Abstract
A 59-year-old man with diabetes mellitus, prior hepatitis B infection and recently diagnosed cirrhosis with prior Babesiosis presented to our institution from an outside hospital with six months of worsening abdominal pain, myalgias and fevers. On admission, physical examination revealed jaundice, hepatosplenomegaly and diffuse lymphadenopathy. Laboratory investigations demonstrated mild anemia, thrombocytopenia, hyperbilirubinemia and elevated lactate dehydrogenase. Tests for human immunodeficiency virus, and active Babesia microti infection were negative, however Epstein-Barr virus DNA by quantitative PCR was markedly elevated. CT scan revealed features suggestive of a cirrhotic liver without focal mass lesions as well as massive splenomegaly with axillary, retroperitoneal and inguinal lymphadenopathy. Bone marrow and lymph node biopsies were obtained which ultimately revealed hepatosplenic T-cell lymphoma. The patient’s initial liver biopsy from five months prior to presentation was re-evaluated by our institution’s pathologists. Histologic analysis showed hepatic sinusoidal and portal infiltration of atypical lymphocytes morphologically identical to those present on the more recently excised lymph node tissue. The hepatic sinusoidal lymphoid cells were strongly positive for CD2, CD3 and CD5 whereas CD4, CD8 stained only minor subsets of the T cells. Subsequent flow cytometric immunophenotypying of peripheral blood identified T-cell receptor alpha/beta positive cells that lacked CD4 and CD8 (double negative alpha/beta T cells). Given the established bone marrow involvement, he was diagnosed with stage IV disease and treated with chemotherapy. His clinical course involved multiple hospitalizations complicated by hyponatremia, neutropenic fevers and pulmonary emboli. Following his fourth cycle of chemotherapy, he developed worsening liver failure and expired approximately three months after initial diagnosis of lymphoma. Hepatosplenic lymphoma of alpha/beta T cells is a rare malignancy with largely unclear risk factors and varied clinical presentations. Notably, diffuse infiltration of liver parenchyma is a prominent feature and the disease can mimic cirrhosis clinically as well as radiographically. Early recognition of this aggressive lymphoma is important and should be considered in the evaluation of patients in whom the etiology of cirrhosis remains in question.
Key Words: Liver cirrhosis, T-cell lymphoma, autoimmune hepatitis, Babesia microti
The case
13virus (HSV) 1 and 2 DNA, Erhlichia chaffeensis/canis/ewingii, cytomegalovirus (CMV) IgM, Epstein-Barr virus (EBV) IgM and Leptospirosis. Babesia microti IgM returned positive at 1:320. Workup for autoimmune disease was negative (ANA, ANCA, Liver-kidney microsome and AMA) except for anti-smooth muscle antibodies, which were positive at 1:20 dilution. Lastly, serum ceruloplasmin and alpha-1-antitrypsin levels were normal. Abdominal MRI showed a normal liver without nodularity or steatosis but marked splenomegaly measuring 20 cm. Given the patient’s hepatitis of unclear etiology, a liver biopsy was performed that revealed extensive active inflammation involving the portal regions and hepatic lobules. No significant iron deposition or fatty change was present. The trichrome stain revealed fibrosis. He was subsequently diagnosed with cirrhosis of unknown etiology. With evidence of active Babesial infection, he was treated with atovaquone and discharged home.
Due to the uncertain source of his cirrhosis and persistent symptoms of anorexia with fatigue, repeat comprehensive laboratory testing was performed two months prior to admission to our institution. These studies were remarkable for prior hepatitis B infection evidenced by negative HBsAg, positive HBsAb, positive HBcAb and subsequent undetectable HBV DNA as well as mildly elevated Babesia microti IgM at 1:40 without detectable Babesial DNA by PCR analysis. Given the patient’s history of diabetes mellitus in the absence of prominent alcohol use, non-alcoholic steatohepatitis (NASH) was considered as a possible etiology of his cirrhosis. Liver biopsy however did not show any evidence of steatosis. He was subsequently referred to outpatient infectious disease clinic given his persistently elevated Babesia titer but was lost to follow-up.
Approximately eight weeks later, he presented to our institution complaining of diffuse abdominal pain, weight loss and fevers. Physical examination revealed hepatosplenomegaly and diffuse lymphadenopathy without ascites or peripheral edema. Laboratory investigations demonstrated mild anemia, thrombocytopenia, elevated lactate dehydrogenase as well as hyperbilirubinemia; other results are shown in Table 1. In addition, laboratory analysis showed mild eosinophilia, positive anti-smooth muscle antibody at 1:40 dilution and elevated serum total IgG. Tests for acute/reactivation viral hepatitis, HIV, and active Babesia microti infection were again negative. However, EBV DNA by quantitative PCR was markedly elevated at approximately 20,000 copies/mL. A CT scan revealed a liver with cirrhotic changes without focal lesions and massive splenomegaly with axillary, retroperitoneal and inguinal lymphadenopathy (Figure 1). Bone marrow and lymph node biopsies were performed. Immunophenotyping by fluorescence-activated cell sorting revealed findings highly suggestive for T-cell lymphoma with the phenotype CD2+, CD3+, CD4–, CD5+, CD8–, CD15–, CD30–, CD45+, and T-cell receptor (TCR) alpha/beta positive. Staining was positive for granzyme, perforin and TIA1. Cytogenetics revealed a clonal process with additional material in chromosomes 2 and 10 at bands 2p23 and 10q21. There were no other karyotypic abnormalities.
Table 1. Laboratory data.
| Variable | Reference range | 5 Mo before admission | On admission, this hospital |
|---|---|---|---|
| White-cell count (per mm3) | 4,000-11,000 | 4,800 | 7,500 |
| Differential count (%) | |||
| Neutrophils | 50-70 | 47 | 31 |
| Band forms | 0-5 | 0 | 7 |
| Lymphocytes | 18-42 | 40 | 40 |
| Monocytes | 2-11 | 8 | 6 |
| Eosinphils | 0-4 | 3 | 10 |
| Basophils | 0-2 | 2 | 0 |
| Atypical lymphocytes | 0 | 0 | 5 |
| Metamyelocytes | 0 | 0 | 1 |
| Hematocrit (%) | 40.0-52.0 | 42.0 | 34.8 |
| Platelet count (per mm3) | 150,000-400,000 | 103,000 | 72,000 |
| International normalized ratio | 1.2 | 1.7 | |
| Bilirubin (mg/dL) | |||
| Total | 0.0-1.5 | 5.0 | 1.8 |
| Direct | 0.0-0.3 | 2.6 | 0.8 |
| Aspartate aminotransferase (U/liter) | 0-40 | 610 | 58 |
| Alanine aminotransferase (U/liter) | 0-40 | 495 | 18 |
| Alkaline phosphatase (U/liter) | 40-130 | 126 | 77 |
| Lactate dehydrogenase (U/liter) | 94-250 | 240 | 556 |
| Antibody to hepatitis B surface antigen | Positive | Positive | |
| Hepatitis B virus surface antigen | Nonreactive | Nonreactive | Nonreactive |
| Antinuclear antibodies | Negative | Negative | Negative |
| Antimitochondrial antibodies | Negative | Negative | Negative |
| Anti-smooth-muscle antibodies | Negative | Positive at 1:20 dilution | Positive at 1:80 dilution |
| Babesia microti antibodies | |||
| lgM | <1:20 | 1:320 | <1:20 |
| lgG | <1:64 | <1:64 | <1:64 |
| Protein | |||
| Total (g/dL) | 6.4-8.3 | 8.1 | 7.3 |
| Albumin (g/dL) | 3.5-5.2 | 2.7 | 3.3 |
| lgG (mg/dL) | 700-1,600 | 3,702 | 2,419 |
| lgA (mg/dL) | 70-400 | 378 | 541 |
| lgM (mg/dL) | 40-230 | 332 | 404 |
Figure 1.
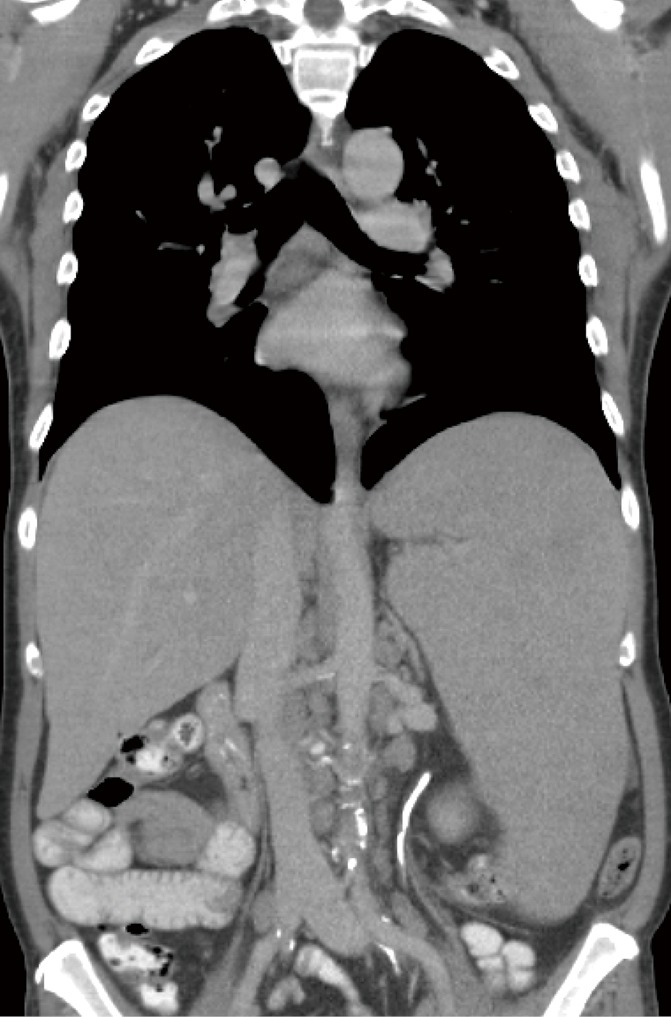
A coronal view of the chest and abdomen obtained with the administration of contrast material, shows a cirrhotic liver with massive splenomegaly
Notably, the patient’s initial liver biopsy from five months prior to presentation was obtained from the outside hospital and re-evaluated by our institution’s pathologists. Histologic analysis showed sinusoidal and portal infiltration with atypical lymphocytes morphologically identical to those present on the more recently excised nodal tissue (Figure 2). The lymphocytes infiltrating the liver were strongly positive for CD2, CD3 and CD5, while CD4 and CD8 stained only small subsets. A fraction of the cells were immunoreactive for granzyme, perforin and TIA1 (Figure 3). Thus, monomorphic, phenotypically aberrant T lymphocytes, were already present in the patient’s liver at the time of his initial diagnosis of cryptogenic cirrhosis. Together, these findings were consistent with a final diagnosis of primary hepatosplenic T-cell lymphoma (HSTCL) of the alpha/beta subtype.
Figure 2.
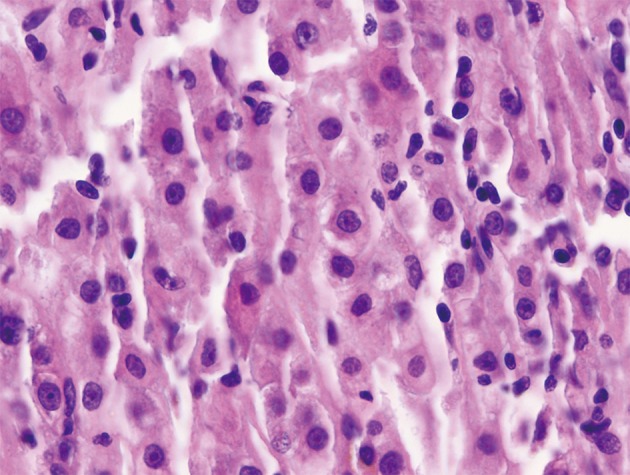
Liver biopsy demonstrating a monomorphic, mostly intrasinusoidal, lymphoid infiltrate composed of small to medium size cells with irregular hyperchromatic nuclei (H&E stain, 500×)
Figure 3.
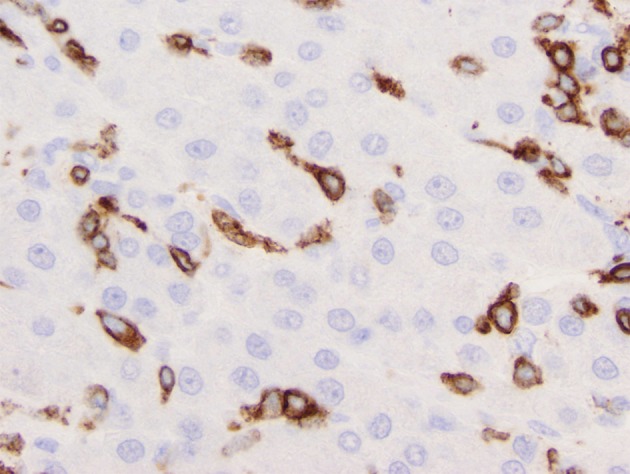
Liver biopsy immunostained for CD3. Note the distribution of immunolabeled cells almost exclusively within hepatic sinusoids, (immunoperoxidase technique, 500×)
Given bone marrow involvement, he was diagnosed with stage IV disease and treated with two cycles of EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide and prednisone) followed by two cycles of alemtuzumab/cladribine and pentostatin. His clinical course involved multiple hospitalizations complicated by hyponatremia, neutropenic fevers and pulmonary emboli. Following his fourth cycle of chemotherapy, he developed worsening liver failure and expired approximately three months after diagnosis of HSTCL.
Discussion
HSTCL was first described in 1990 and is a rare malignancy and accounts for less than 1% of all non-Hodgkin’s lymphomas (1). HSTCL predominantly affects young males with median age at diagnosis of approximately 35 years (2). While the pathogenesis of HSTCL is largely unknown, there is an association with chronic immune suppression, particularly in patients who are on immunosuppressive agents for solid organ transplant, lymphoproliferative disorders, or inflammatory bowel disease. Anti-tumor necrosis factor therapies have been implicated especially in patients with Crohn’s disease (3). However, up to 80% of cases of HSTCL occur in patients without chronic immune suppression. Associations with viral infections such as hepatitis B virus and EBV have only been reported in several cases (4-6). While the clinical presentation of HSTCL is variable, most patients initially present with prominent hepatosplenomegaly and thrombocytopenia in the absence of prominent lymphadenopathy (7). Abnormal liver-function tests as well as constitutional symptoms such as fevers and anorexia are also common. Histologically, HSTCL is characterized by sinusoidal infiltration of T-cells in the liver, spleen and bone marrow. In most cases, tumor cells express CD2 and surface CD3 while CD4, CD8 are absent. Cytogenetics frequently reveal isochromosome 7q or trisomy of chromosome 8 (8). Most tumors exhibit the gamma/delta TCR but few cases such as with our patient express the alpha/beta TCR instead. While HSTCL is already very rare with only several hundred cases reported in the literature to date, less than 30 cases of the alpha/beta subtype have been described (5).
Complicating and perhaps delaying the diagnosis of HSTCL in our patient was the presence of active Babesia microti infection at the time his initial presentation and diagnosis of cirrhosis. Furthermore, diffuse infiltration of liver parenchyma has been described in both hematologic and solid tumors which can mimic cirrhosis clinically as well as radiographically (9,10). This clinical presentation has been described as pseudocirrhosis, and is most commonly seen with hematological malignancies, notably in non-Hodgkin’s lymphoma (11). Reports implicate the desmosplastic response to infiltrating tumor cells as the cause for extensive fibrosis seen in several cases of pseudocirrhosis (12).
In retrospect, our patient’s history of diabetes mellitus was likely a confounding characteristic, which contributed to suspicion of NASH as a potential etiology of his cryptogenic cirrhosis. While absence of steatosis on biopsy strongly challenged metabolic liver disease as an explanatory diagnosis (13), the patient was unfortunately lost to follow-up before further investigations could be conducted. Additionally, invoking prior hepatitis B virus infection as a contributing factor in the development of his malignancy is plausible however only associations with active hepatitis B infection and HSTCL have been reported in the literature (5,14). Of note, the patient’s social history was remarkable for having sex with men in the absence of intravenous drug use or blood transfusions which may explain how he contracted hepatitis B. Alternatively, based on a simplified scoring system for autoimmune hepatitis (incorporating titers of autoantibodies, IgG levels, liver histology, and the exclusion of active viral hepatitis), a diagnosis of autoimmune hepatitis was possible in our patient however infiltrating plasma cells on liver biopsy were notably absent (15). While there are only three previously reported cases of hepatosplenic T-cell lymphoma presenting with autoimmune hepatitis, it is unclear whether the initial diagnosis was correct or whether lymphoma was present all along with incidentally positive autoimmune hepatitis serologies (16-18). Interestingly however, mechanistic links between autoimmunity and tumorigenesis have been described in many other lymphomas (19). Finally, other cases have been reported of hepatosplenic T-cell lymphoma that mimic acute hepatitis in the absence of viral, toxic, autoimmune or metabolic etiologies (20,21).
HSTCL is an aggressive malignancy with a median survival of less than two years (22). While patients often initially respond to chemotherapy, remissions are typically short lived before relapse occurs. If patients achieve a complete remission with chemotherapy, autologous or allogeneic hematopoietic cell transplantation should be considered. Most patients described in the literature undergo induction therapy with EPOCH or CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) with or without etoposide. One report suggests that patients with HSTCL might respond well to an experimental combination therapy of purine analogue, cladribine, and anti-CD52 antibody, alemtuzumab (23), while another report demonstrated therapeutic benefit of antimetabolite, pentostatin which were all utilized in the treatment of our patient (24). Finally, in some cases splenectomy is performed, however only modest improvement of median survival has been observed (25,26).
Conclusions
HSTCL is a rare malignancy with largely unclear risk factors and heterogeneous clinical presentations. The diagnosis of HSTCL in this patient was challenging given the concomitant presentation of Babesiosis and cirrhosis. In retrospect, recognition of the absence of histologic confirmation of NASH or autoimmune hepatitis on initial liver biopsy could have led to an earlier review of the pathology, which ultimately revealed a sinusoidal pattern of monomorphic lymphoid cells. Given its rapidly progressive clinical course, early detection of this lymphoma is important and should be considered in the evaluation of patients in whom the etiology of cirrhosis remains in question.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.1. Farcet JP, Gaulard P, Marolleau JP, et al. Hepatosplenic T-cell lymphoma: sinusal/sinusoidal localization of malignant cells expressing the T-cell receptor gamma delta. Blood 1990;75:2213-9. [PubMed]
- 2.Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011;117:5019-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotlyar DS, Osterman MT, Diamond RH, et al. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2011;9:36-41.e1. [DOI] [PubMed]
- 4.Ohshima K, Haraoka S, Harada N, et al. Hepatosplenic gammadelta T-cell lymphoma: relation to Epstein-Barr virus and activated cytotoxic molecules. Histopathology 2000;36:127-35 [DOI] [PubMed] [Google Scholar]
- 5.Nagai Y, Ikegame K, Mori M, et al. Hepatosplenic alphabeta T cell lymphoma. Int J Clin Oncol 2010;15:215-9 [DOI] [PubMed] [Google Scholar]
- 6.Macon WR, Levy NB, Kurtin PJ, et al. Hepatosplenic alphabeta T-cell lymphomas: a report of 14 cases and comparison with hepatosplenic gammadelta T-cell lymphomas. Am J Surg Pathol 2001;25:285-96 [DOI] [PubMed] [Google Scholar]
- 7.Falchook GS, Vega F, Dang NH, et al. Hepatosplenic gamma-delta T-cell lymphoma: clinicopathological features and treatment. Ann Oncol 2009;20:1080-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonveaux P, Daniel MT, Martel V, et al. Isochromosome 7q and trisomy 8 are consistent primary, non-random chromosomal abnormalities associated with hepatosplenic T gamma/delta lymphoma. Leukemia 1996;10:1453-5 [PubMed] [Google Scholar]
- 9.Nascimento AB, Mitchell DG, Rubin R, et al. Diffuse desmoplastic breast carcinoma metastases to the liver simulating cirrhosis at MR imaging: report of two cases. Radiology 2001;221:117-21 [DOI] [PubMed] [Google Scholar]
- 10.Jha P, Poder L, Wang ZJ, et al. Radiologic mimics of cirrhosis. AJR Am J Roentgenol 2010;194:993-9 [DOI] [PubMed] [Google Scholar]
- 11.Rowbotham D, Wendon J, Williams R.Acute liver failure secondary to hepatic infiltration: a single centre experience of 18 cases. Gut 1998;42:576-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sass DA, Clark K, Grzybicki D, et al. Diffuse desmoplastic metastatic breast cancer simulating cirrhosis with severe portal hypertension: a case of “pseudocirrhosis”. Dig Dis Sci 2007;52:749-52 [DOI] [PubMed] [Google Scholar]
- 13.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology 2003;37:1202-19 [DOI] [PubMed] [Google Scholar]
- 14.Ozaki S, Ogasahara K, Kosaka M, et al. Hepatosplenic gamma delta T-cell lymphoma associated with hepatitis B virus infection. J Med Invest 1998;44:215-7 [PubMed] [Google Scholar]
- 15.Hennes EM, Zeniya M, Czaja AJ, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48:169-76 [DOI] [PubMed] [Google Scholar]
- 16.Veldt BJ, Meijers C, Zondervan PE, et al. Hepatosplenic gammadelta T cell lymphoma: a diagnostic pitfall. J Hepatol 2003;39:455-7 [DOI] [PubMed] [Google Scholar]
- 17.Przybylski GK, Wu H, Macon WR, et al. Hepatosplenic and subcutaneous panniculitis-like gamma/delta T cell lymphomas are derived from different Vdelta subsets of gamma/delta T lymphocytes. J Mol Diagn 2000;2:11-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen-Benz C, Hoffmann N, Beckurts T, et al. Fulminant liver failure induced by hepatosplenic alphabeta T-cell lymphoma. Z Gastroenterol 2003;41:1083-6 [DOI] [PubMed] [Google Scholar]
- 19.Tvarůzková Z, Pavlová S, Doubek M, et al. Lymphoproliferative disease in patients with autoimmune and inflammatory diseases: significance of antigenic stimulation and inflammatory processes. Cas Lek Cesk 2011;150:161-8 [PubMed] [Google Scholar]
- 20.Perfetto F, Tarquini R, Mancuso F, et al. Hepato-splenic lymphoma: a rare entity mimicking acute hepatitis: a case report. World J Gastroenterol 2003;9:1381-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta E, Rose JE, Straughen JE, et al. Hepatic failure and death due to new onset T cell lymphoma. J Gastrointest Cancer 2011;42:179-82 [DOI] [PubMed] [Google Scholar]
- 22.Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood 2003;102:4261-9 [DOI] [PubMed] [Google Scholar]
- 23.Jaeger G, Bauer F, Brezinschek R, et al. Hepatosplenic gammadelta T-cell lymphoma successfully treated with a combination of alemtuzumab and cladribine. Ann Oncol 2008;19:1025-6 [DOI] [PubMed] [Google Scholar]
- 24.Aldinucci D, Poletto D, Zagonel V, et al. In vitro and in vivo effects of 2'-deoxycoformycin (Pentostatin) on tumour cells from human gammadelta+ T-cell malignancies. Br J Haematol 2000;110:188-96 [DOI] [PubMed] [Google Scholar]
- 25.Khan WA, Yu L, Eisenbrey AB, et al. Hepatosplenic gamma/delta T-cell lymphoma in immunocompromised patients. Report of two cases and review of literature. Am J Clin Pathol 2001;116:41-50 [DOI] [PubMed] [Google Scholar]
- 26.Gopcsa L, Bányai A, Tamáska J, et al. Hepatosplenic gamma delta T-cell lymphoma with leukemic phase successfully treated with 2-chlorodeoxyadenosine. Haematologia (Budap) 2002;32:519-27 [PubMed] [Google Scholar]


