Swerdlow 2011; Swerdlow and Kish 2002). In 2001 Mark Smith and colleagues published a highly influential immunochemical and electron microscopy study of AD brain mitochondria (Hirai et al. 2001). In this study the number of normal-appearing mitochondria within AD brain hippocampal neurons was reduced relative to controls, but the number of degraded or degrading mitochondria was increased. The amount of PCR-amplifiable mtDNA was reduced but mtDNA deletion levels were increased. In control subject brains levels of a COX protein subunit, COX1, increased with advancing age and interestingly COX1 protein was even higher in AD brains. All these characteristics were most prominent in hippocampal neurons that showed the highest degree of concomitant oxidative stress.
With the exception of its rare autosomal dominant variants, AD and aging are closely associated (Swerdlow 2007a, c). In some elderly demographics the percentage of those with AD probably exceeds the percentage of those that don’t have AD (Corrada et al. 2010). In addition to being abnormal in the AD brain, particular mitochondrial alterations are observed in the brains of aged humans and animals (Boveris and Navarro 2008; Navarro and Boveris 2007). In some instances age and AD-associated mitochondrial defects overlap but differ in magnitude. For example, brain COX activity is probably lower in elderly individuals than it is in young individuals, and COX activity is even lower in AD subjects than it is in non-demented elderly subjects (Lin et al. 2002; Swerdlow 2011; Swerdlow and Kish 2002). Observational and experimental data suggest mitochondria may contribute to the aging process itself (Trifunovic et al. 2004; Kujoth et al. 2005). Mitochondria therefore constitute a nexus through which aging and AD may relate.
AD-associated mitochondrial defects were recently reviewed in detail elsewhere (Swerdlow 2011). Several of these details, though, seem particularly relevant to the current discussion of whether mitochondria initiate an AD-typical pathologic cascade. Of note, some AD mitochondrial defects are not brain-limited. In addition to its reduction in the AD brain, COX activity is lower in platelet and fibroblast mitochondria from AD subjects than it is in age-matched control subject platelet and fibroblast mitochondria (Parker et al. 1990; Curti et al. 1997). From a mitochondrial perspective, then, AD is not a brain-limited condition and therefore AD-associated mitochondrial characteristics are not simply a consequence of neurodegeneration or, it appears, any other brain limited pathology.
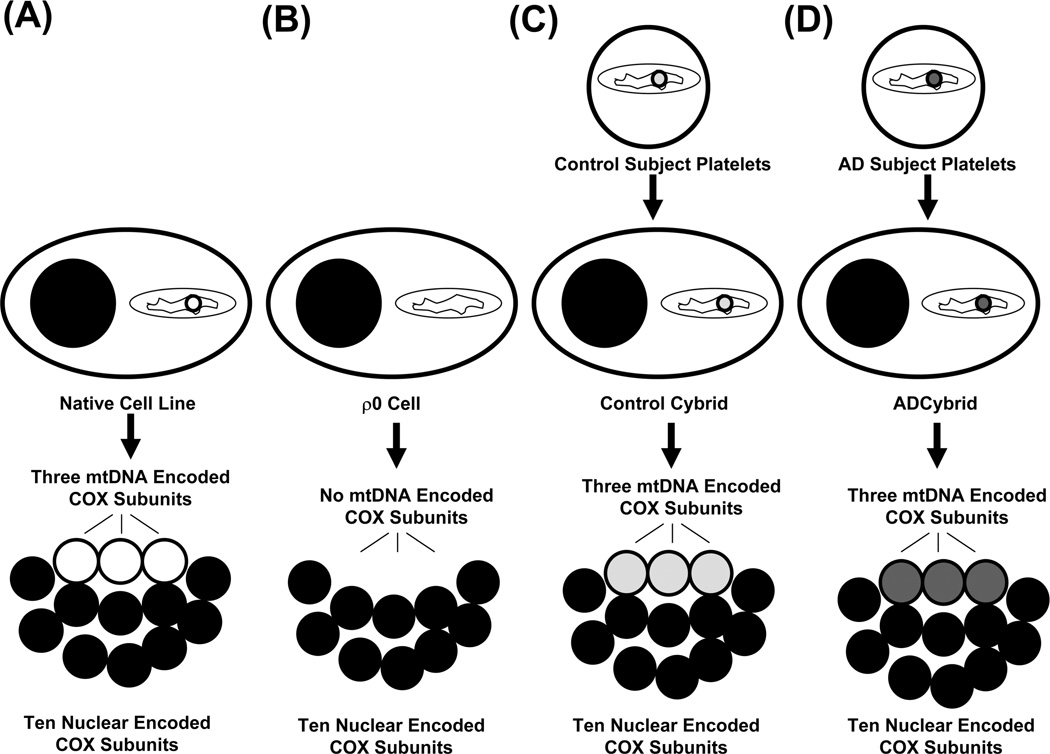
Data from cytoplasmic hybrid (cybrid) studies suggest AD-specific mitochondrial dysfunction can initiate key AD-associated pathologies (Swerdlow 2007b, d; Swerdlow 2011). Cybrid cell lines are created when mitochondria from a particular source are transferred to cultured cell lines (Figure 2). In the case of AD cybrid studies, mitochondria from AD subject platelets were transferred to mtDNA-depleted human neuroblastoma or teratocarcinoma cell lines with neuronal characteristics. Because these cell lines contained no endogenous mtDNA (such cells are called ρ0 cells), all mtDNA-encoded proteins in the resulting cybrid cell lines derive from AD subject mtDNA.
Figure 2. Cybrid studies conclude mtDNA is at least partly responsible for low COX activity in AD subjects.
COX has 13 subunits, 10 of which are nuclear-gene encoded and three of which are encoded on mtDNA genes (A). If a cell’s mtDNA is removed, it cannot produce its three crucial mtDNA-encoded subunits; these “ρ0” cells have virtually no detectable COX activity (B). Transferring mtDNA-containing mitochondria from an exogenous source such as platelets restores the ρ0 cell’s mtDNA, allows it to again produce mtDNA-encoded COX subunits, and creates a complete COX holoenzyme (C and D). Because the ten nuclear subunits are equivalent between cybrid lines containing mtDNA from different sources, differences in COX function between cybrid lines reflect differences in their mtDNA. Cybrid cell lines containing mtDNA from AD subjects (D) have a lower COX activity than cybrid cell lines containing mtDNA from control subjects (C).
Several respiratory chain holoenzymes contain a mix of both nuclear and mtDNA-encoded protein subunits. These include complex I, complex III, complex IV (COX), and complex V. In newly created cybrid cell lines, a point is eventually reached in which the nuclear-encoded subunits all derive from the cell line nucleus, and the mtDNA-encoded subunits all derive from the mtDNA that was transferred with the original platelet mitochondria (Figure 2). All cybrid cell lines produced from a common ρ0 stock have the same nuclear genes and therefore their nuclear DNA-derived respiratory chain subunits should be identical. Differences in respiratory chain function between cybrid cell lines, therefore, most likely reflect differences in their mtDNA. These mtDNA differences in turn reflect the fact that each cybrid cell line’s mtDNA originated from a different platelet donor.
COX activity is lower in cybrid cell lines prepared through the transfer of AD subject platelet mitochondria than it is in cybrid cell lines prepared through the transfer of control subject platelet mitochondria (Swerdlow 2007b, d; Swerdlow 2011). The most straightforward interpretation for this is that mtDNA from AD subjects differs from that of control subjects, these differences result in lower AD cybrid COX activity, and mtDNA at least partly accounts for low COX activity in AD subjects.
ROS production is also higher in AD cybrid cell lines than it is in control cybrid cell lines (Swerdlow et al. 1997; Swerdlow 2007b, d; Swerdlow 2011). This occurs despite the fact that the mitochondrial membrane potential is reduced in AD cybrids. The most likely source of increased AD cybrid ROS production is a defective AD cybrid ETC.
Compared to control cybrid cell lines, AD cybrid cell lines also generate increased amounts of Aβ (Khan et al. 2000; Swerdlow 2007b, d; Swerdlow 2011). Administering antioxidants prevents this. Overall, cybrid data suggest AD subject mtDNA generates defective ETC subunits that alter respiratory chain function, altered respiratory chain function produces oxidative stress, and oxidative stress shifts APP processing towards its Aβ derivative.
AD cybrid data in general are consistent with other data that indicate respiratory chain inhibition or bioenergetic stress shift APP processing away from α secretase-mediated processing, which should enhance its BACE-mediated processing and produce Aβ (Gabuzda et al. 1994; Gasparini et al. 1997; Webster et al. 1998; Khan et al. 2000; Tamagno et al. 2002). Cybrid data are also consistent with other data that show ETC inhibition increases ROS production (Barrett et al. 2004).
ETC inhibition and bioenergetic stress have also been shown to increase tau phosphorylation and aggregation (Blass et al. 1990; Szabados et al. 2004). In general, the more aerobic a cell is the more differentiated it tends to be, and more anaerobic cells tend to be more mitotically active (Swerdlow and Khan 2004).
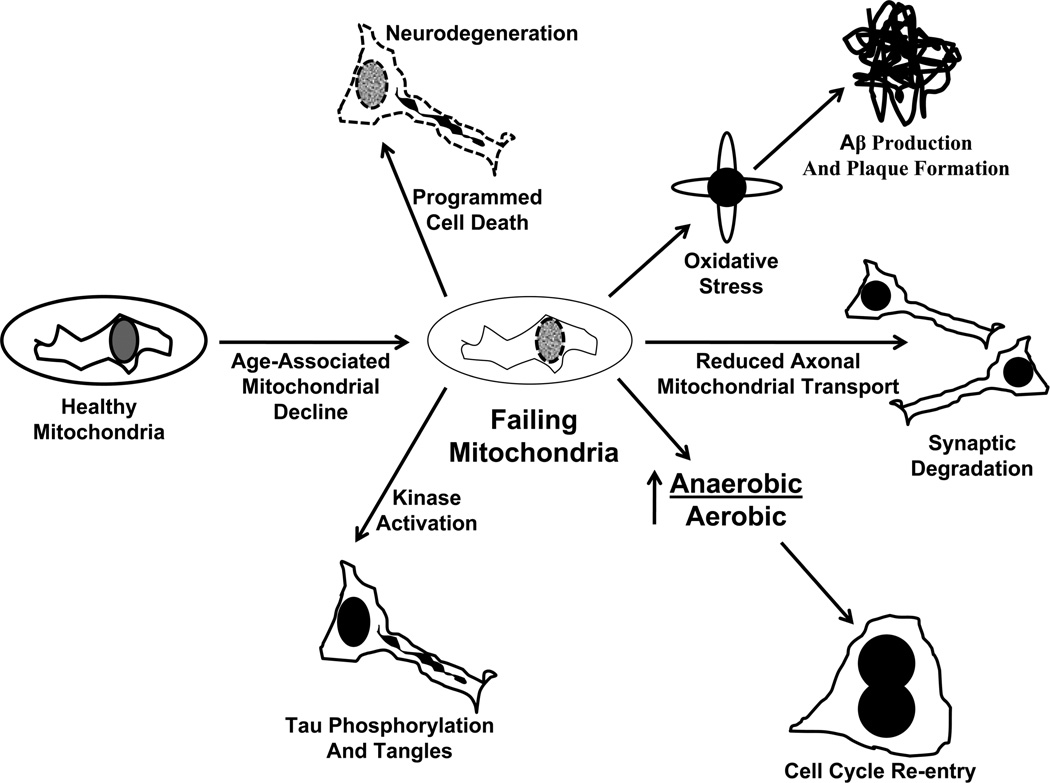
For all these reasons, an upstream role for mitochondria in AD pathology cascades seems feasible, at least for sporadic AD. The “mitochondrial cascade hypothesis” places mitochondrial dysfunction at the apex of the late-onset, non-autosomal dominant AD pathology cascade (Swerdlow 2007c; Swerdlow 2011; Swerdlow et al. 2010; Swerdlow and Khan 2004, 2009) (Figure 3). The mitochondrial cascade hypothesis proposes an individual’s genetic composition determines their baseline mitochondrial function and durability. This in turn determines the rate at which age-dependent mitochondrial functional decline proceeds within that individual. When and if mitochondrial functional capacity falls below a critical threshold other AD-typical pathologies ensue. These events are initiated by mitochondrial dysfunction and include Aβ production, tau phosphorylation, synaptic degeneration, oxidative stress, and neuron cell cycle re-entry.
Figure 3. The mitochondrial cascade hypothesis.
According to this hypothesis, an individual’s genetic make-up determines baseline mitochondrial function and durability. With advancing age mitochondrial function declines. The rate of mitochondrial decline is determined by a combination of the individual’s genetic make-up and environmental factors. When mitochondrial function surpasses a critical threshold beyond which compensation is not possible, AD-associated phenomena occur. Apoptosis pathways are activated and neurodegeneration results; faulty electron transport chains produce superoxide, which activates BACE and causes Aβ production; bioenergetic failure at the synapse causes synaptic degradation; shifting anaerobic/aerobic bioenergetic flux relationships activate cell cycle re-entry; and oxidative and bioenergetic stresses activate the kinases that phosphorylate tau.
Conclusions
Mark Smith’s research into the causes of AD challenged the field’s prevailing wisdom. His exceptional courage, energy, and intellectual prowess made him one of the AD research community’s most important and highly regarded gadflies. In losing Mark the field lost an important voice, and some lost a valued and beloved colleague.
The amyloid cascade hypothesis continues to dominate AD research and how many investigators conceptually approach this disease. The amyloid cascade hypothesis presumes all AD variants are primary amyloidoses. According to this hypothesis Aβ in all cases initiates AD neuropathology, neurodysfunction, and neurodegeneration. Indeed, newly revised criteria define AD as the presence of altered brain Aβ (Sperling et al. 2011). Research conducted by Mark and others, though, raises serious doubts as to whether Aβ could possibly constitute the “head of the snake”. After all, APP processing and Aβ handling are exquisitely regulated processes, and some of these regulatory processes are perturbed in the AD brain. While much research is still required to settle this debate, as far as AD’s etiology goes the head of the snake is increasingly shaped like a mitochondrion. At the time of his death, Mark certainly seemed to be headed in this direction (Moreira et al. 2010a; Moreira et al. 2010b).
Acknowledgements
The author is supported by P30AG035982 and the Morgan Family Foundation.
Abbreviations
- Aβ
beta amyloid
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- BACE
Beta secretase
- COX
Cytochrome oxidase
- ETC
Electron transport chain
- FBD
Familial British dementia
- FDD
Familial Danish dementia
- GWAS
Genome wide association study
- HCHWA
Hereditary cerebral hemorrhage with amyloidosis
- mtDNA
mitochondrial DNA
- NOS
nitric oxide synthase
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
References
- Adalbert R, Gilley J, Coleman MP. Abeta, tau and ApoE4 in Alzheimer's disease: the axonal connection. Trends Mol Med. 2007;13(4):135–142. doi: 10.1016/j.molmed.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Alzheimer A. Uber eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiat Psych-Gerichtl Med. 1907;64:146–148. [Google Scholar]
- Alzheimer A. Uber eigenartige Krankheitsfalle des spateren Alters. Z die Gesamte Neurologie Pscyhiatrie. 1911;4:456–485. [Google Scholar]
- Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer's 1907 paper, "Uber eine eigenartige Erkankung der Hirnrinde". Clin Anat. 1995;8(6):429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- Arendt T, Holzer M, Stobe A, Gartner U, Luth HJ, Bruckner MK, Ueberham U. Activated mitogenic signaling induces a process of dedifferentiation in Alzheimer's disease that eventually results in cell death. Ann N Y Acad Sci. 2000;920:249–255. doi: 10.1111/j.1749-6632.2000.tb06931.x. [DOI] [PubMed] [Google Scholar]
- Arendt T, Stieler J, Strijkstra AM, Hut RA, Rudiger J, Van der Zee EA, Harkany T, Holzer M, Hartig W. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci. 2003;23(18):6972–6981. doi: 10.1523/JNEUROSCI.23-18-06972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 1992;42(9):1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, Hardy J, Lynch T, Bigio E, Hutton M. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8(4):711–715. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- Barrett MJ, Alones V, Wang KX, Phan L, Swerdlow RH. Mitochondria-derived oxidative stress induces a heat shock protein response. J Neurosci Res. 2004;78(3):420–429. doi: 10.1002/jnr.20249. [DOI] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Baty J, Morris JC. Neuropathological indexes of Alzheimer's disease in demented and nondemented persons aged 80 years and older. Arch Neurol. 1993;50(4):349–358. doi: 10.1001/archneur.1993.00540040011008. [DOI] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55(3):326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Blass JP, Baker AC, Ko L, Black RS. Induction of Alzheimer antigens by an uncoupler of oxidative phosphorylation. Arch Neurol. 1990;47(8):864–869. doi: 10.1001/archneur.1990.00530080046009. [DOI] [PubMed] [Google Scholar]
- Blocq P, Marinesco G. Sur les lesions et la pathogenie de l’epilepsie dite essentielle. La Semaine Medicale. 1892;12:445–446. [Google Scholar]
- Boveris A, Navarro A. Brain mitochondrial dysfunction in aging. IUBMB Life. 2008;60(5):308–314. doi: 10.1002/iub.46. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16(3):271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278–284. [DOI] [PubMed] [Google Scholar]
- Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37(6):755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Brown GC. Nitric oxide and mitochondrial respiration. Biochim Biophys Acta. 1999;1411(2–3):351–369. doi: 10.1016/s0005-2728(99)00025-0. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, White CL, Gupta S, Knight AG, Pistell PJ, Ingram DK, Morrison CD, Keller JN. NOX activity in brain aging: exacerbation by high fat diet. Free Radic Biol Med. 2010;49(1):22–30. doi: 10.1016/j.freeradbiomed.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canevari L, Clark JB, Bates TE. beta-Amyloid fragment 25–35 selectively decreases complex IV activity in isolated mitochondria. FEBS Lett. 1999;457(1):131–134. doi: 10.1016/s0014-5793(99)01028-5. [DOI] [PubMed] [Google Scholar]
- Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem. 2002;80(1):91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Lee HG, Siedlak SL, Nunomura A, Hayashi T, Nakamura M, Zhu X, Perry G, Smith MA. Reexamining Alzheimer's disease: evidence for a protective role for amyloid-beta protein precursor and amyloid-beta. J Alzheimers Dis. 2009;18(2):447–452. doi: 10.3233/JAD-2009-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, Demattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE Isoforms Differentially Regulate Brain Amyloid-{beta} Peptide Clearance. Sci Transl Med. 2011;3(89) doi: 10.1126/scitranslmed.3002156. 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PD, Yao PJ. Synaptic slaughter in Alzheimer's disease. Neurobiol Aging. 2003;24(8):1023–1027. doi: 10.1016/j.neurobiolaging.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann Neurol. 2010;67(1):114–121. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley M. The nature and significance of senile plaques. J Neurol Psychopath. 1929;10:124–139. doi: 10.1136/jnnp.s1-10.38.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42. J Neurosci. 2005;25(3):672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti D, Rognoni F, Gasparini L, Cattaneo A, Paolillo M, Racchi M, Zani L, Bianchetti A, Trabucchi M, Bergamaschi S, Govoni S. Oxidative metabolism in cultured fibroblasts derived from sporadic Alzheimer's disease (AD) patients. Neurosci Lett. 1997;236(1):13–16. doi: 10.1016/s0304-3940(97)00741-6. [DOI] [PubMed] [Google Scholar]
- Davis JN, 2nd, Chisholm JC. Alois Alzheimer and the amyloid debate. Nature. 1999;400(6747):810. doi: 10.1038/23571. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW, Styren SD. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration. 1996;5(4):417–421. doi: 10.1006/neur.1996.0056. [DOI] [PubMed] [Google Scholar]
- Divry P. Etude histochimique des plaques seniles. J Belg Neurol Psychiatry. 1927;9:643–657. [Google Scholar]
- Dong H, Martin MV, Chambers S, Csernansky JG. Spatial relationship between synapse loss and beta-amyloid deposition in Tg2576 mice. J Comp Neurol. 2007;500(2):311–321. doi: 10.1002/cne.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragicevic N, Mamcarz M, Zhu Y, Buzzeo R, Tan J, Arendash GW, Bradshaw PC. Mitochondrial amyloid-beta levels are associated with the extent of mitochondrial dysfunction in different brain regions and the degree of cognitive impairment in Alzheimer's transgenic mice. J Alzheimers Dis. 2010;20(Suppl 2):S535–S550. doi: 10.3233/JAD-2010-100342. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc Natl Acad Sci U S A. 2010;107(43):18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A, Ross OA, Ioannidis JP, Soto-Ortolaza AI, Moisan F, Aasly J, Annesi G, Bozi M, Brighina L, Chartier-Harlin MC, Destee A, Ferrarese C, Ferraris A, Gibson JM, Gispert S, Hadjigeorgiou GM, Jasinska-Myga B, Klein C, Kruger R, Lambert JC, Lohmann K, van de Loo S, Loriot MA, Lynch T, Mellick GD, Mutez E, Nilsson C, Opala G, Puschmann A, Quattrone A, Sharma M, Silburn PA, Stefanis L, Uitti RJ, Valente EM, Vilarino-Guell C, Wirdefeldt K, Wszolek ZK, Xiromerisiou G, Maraganore DM, Farrer MJ. Independent and joint effects of the MAPT and SNCA genes in Parkinson disease. Ann Neurol. 2011;69(5):778–792. doi: 10.1002/ana.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Reactive oxygen species and signal transduction. IUBMB Life. 2001;52(1–2):3–6. doi: 10.1080/15216540252774694. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15(2):247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Fischer O. Miliare Nekrosen mit drusigen Wucherungen der Neurofibrillen, eine regelmabige Veranderung der Hirnrinde bei seniler Demenz. Monatsschr Psychiatr Neurol. 1907;22:361–372. [Google Scholar]
- Gabuzda D, Busciglio J, Chen LB, Matsudaira P, Yankner BA. Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentially amyloidogenic derivative. J Biol Chem. 1994;269(18):13623–13628. [PubMed] [Google Scholar]
- Gasparini L, Racchi M, Benussi L, Curti D, Binetti G, Bianchetti A, Trabucchi M, Govoni S. Effect of energy shortage and oxidative stress on amyloid precursor protein metabolism in COS cells. Neurosci Lett. 1997;231(2):113–117. doi: 10.1016/s0304-3940(97)00536-3. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, Morrison JH, Gold G, Hof PR. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60(9):1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984a;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984b;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Goedert M. Neurofibrillary pathology of Alzheimer's disease and other tauopathies. Prog Brain Res. 1998;117:287–306. doi: 10.1016/s0079-6123(08)64022-4. [DOI] [PubMed] [Google Scholar]
- Goedert M, Klug A, Crowther RA. Tau protein, the paired helical filament and Alzheimer's disease. J Alzheimers Dis. 2006;9(3 Suppl):195–207. doi: 10.3233/jad-2006-9s323. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyant-Marechal L, Rovelet-Lecrux A, Goumidi L, Cousin E, Hannequin D, Raux G, Penet C, Ricard S, Mace S, Amouyel P, Deleuze JF, Frebourg T, Brice A, Lambert JC, Campion D. Variations in the APP gene promoter region and risk of Alzheimer disease. Neurology. 2007;68(9):684–687. doi: 10.1212/01.wnl.0000255938.33739.46. [DOI] [PubMed] [Google Scholar]
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991;12(10):383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41(10):1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig W, Stieler J, Boerema AS, Wolf J, Schmidt U, Weissfuss J, Bullmann T, Strijkstra AM, Arendt T. Hibernation model of tau phosphorylation in hamsters: selective vulnerability of cholinergic basal forebrain neurons - implications for Alzheimer's disease. Eur J Neurosci. 2007;25(1):69–80. doi: 10.1111/j.1460-9568.2006.05250.x. [DOI] [PubMed] [Google Scholar]
- Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, Leuner K, Eckert A, Muller WE. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging. 2009;30(10):1574–1586. doi: 10.1016/j.neurobiolaging.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Herrup K. Reimagining Alzheimer's disease--an age-based hypothesis. J Neurosci. 2010;30(50):16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K, Arendt T. Re-expression of cell cycle proteins induces neuronal cell death during Alzheimer's disease. J Alzheimers Dis. 2002;4(3):243–247. doi: 10.3233/jad-2002-4315. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21(9):3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Glannakopoulos P, Bouras C. The neuropathological changes associated with normal brain aging. Histol Histopathol. 1996;11(4):1075–1088. [PubMed] [Google Scholar]
- Holton JL, Ghiso J, Lashley T, Rostagno A, Guerin CJ, Gibb G, Houlden H, Ayling H, Martinian L, Anderton BH, Wood NW, Vidal R, Plant G, Frangione B, Revesz T. Regional distribution of amyloid-Bri deposition and its association with neurofibrillary degeneration in familial British dementia. Am J Pathol. 2001;158(2):515–526. doi: 10.1016/S0002-9440(10)63993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton JL, Lashley T, Ghiso J, Braendgaard H, Vidal R, Guerin CJ, Gibb G, Hanger DP, Rostagno A, Anderton BH, Strand C, Ayling H, Plant G, Frangione B, Bojsen-Moller M, Revesz T. Familial Danish dementia: a novel form of cerebral amyloidosis associated with deposition of both amyloid-Dan and amyloid-beta. J Neuropathol Exp Neurol. 2002;61(3):254–267. doi: 10.1093/jnen/61.3.254. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun G, Naj AC, Beecham GW, Wang LS, Buros J, Gallins PJ, Buxbaum JD, Ertekin-Taner N, Fallin MD, Friedland R, Inzelberg R, Kramer P, Rogaeva E, St George-Hyslop P, Cantwell LB, Dombroski BA, Saykin AJ, Reiman EM, Bennett DA, Morris JC, Lunetta KL, Martin ER, Montine TJ, Goate AM, Blacker D, Tsuang DW, Beekly D, Cupples LA, Hakonarson H, Kukull W, Foroud TM, Haines J, Mayeux R, Farrer LA, Pericak-Vance MA, Schellenberg GD. Meta-analysis confirms CR1, CLU, and PICALM as alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch Neurol. 2010;67(12):1473–1484. doi: 10.1001/archneurol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Trimmer PA, Krebs CT, Bennett JC, Parks JK, Swerdlow RH, Parker WD, Jr, Bennett JP., Jr Alzheimer's disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Ann Neurol. 2000;48(2):148–155. [PubMed] [Google Scholar]
- Kraepelin E. Ein Lehrbuch fur Studierende und Arzte. Lepzig: Klinishce Psychiatrie Verlag Johann Ambrosius Barth; 1910. Psychiatrie. [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309(5733):481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Lee HG, Zhu X, Nunomura A, Perry G, Smith MA. Amyloid beta: the alternate hypothesis. Curr Alzheimer Res. 2006;3(1):75–80. doi: 10.2174/156720506775697124. [DOI] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Bots GT, Luyendijk W, Frangione B. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248(4959):1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer's disease brain. Hum Mol Genet. 2002;11(2):133–145. doi: 10.1093/hmg/11.2.133. [DOI] [PubMed] [Google Scholar]
- Lovestone S, Reynolds CH. The phosphorylation of tau: a critical stage in neurodevelopment and neurodegenerative processes. Neuroscience. 1997;78(2):309–324. doi: 10.1016/s0306-4522(96)00577-5. [DOI] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShea A, Harris PL, Webster KR, Wahl AF, Smith MA. Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer's disease. Am J Pathol. 1997;150(6):1933–1939. [PMC free article] [PubMed] [Google Scholar]
- McShea A, Lee HG, Petersen RB, Casadesus G, Vincent I, Linford NJ, Funk JO, Shapiro RA, Smith MA. Neuronal cell cycle re-entry mediates Alzheimer disease-type changes. Biochim Biophys Acta. 2007;1772(4):467–472. doi: 10.1016/j.bbadis.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Mead S, James-Galton M, Revesz T, Doshi RB, Harwood G, Pan EL, Ghiso J, Frangione B, Plant G. Familial British dementia with amyloid angiopathy: early clinical, neuropsychological and imaging findings. Brain. 2000;123(Pt 5):975–991. doi: 10.1093/brain/123.5.975. [DOI] [PubMed] [Google Scholar]
- Minnick DT, Pavlov YI, Kunkel TA. The fidelity of the human leading and lagging strand DNA replication apparatus with 8-oxodeoxyguanosine triphosphate. Nucleic Acids Res. 1994;22(25):5658–5664. doi: 10.1093/nar/22.25.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer's disease pathophysiology. Biochim Biophys Acta. 2010a;1802(1):2–10. doi: 10.1016/j.bbadis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Zhu X, Wang X, Lee HG, Nunomura A, Petersen RB, Perry G, Smith MA. Mitochondria: a therapeutic target in neurodegeneration. Biochim Biophys Acta. 2010b;1802(1):212–220. doi: 10.1016/j.bbadis.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T. Aneuploidy and DNA replication in the normal human brain and Alzheimer's disease. J Neurosci. 2007;27(26):6859–6867. doi: 10.1523/JNEUROSCI.0379-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee O, Kauwe JS, Mayo K, Morris JC, Goate AM. Haplotype-based association analysis of the MAPT locus in late onset Alzheimer's disease. BMC Genet. 2007;8:3. doi: 10.1186/1471-2156-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AJ, Kaleem M, Marlowe L, Pittman AM, Lees AJ, Fung HC, Duckworth J, Leung D, Gibson A, Morris CM, de Silva R, Hardy J. The H1c haplotype at the MAPT locus is associated with Alzheimer's disease. Hum Mol Genet. 2005;14(16):2399–2404. doi: 10.1093/hmg/ddi241. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Esiri MM, Smith AD. The cell division cycle and the pathophysiology of Alzheimer's disease. Neuroscience. 1998;87(4):731–739. doi: 10.1016/s0306-4522(98)00293-0. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Jobst KA, Esiri MM, Morris JH, King EM, MacDonald B, Litchfield S, Barnetson L, Smith AD. Hippocampal pathology reflects memory deficit and brain imaging measurements in Alzheimer's disease: clinicopathologic correlations using three sets of pathologic diagnostic criteria. Dementia. 1996;7(2):76–81. doi: 10.1159/000106857. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292(2):C670–C686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60(8):759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J Neurosci. 1999;19(6):1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43(3):321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Ozawa T. Genetic and functional changes in mitochondria associated with aging. Physiol Rev. 1997;77(2):425–464. doi: 10.1152/physrev.1997.77.2.425. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer's disease. Neurology. 1990;40(8):1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- Pavlov YI, Minnick DT, Izuta S, Kunkel TA. DNA replication fidelity with 8-oxodeoxyguanosine triphosphate. Biochemistry. 1994;33(15):4695–4701. doi: 10.1021/bi00181a029. [DOI] [PubMed] [Google Scholar]
- Pereira C, Santos MS, Oliveira C. Mitochondrial function impairment induced by amyloid beta-peptide on PC12 cells. Neuroreport. 1998;9(8):1749–1755. doi: 10.1097/00001756-199806010-00015. [DOI] [PubMed] [Google Scholar]
- Perry G, Nunomura A, Hirai K, Zhu X, Perez M, Avila J, Castellani RJ, Atwood CS, Aliev G, Sayre LM, Takeda A, Smith MA. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer's and other neurodegenerative diseases? Free Radic Biol Med. 2002;33(11):1475–1479. doi: 10.1016/s0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- Perry G, Nunomura A, Raina AK, Smith MA. Amyloid-beta junkies. Lancet. 2000;355(9205):757. doi: 10.1016/S0140-6736(05)72173-5. [DOI] [PubMed] [Google Scholar]
- Petersen RB, Nunomura A, Lee HG, Casadesus G, Perry G, Smith MA, Zhu X. Signal transduction cascades associated with oxidative stress in Alzheimer's disease. J Alzheimers Dis. 2007;11(2):143–152. doi: 10.3233/jad-2007-11202. [DOI] [PubMed] [Google Scholar]
- Pittman AM, Fung HC, de Silva R. Untangling the tau gene association with neurodegenerative disorders. Hum Mol Genet. 2006;15(Spec No 2):R188–R195. doi: 10.1093/hmg/ddl190. [DOI] [PubMed] [Google Scholar]
- Redlich E. Über miliare Sklerosen der Hirnrinde bei seniler Atrophie. Jahrb Psychiat Neurol. 1898;17:208–216. [Google Scholar]
- Revesz T, Holton JL, Lashley T, Plant G, Frangione B, Rostagno A, Ghiso J. Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 2009;118(1):115–130. doi: 10.1007/s00401-009-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riobo NA, Clementi E, Melani M, Boveris A, Cadenas E, Moncada S, Poderoso JJ. Nitric oxide inhibits mitochondrial NADH:ubiquinone reductase activity through peroxynitrite formation. Biochem J. 2001;359(Pt 1):139–145. doi: 10.1042/0264-6021:3590139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, Chen F, Shibata N, Lunetta KL, Pardossi-Piquard R, Bohm C, Wakutani Y, Cupples LA, Cuenco KT, Green RC, Pinessi L, Rainero I, Sorbi S, Bruni A, Duara R, Friedland RP, Inzelberg R, Hampe W, Bujo H, Song YQ, Andersen OM, Willnow TE, Graff-Radford N, Petersen RC, Dickson D, Der SD, Fraser PE, Schmitt-Ulms G, Younkin S, Mayeux R, Farrer LA, St George-Hyslop P. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39(2):168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelaar H, Rohrer JD, Pijnenburg YA, Fox NC, van Swieten JC. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry. 2011;82(5):476–486. doi: 10.1136/jnnp.2010.212225. [DOI] [PubMed] [Google Scholar]
- Sergeant N, Wattez A, Delacourte A. Neurofibrillary degeneration in progressive supranuclear palsy and corticobasal degeneration: tau pathologies with exclusively "exon 10" isoforms. J Neurochem. 1999;72(3):1243–1249. doi: 10.1046/j.1471-4159.1999.0721243.x. [DOI] [PubMed] [Google Scholar]
- Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Greenwald JE, Bianchine J, Metz EN, Sagone AL., Jr Evidence for the generation of hydroxyl radical during arachidonic acid metabolism by human platelets. Am J Hematol. 1981;11(3):233–240. doi: 10.1002/ajh.2830110303. [DOI] [PubMed] [Google Scholar]
- Sipe JD, Cohen AS. Review: history of the amyloid fibril. J Struct Biol. 2000;130(2–3):88–98. doi: 10.1006/jsbi.2000.4221. [DOI] [PubMed] [Google Scholar]
- Smith MA, Drew KL, Nunomura A, Takeda A, Hirai K, Zhu X, Atwood CS, Raina AK, Rottkamp CA, Sayre LM, Friedland RP, Perry G. Amyloid-beta, tau alterations and mitochondrial dysfunction in Alzheimer disease: the chickens or the eggs? Neurochem Int. 2002;40(6):527–531. doi: 10.1016/s0197-0186(01)00123-1. [DOI] [PubMed] [Google Scholar]
- Smith MA, Kutty RK, Richey PL, Yan SD, Stern D, Chader GJ, Wiggert B, Petersen RB, Perry G. Heme oxygenase-1 is associated with the neurofibrillary pathology of Alzheimer's disease. Am J Pathol. 1994;145(1):42–47. [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Rudnicka-Nawrot M, Richey PL, Praprotnik D, Mulvihill P, Miller CA, Sayre LM, Perry G. Carbonyl-related posttranslational modification of neurofilament protein in the neurofibrillary pathology of Alzheimer's disease. J Neurochem. 1995;64(6):2660–2666. doi: 10.1046/j.1471-4159.1995.64062660.x. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Is aging part of Alzheimer's disease, or is Alzheimer's disease part of aging? Neurobiol Aging. 2007a;28(10):1465–1480. doi: 10.1016/j.neurobiolaging.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. J Neurosci Res. 2007b;85(15):3416–3428. doi: 10.1002/jnr.21167. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Pathogenesis of Alzheimer's disease. Clin Interv Aging. 2007c;2(3):347–359. [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Treating neurodegeneration by modifying mitochondria: potential solutions to a "complex" problem. Antioxid Redox Signal. 2007d;9(10):1591–1603. doi: 10.1089/ars.2007.1676. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. The neurodegenerative mitochondriopathies. J Alzheimers Dis. 2009;17(4):737–751. doi: 10.3233/JAD-2009-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondria and cell bioenergetics: Increasingly recognized components and a possible etiologic cause of Alzheimer's disease. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2011.4149. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Burns JM, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20(Suppl 2):S265–S279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. A "mitochondrial cascade hypothesis" for sporadic Alzheimer's disease. Med Hypotheses. 2004;63(1):8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis: an update. Exp Neurol. 2009;218(2):308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Kish SJ. Mitochondria in Alzheimer's disease. Int Rev Neurobiol. 2002;53:341–385. doi: 10.1016/s0074-7742(02)53013-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP, Jr, Davis RE, Parker WD., Jr Cybrids in Alzheimer's disease: a cellular model of the disease? Neurology. 1997;49(4):918–925. doi: 10.1212/wnl.49.4.918. [DOI] [PubMed] [Google Scholar]
- Szabados T, Dul C, Majtenyi K, Hargitai J, Penzes Z, Urbanics R. A chronic Alzheimer's model evoked by mitochondrial poison sodium azide for pharmacological investigations. Behav Brain Res. 2004;154(1):31–40. doi: 10.1016/j.bbr.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O, Smith MA, Perry G, Tabaton M. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10(3):279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429(6990):417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- Varadarajan S, Yatin S, Kanski J, Jahanshahi F, Butterfield DA. Methionine residue 35 is important in amyloid beta-peptide-associated free radical oxidative stress. Brain Res Bull. 1999;50(2):133–141. doi: 10.1016/s0361-9230(99)00093-3. [DOI] [PubMed] [Google Scholar]
- Vincent I, Rosado M, Davies P. Mitotic mechanisms in Alzheimer's disease? J Cell Biol. 1996;132(3):413–425. doi: 10.1083/jcb.132.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent I, Zheng JH, Dickson DW, Kress Y, Davies P. Mitotic phosphoepitopes precede paired helical filaments in Alzheimer's disease. Neurobiol Aging. 1998;19(4):287–296. doi: 10.1016/s0197-4580(98)00071-2. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101(5):1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Webster MT, Pearce BR, Bowen DM, Francis PT. The effects of perturbed energy metabolism on the processing of amyloid precursor protein in PC12 cells. J Neural Transm. 1998;105(8–9):839–853. doi: 10.1007/s007020050098. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Planel E, Ishiguro K, Fujita SC. Starvation induces tau hyperphosphorylation in mouse brain: implications for Alzheimer's disease. FEBS Lett. 1999;461(3):329–333. doi: 10.1016/s0014-5793(99)01480-5. [DOI] [PubMed] [Google Scholar]
- Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer's disease. J Neurosci. 2001;21(8):2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer's disease. J Neurosci. 2003;23(7):2557–2563. doi: 10.1523/JNEUROSCI.23-07-02557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Varvel NH, Lamb BT, Herrup K. Ectopic cell cycle events link human Alzheimer's disease and amyloid precursor protein transgenic mouse models. J Neurosci. 2006;26(3):775–784. doi: 10.1523/JNEUROSCI.3707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, Neve RL. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science. 1989;245(4916):417–420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]
- Zhu X, Castellani RJ, Takeda A, Nunomura A, Atwood CS, Perry G, Smith MA. Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: the ‘two hit’ hypothesis. Mech Ageing Dev. 2001;123(1):39–46. doi: 10.1016/s0047-6374(01)00342-6. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lee HG, Perry G, Smith MA. Alzheimer disease, the two-hit hypothesis: an update. Biochim Biophys Acta. 2007;1772(4):494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Zhu X, McShea A, Harris PL, Raina AK, Castellani RJ, Funk JO, Shah S, Atwood C, Bowen R, Bowser R, Morelli L, Perry G, Smith MA. Elevated expression of a regulator of the G2/M phase of the cell cycle, neuronal CIP-1-associated regulator of cyclin B, in Alzheimer's disease. J Neurosci Res. 2004a;75(5):698–703. doi: 10.1002/jnr.20028. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Perry G, Smith MA. Alzheimer's disease: the two-hit hypothesis. Lancet Neurol. 2004b;3(4):219–226. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]