Abstract
Parasitoids are a major top-down cause of mortality of coastal harmful algae, but the mechanisms and strategies they have evolved to efficiently infect ephemeral blooms are largely unknown. Here, we show that the generalist dinoflagellate parasitoid Parvilucifera sinerae (Perkinsozoa, Alveolata) is activated from dormancy, not only by Alexandrium minutum cells but also by culture filtrates. We unequivocally identified the algal metabolite dimethylsulphide (DMS) as the density-dependent cue of the presence of potential host. This allows the parasitoid to alternate between a sporangium-hosted dormant stage and a chemically-activated, free-living virulent stage. DMS-rich exudates of resistant dinoflagellates also induced parasitoid activation, which we interpret as an example of coevolutionary arms race between parasitoid and host. These results further expand the involvement of dimethylated sulphur compounds in marine chemical ecology, where they have been described as foraging cues and chemoattractants for mammals, turtles, birds, fish, invertebrates and plankton microbes.
Keywords: Alexandrium, dimethylsulphide, dinoflagellates, infochemistry, parasitoid, Parvilucifera
Marine harmful algal blooms are dense ephemeral proliferations typically of dinoflagellates, cyanobacteria or diatoms, that can directly cause illness and death in humans and marine life through the production of toxins, or cause ecosystem alterations affecting food provision and recreational activities (Zingone et al., 2000; MEA, 2005). Even though marine harmful algal blooms have been recognized as a major environmental challenge (MEA, 2005), little is known about what makes them thrive and wane. In the case of dinoflagellates, which account for 75% of marine harmful algal bloom-forming phytoplankton species, bottom-up factors (including man-enhanced eutrophication, climate shifts and species dispersal) are usually invoked as triggers (Zingone et al., 2000; Heisler et al., 2008; Anderson, 2009), but the causes and mechanisms of termination remain obscure.
Parasitoids have been identified as a main cause of mortality of harmful dinoflagellates (Taylor, 1968; Chambouvet et al., 2008), to the extent that their deliberate use has been suggested as a biological mitigation of marine harmful algal blooms (Taylor, 1968), in the same manner it is done in agricultural applications on land. The suggestion has faced opposition on the basis of the lack of knowledge on their specificity, the mechanisms of infection and the potential side effects (Anderson, 2009). The debate has prompted the need for a better understanding of parasitoid−host interactions, along the same lines as the increasing interest in chemical ecology and interspecific communication in oceanic plankton (Ianora et al., 2011).
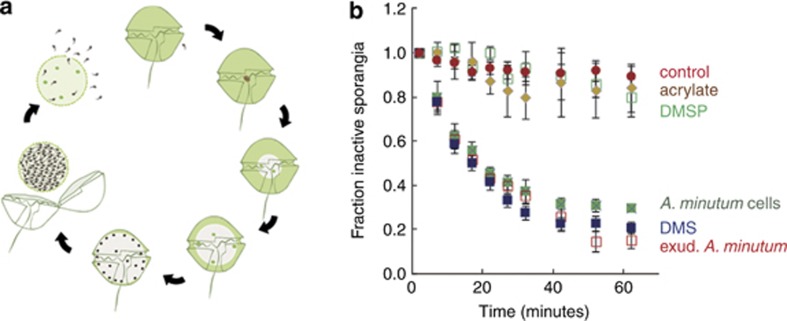
Parvilucifera sinerae (Perkinsozoa, Alveolata) is a flagellate parasitoid that efficiently infects and kills a taxonomically broad variety of dinoflagellates, including harmful bloom forming species within the genera Alexandrium, Dinophysis, Gambierdiscus, Gymnodinium, Ostreopsis and Protoceratium (Garcés et al., 2012). The infection cycle proceeds as follows (Figure 1a): a flagellate zoospore penetrates the host cell, destroys its content, forms a spherical sporangium the size of the host, and divides to fill up the sporangium with dormant zoospores. They remain dormant until a wakeup call signals the presence of a sufficient density of host cells; then the zoospores activate into quick tumbling motion and eventually leave the sporangium through one or several opercula opened in the wall (Supplementary Movie S1 in the online Supplementary Material). The nature and origin of the signal were hitherto unknown.
Figure 1.
Response of P. sinerae sporangia to chemical cues. (a) The infection cycle of A. minutum: a zoospore enters the host, destroys its content, forms and sporangium and fills it with dormant zoospores; host-signalling cues cause the activation of P. sinerae zoospores and their release from the sporangium. (b) Decay in the number of inactive sporangia upon exposure to L1 medium (control), A. minutum AMP4 cells and exudates (which contain 300 nℳ of DMS), 270 nℳ DMSP in L1, 270 nℳ acrylate in L1, and 270 nℳ DMS in L1. Data are expressed as mean±s.e. of 3–11 replicates.
When studying the infection of the toxic dinoflagellate Alexandrium minutum by P. sinerae, we observed that the presence of filtered exudates of the dinoflagellate was enough to activate the dormant zoospores into motion and induce their release from the sporangia as efficiently as the presence of host cells did (Figure 1b), pointing towards the involvement of a chemical signal. A. minutum is a strong producer of the osmolyte dimethylsulphoniopropionate (DMSP), which occurs at intracellular concentrations as high as 0.3 M, or 7% of total cell carbon (Berdalet et al., 2011; Caruana et al., 2012). Considering that A. minutum exudates contained DMSP concentrations in the order of 100–800 nℳ, and that this compound has been shown to induce positive chemotaxis in a variety of plankton microorganisms (Seymour et al., 2010), it stood as a good candidate for the activation of the parasitoid. Additions of a lab-prepared solution of 270 nℳ DMSP, however, did not give any activation response significantly different from the negative control (Figure 1b).
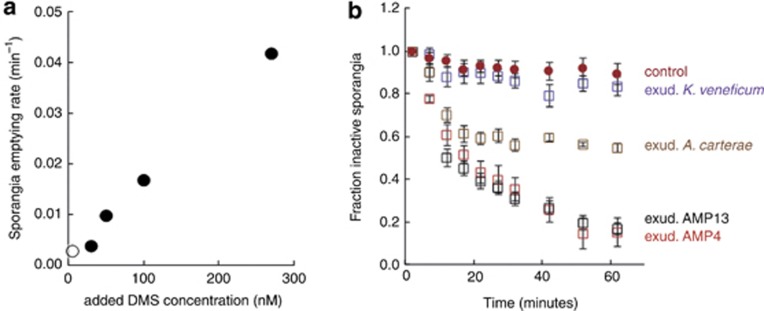
A. minutum also harbours high activity of DMSP lyases (Caruana et al., 2012), the enzymes that cleave DMSP into equimolar amounts of acrylate and dimethylsulphide (DMS). Indeed, A. minutum blooms and cultures have the characteristic seafood smell of DMS, and exudates used in this study had DMS concentrations of ca. 300 nℳ. Additions of an acrylate solution did not induce any response, but additions of DMS at a concentration similar to that in the exudates did (Figure 1b). Moreover, the response rate was proportional to the added DMS concentration down to a threshold close to 30 nℳ (Figure 2a).
Figure 2.
DMS-dependent parasitoid activation. (a) Rate constants of the emptying of P. sinerae sporangia upon exposure to L1 medium (open circle) and increasing concentrations of DMS in medium (filled circles). (b) Decay in the number of inactive P. sinerae sporangia upon exposure to L1 medium (control) and exudates of A. minutum AMP4 (susceptible host, 300 nℳ DMS), A. minutum AMP13 (resistant host, 270 nℳ DMS), Amphidinium carterae (resistant host, 140 nℳ DMS), and Karlodinium veneficum (resistant host, 1 nℳ DMS). Data are expressed as mean±s.e. of 3–11 replicates.
We showed that DMS alone was enough for parasitoid activation, that is, for the necessary step before infection. As many dinoflagellates produce DMS (Stefels et al., 2007; Caruana et al., 2012), including susceptible and resistant strains, how host-specific is this mechanism? Could it be that DMS acts in concert with other chemicals contained only in the exudates of the susceptible dinoflagellates? Assays with culture filtrates of nonsusceptible A. minutum, Amphidinium carterae and Karlodinium veneficum strains showed parasitoid activation rates proportional to their DMS content (Figure 2b). In other words, exudates of dinoflagellates caused parasitoid activation as long as they had enough DMS, independently of the strains' susceptibility to infection.
Our experiments demonstrated that P. sinerae perceives DMS as the wakeup call for activation. In the coastal ocean, background DMS concentrations are typically 0.5–10 nℳ (Lana et al., 2011), while a confined coastal bloom of A. minutum (ca. 1000 cells per ml−1) on 7 March 2012 had a DMS concentration of 217 nℳ. As DMS is a short-lived substance in seawater (Pinhassi et al., 2005), where it is consumed by bacteria and photolysis, the threshold found here for the activation of the dormant zoospores (a few tens of nM) allows the parasitoid to activate only in the presence of relatively high densities of potential host cells, and do it more rapidly within denser blooms. This alternation between a sporangium-hosted dormant stage and a chemically-activated, free-living virulent stage stands as an efficient strategy for success in the maintenance of the parasitoid population. How do they survive between host blooms, either by serially infecting a sequence of dinoflagellate hosts, as shown for other parasites (Chambouvet et al., 2008), or by sporangia sinking to the sediments along with host cysts and remaining dormant therein until favourable conditions for resuspension and activation occur, remains to be solved.
DMS is a by-product of both algal physiology (Stefels et al., 2007) and food web interactions, including herbivore grazing and bacterial catabolism (Simó, 2001). In dense monospecific microalgal blooms under some degree of physiological stress due to nutrient scarcity or high sunlight exposure, DMS leakage from the algal cell is suggested to occur as part of an overflow of excess of energy and sulphur (Stefels et al., 2007), and/or as part of a protection mechanism against oxidative stress (Sunda et al., 2002). P. sinerae has evolved a sensory response to this by-product and, because of its chemotactic characteristics for protists (Seymour et al., 2010), it is conceivable that the zoospores further use DMS gradients for an oriented swimming towards the potential host. However, the occurrence of an eventual infection depends on host resistance mechanisms that are still unknown.
Alexandrium species are known to produce allelochemicals with deleterious (lytic) effects on autotrophic and heterotrophic protists (Tillman et al., 2008) as a mechanism to overcome competition and grazing. Rather, we show that DMS behaves as a ‘kairomone', that is, a chemical signal released by the dinoflagellate, which mediates an interspecific interaction that benefits the receiving organism (the parasitoid) without benefiting the producer (Pohnert et al., 2007). In this case, the kairomone is even disadvantageous to the producer, as it induces infection and subsequent death. Therefore, its release must be unavoidable or its costs must be outweighed by the aforementioned physiological benefits. In any case, this stands as one of the scarce examples of a chemically-mediated arms race in the coevolution of plankton microbes (Smetacek, 2001; Ianora et al., 2011).
Our study further expands the importance of ubiquitous dimethylated sulphur compounds (DMSP and DMS) in the chemical ecology of the oceans. These compounds have been described as foraging cues for seals, turtles, penguins, procellariiform birds, fishes, some macroinvertebrates and copepods (van Alstyne, 2008; Nevitt, 2011; Endres and Lohmann, 2012; and refs. therein), and chemotactic attractants for protists, microalgae and bacteria (Seymour et al., 2010, and refs. therein), in what possibly stands as a unique case amidst the infochemical landscape of the biosphere. Here, we discover their involvement in planktonic host−parasitoid interactions.
Acknowledgments
Support was provided by the (former) Spanish Ministry of Science and Innovation through projects PARAL (to E.G.) and SUMMER (to R.S.). K.P. acknowledges a grant from Australian Endeavour Foundation (Australian Government). We thank Beatriz Garriz for the drawing of the infection cycle.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Anderson DM. Approaches to monitoring, control and management of harmful algal blooms (HABs) Ocean Coast Manage. 2009;52:342–347. doi: 10.1016/j.ocecoaman.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdalet E, Llaveria G, Simó R. Modulation of dimethylsulfoniopropionate (DMSP) concentration in an Alexandrium minutum (Dinophyceae) culture by small-scale turbulence: a link to toxin production. Harmful Algae. 2011;11:88–95. [Google Scholar]
- Caruana AMN, Steinke M, Turner SM, Malin G. Concentrations of dimethylsulphoniopropionate and activities of dimethylsulphide-producing enzymes in batch cultures of nine dinoflagellate species. Biogeochemistry. 2012;110:87–107. [Google Scholar]
- Chambouvet A, Morin P, Marie D, Guillou L. Control of toxic marine dinoflagellate blooms by serial parasitic killers. Science. 2008;322:1254–1257. doi: 10.1126/science.1164387. [DOI] [PubMed] [Google Scholar]
- Endres CS, Lohmann KJ. Perception of dimethyl sulfide (DMS) by loggerhead sea turtles: a posible mechanism for locating high-productivity oceanic regions for foraging. J Exp Biol. 2012;215:3535–3538. doi: 10.1242/jeb.073221. [DOI] [PubMed] [Google Scholar]
- Garcés E, Alacid E, Bravo I, Figueroa R, Fraga S.2012Parvilucifera sinerae (Alveolata, myzozoa) is a generalist parasitoid of dinoflagellates Protistdoi: 10.1016/j.protis.2012.11.004(in press). [DOI] [PubMed]
- Heisler J, Gilbert PM, Burkholder JM, Anderson DM, Cochlan W, Dennison WC, et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae. 2008;8:3–13. doi: 10.1016/j.hal.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianora A, Bentley MG, Caldwell GS, Casotti R, Cembella AD, Engström-Öst J, et al. The relevance of marine chemical ecology to plankton and ecosystem function: an emerging field. Mar Drugs. 2011;9:1625–1648. doi: 10.3390/md9091625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lana A, Bell TG, Simó R, Vallina SM, Ballabrera-Poy J, Kettle AJ, et al. 2011An updated climatology of surface dimethlysulfide concentrations and emission fluxes in the global ocean Global Biogeochem Cy 25GB1004doi: 10.1029/2010GB003850 [DOI] [Google Scholar]
- Millennium Ecosystem Assessment . Ecosystems and Human Well-being: Synthesis. Island Press: Washington DC; 2005. [Google Scholar]
- Nevitt GA. The neuroecology of dimethyl sulfide: a global-climate regulator turned marine infochemical. Integr Comp Biol. 2011;51:819–825. doi: 10.1093/icb/icr093. [DOI] [PubMed] [Google Scholar]
- Pinhassi J, Simó R, González JM, Vila M, Alonso-Sáez L, Kiene RP, et al. Dimethylsulfoniopropionate turnover is linked to the composition and dynamics of the bacterioplankton assemblage during a microcosm phytoplankton bloom. Appl Environ Microbiol. 2005;71:7650–7660. doi: 10.1128/AEM.71.12.7650-7660.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohnert G, Steinke M, Tollrian R. Chemical cues, defence metabolites and the shaping of pelagic interspecific interactions. Trends Ecol Evol. 2007;22:198–203. doi: 10.1016/j.tree.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Seymour JR, Simó R, Ahmed T, Stocker R. Chemoattraction to dimethylsulfoniopropionate throughout the marine microbial food web. Science. 2010;329:342–345. doi: 10.1126/science.1188418. [DOI] [PubMed] [Google Scholar]
- Simó R. Production of atmospheric sulfur by oceanic plankton: biogeochemical, ecological and evolutionary links. Trends Ecol Evol. 2001;16:287–294. doi: 10.1016/s0169-5347(01)02152-8. [DOI] [PubMed] [Google Scholar]
- Smetacek V. A watery arms race. Nature. 2001;411:745. doi: 10.1038/35081210. [DOI] [PubMed] [Google Scholar]
- Stefels J, Steinke M, Turner S, Malin G, Belviso S. Environmental constraints on the production and removal of the climatically active gas dimethylsulphide (DMS) and implications for ecosystem modelling. Biogeochemistry. 2007;83:245–275. [Google Scholar]
- Sunda W, Kieber DJ, Kiene RP, Huntsman S. An antioxidant function for DMSP and DMS in marine algae. Nature. 2002;418:317–319. doi: 10.1038/nature00851. [DOI] [PubMed] [Google Scholar]
- Taylor FJR. Parasitism of the toxin producing dinoflagellate Gonyaulax catenella by the endoparasitic dinoflagellate Amoebophrya ceratii. J Fish Res Bd Canada. 1968;25:2241–2245. [Google Scholar]
- Tillman U, Alpermann T, John U, Cembella A. Allelochemical interactions and short-term effects of the dinoflagellate Alexandrium on selected photoautotrophic and heterotrophic protists. Harmful Algae. 2008;7:52–64. [Google Scholar]
- van Alstyne KL.2008Ecological and physiological roles of dimethylsulfoniopropionate and its products in marine macroalgaeIn: Amsler CD, (eds)Algal chemical ecology Springer: Heidelberg, Berlin; 173–194. [Google Scholar]
- Zingone A, Enevoldsen HO. The diversity of harmful algal blooms: a challenge for science and management. Ocean Coast Manage. 2000;43:725–748. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.