Abstract
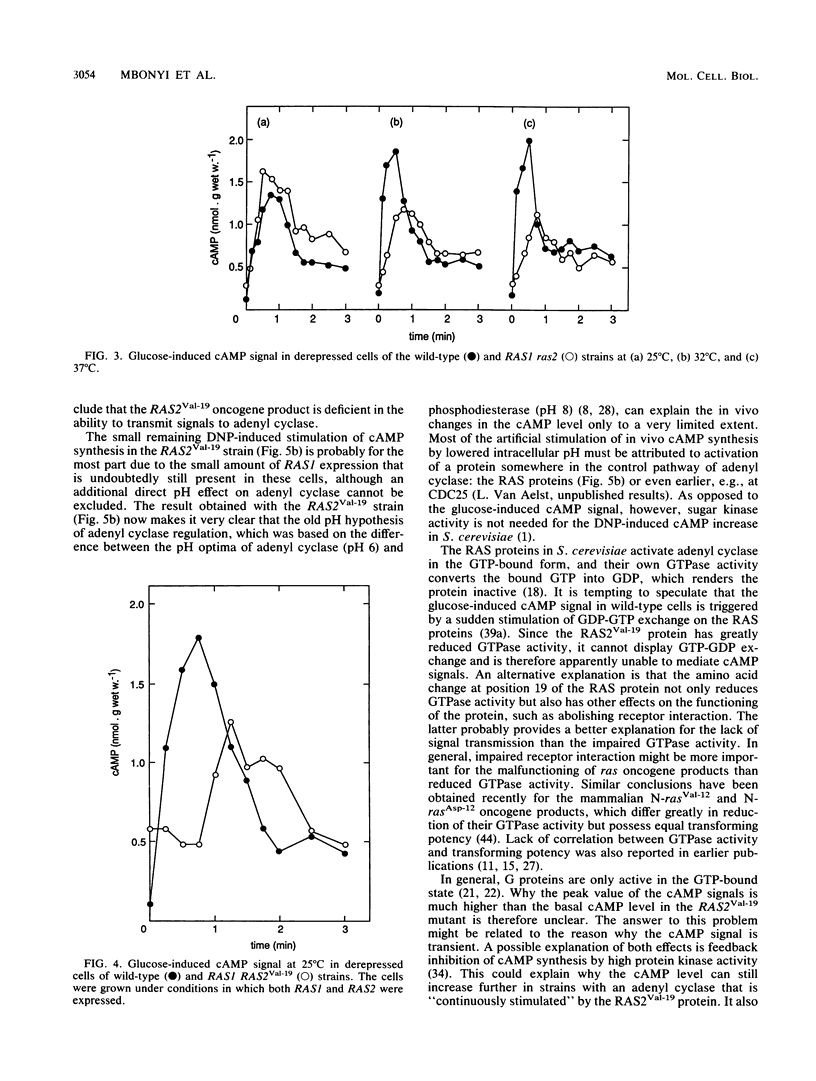
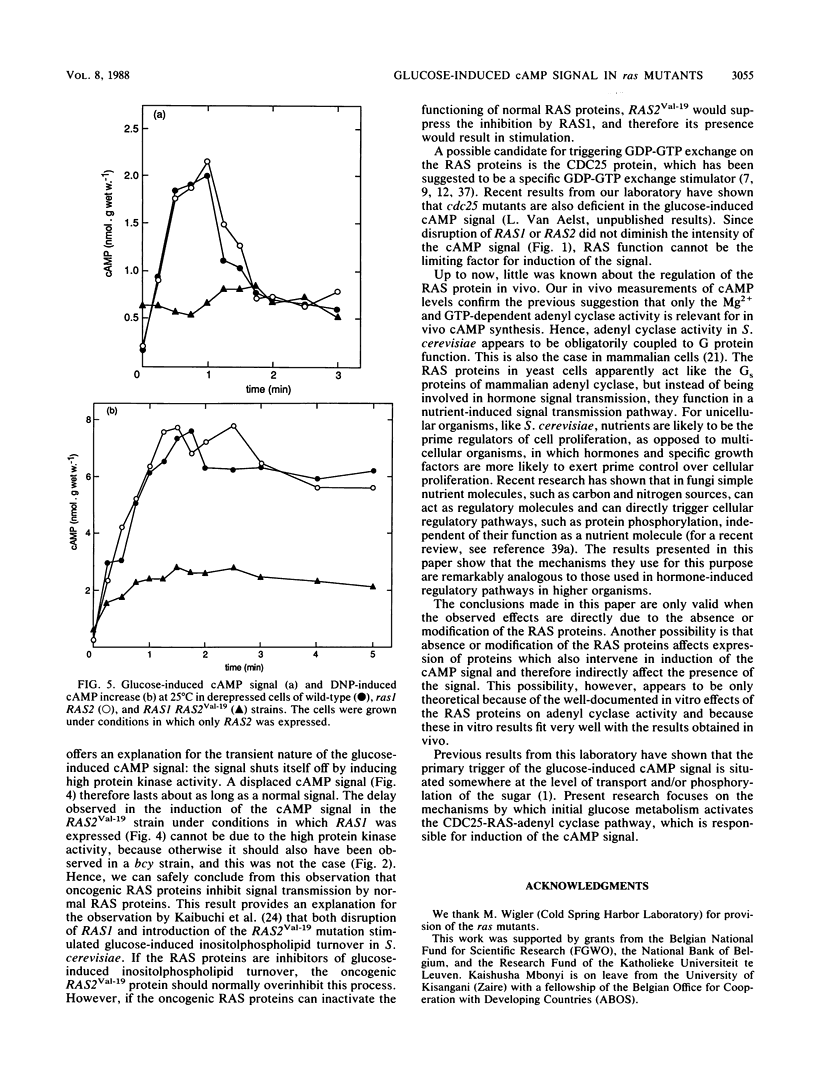
Addition of glucose to Saccharomyces cerevisiae cells grown on a nonfermentable carbon source triggers a cyclic AMP (cAMP) signal, which induces a protein phosphorylation cascade. In a yeast strain lacking functional RAS1 and RAS2 genes and containing a bcy mutation to suppress the lethality of RAS deficiency, the cAMP signal was absent. Addition of dinitrophenol, which stimulates in vivo cAMP synthesis by lowering intracellular pH, also did not enhance the cAMP level. A bcy control strain, with functional RAS genes present, showed cAMP responses similar to those of a wild-type strain. In disruption mutants containing either a functional RAS1 gene or a functional RAS2 gene, the cAMP signal was not significantly different from the one in wild-type cells, indicating that RAS function cannot be a limiting factor for cAMP synthesis during induction of the signal. Compared with wild-type cells, the cAMP signal decreased in intensity with increasing temperature in a ras2 disruption mutant. When the mutant RAS2Val-19, which carries the equivalent of the human H-rasVal-12 oncogene, was grown under conditions in which RAS1 expression is repressed, the cAMP signal was absent. The oncogene product is known to be deficient in GTPase activity. However, the amino acid change at position 19 (or 12 in the corresponding human oncogene product) might also have other effects, such as abolishing receptor interaction. Such an additional effect probably provides a better explanation for the lack of signal transmission than the impaired GTPase activity. When the RAS2Val-19 mutant was grown under conditions in which RAS1 is expressed, the cAMP signal was present but significantly delayed compared with the signal in wild-type cells. This indicates that oncogenic RAS proteins inhibit normal functioning of wild-type RAS proteins in vivo and also that in spite of the presence of the RAS2(Val-19) oncogene, adenyl cyclase is not maximally stimulated in vivo. Expression of only the RAS(Val-19) gene product also prevented most of the stimulation of cAMP synthesis by dinitrophenol, indicating that lowered intracellular pH does not act directly on adenyl cyclase but on a step earlier in the activation pathway of the enzyme. The results obtained with the control bcy strain, the RAS2(Val-19) strain under conditions in which RAS1 is expressed, and with dinitrophenol show that the inability of the oncogene product to mediate the cAMP signal is not due to feedback inhibition by the high protein kinase activity in strains containing the RAS2(Val-19) oncogene. Hence, the present results show that the RAS protein in S. cerevisiae are involved in the transmission of the glucose-induced cAMP signal and that the oncogenic RAS protein is unable to act as a signal transducer. The RAS protein in S. cerevisiae apparently act similarly to the Gs proteins of mammalian adenyl cyclase, but instead of being involved in hormone signal transmission, they function in a nutrient-induced signal transmission pathway.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beullens M., Mbonyi K., Geerts L., Gladines D., Detremerie K., Jans A. W., Thevelein J. M. Studies on the mechanism of the glucose-induced cAMP signal in glycolysis and glucose repression mutants of the yeast Saccharomyces cerevisiae. Eur J Biochem. 1988 Feb 15;172(1):227–231. doi: 10.1111/j.1432-1033.1988.tb13877.x. [DOI] [PubMed] [Google Scholar]
- Bisson L. F., Fraenkel D. G. Expression of kinase-dependent glucose uptake in Saccharomyces cerevisiae. J Bacteriol. 1984 Sep;159(3):1013–1017. doi: 10.1128/jb.159.3.1013-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Fraenkel D. G. Involvement of kinases in glucose and fructose uptake by Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1730–1734. doi: 10.1073/pnas.80.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Neigeborn L., Carlson M., Fraenkel D. G. The SNF3 gene is required for high-affinity glucose transport in Saccharomyces cerevisiae. J Bacteriol. 1987 Apr;169(4):1656–1662. doi: 10.1128/jb.169.4.1656-1662.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breviario D., Hinnebusch A., Cannon J., Tatchell K., Dhar R. Carbon source regulation of RAS1 expression in Saccharomyces cerevisiae and the phenotypes of ras2- cells. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4152–4156. doi: 10.1073/pnas.83.12.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broek D., Samiy N., Fasano O., Fujiyama A., Tamanoi F., Northup J., Wigler M. Differential activation of yeast adenylate cyclase by wild-type and mutant RAS proteins. Cell. 1985 Jul;41(3):763–769. doi: 10.1016/s0092-8674(85)80057-x. [DOI] [PubMed] [Google Scholar]
- Broek D., Toda T., Michaeli T., Levin L., Birchmeier C., Zoller M., Powers S., Wigler M. The S. cerevisiae CDC25 gene product regulates the RAS/adenylate cyclase pathway. Cell. 1987 Mar 13;48(5):789–799. doi: 10.1016/0092-8674(87)90076-6. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984 Apr;246(4 Pt 2):R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- Camonis J. H., Kalékine M., Gondré B., Garreau H., Boy-Marcotte E., Jacquet M. Characterization, cloning and sequence analysis of the CDC25 gene which controls the cyclic AMP level of Saccharomyces cerevisiae. EMBO J. 1986 Feb;5(2):375–380. doi: 10.1002/j.1460-2075.1986.tb04222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby W. W., Hayflick J. S., Clark S. G., Levinson A. D. Biochemical characterization of polypeptides encoded by mutated human Ha-ras1 genes. Mol Cell Biol. 1986 Feb;6(2):730–734. doi: 10.1128/mcb.6.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J., Becker J. M., Enari E., Levitzki A. The activation of adenylate cyclase by guanyl nucleotides in Saccharomyces cerevisiae is controlled by the CDC25 start gene product. Mol Cell Biol. 1987 Oct;7(10):3857–3861. doi: 10.1128/mcb.7.10.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeo-Jones D., Scolnick E. M., Koller R., Dhar R. ras-Related gene sequences identified and isolated from Saccharomyces cerevisiae. Nature. 1983 Dec 15;306(5944):707–709. doi: 10.1038/306707a0. [DOI] [PubMed] [Google Scholar]
- DeFeo-Jones D., Tatchell K., Robinson L. C., Sigal I. S., Vass W. C., Lowy D. R., Scolnick E. M. Mammalian and yeast ras gene products: biological function in their heterologous systems. Science. 1985 Apr 12;228(4696):179–184. doi: 10.1126/science.3883495. [DOI] [PubMed] [Google Scholar]
- Der C. J., Finkel T., Cooper G. M. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986 Jan 17;44(1):167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Dhar R., Nieto A., Koller R., DeFeo-Jones D., Scolnick E. M. Nucleotide sequence of two rasH related-genes isolated from the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1984 Apr 25;12(8):3611–3618. doi: 10.1093/nar/12.8.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraso P., Mazón M. J., Gancedo J. M. Internal acidification and cAMP increase are not correlated in Saccharomyces cerevisiae. Eur J Biochem. 1987 Jun 15;165(3):671–674. doi: 10.1111/j.1432-1033.1987.tb11493.x. [DOI] [PubMed] [Google Scholar]
- Field J., Broek D., Kataoka T., Wigler M. Guanine nucleotide activation of, and competition between, RAS proteins from Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jun;7(6):2128–2133. doi: 10.1128/mcb.7.6.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. On ras gene function in yeast. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4740–4744. doi: 10.1073/pnas.82.14.4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Miyajima A., Arai K., Matsumoto K. Possible involvement of RAS-encoded proteins in glucose-induced inositolphospholipid turnover in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8172–8176. doi: 10.1073/pnas.83.21.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Powers S., Cameron S., Fasano O., Goldfarb M., Broach J., Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985 Jan;40(1):19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers S., McGill C., Fasano O., Strathern J., Broach J., Wigler M. Genetic analysis of yeast RAS1 and RAS2 genes. Cell. 1984 Jun;37(2):437–445. doi: 10.1016/0092-8674(84)90374-x. [DOI] [PubMed] [Google Scholar]
- Lacal J. C., Srivastava S. K., Anderson P. S., Aaronson S. A. Ras p21 proteins with high or low GTPase activity can efficiently transform NIH/3T3 cells. Cell. 1986 Feb 28;44(4):609–617. doi: 10.1016/0092-8674(86)90270-9. [DOI] [PubMed] [Google Scholar]
- Londesborough J. C., Nurminen T. A manganese-dependent adenyl cyclase in baker's yeast, Saccharomyces cerevisiae. Acta Chem Scand. 1972;26(8):3396–3398. doi: 10.3891/acta.chem.scand.26-3396. [DOI] [PubMed] [Google Scholar]
- Londesborough J. Characterization of an adenosine 3':5'-cyclic monophosphate phosphodiesterase from baker's yeast. Its binding to subcellular particles, catalytic properties and gel-filtration behaviour. Biochem J. 1977 Jun 1;163(3):467–476. doi: 10.1042/bj1630467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M. S., Gibbs J. B., Scolnick E. M., Sigal I. S. Regulatory function of the Saccharomyces cerevisiae RAS C-terminus. Mol Cell Biol. 1987 Jul;7(7):2309–2315. doi: 10.1128/mcb.7.7.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazón M. J., Gancedo J. M., Gancedo C. Phosphorylation and inactivation of yeast fructose-bisphosphatase in vivo by glucose and by proton ionophores. A possible role for cAMP. Eur J Biochem. 1982 Oct;127(3):605–608. doi: 10.1111/j.1432-1033.1982.tb06915.x. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Neigeborn L., Schwartzberg P., Reid R., Carlson M. Null mutations in the SNF3 gene of Saccharomyces cerevisiae cause a different phenotype than do previously isolated missense mutations. Mol Cell Biol. 1986 Nov;6(11):3569–3574. doi: 10.1128/mcb.6.11.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa J., Cameron S., Toda T., Ferguson K. M., Wigler M. Rigorous feedback control of cAMP levels in Saccharomyces cerevisiae. Genes Dev. 1987 Nov;1(9):931–937. doi: 10.1101/gad.1.9.931. [DOI] [PubMed] [Google Scholar]
- Powers S., Kataoka T., Fasano O., Goldfarb M., Strathern J., Broach J., Wigler M. Genes in S. cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell. 1984 Mar;36(3):607–612. doi: 10.1016/0092-8674(84)90340-4. [DOI] [PubMed] [Google Scholar]
- Purwin C., Nicolay K., Scheffers W. A., Holzer H. Mechanism of control of adenylate cyclase activity in yeast by fermentable sugars and carbonyl cyanide m-chlorophenylhydrazone. J Biol Chem. 1986 Jul 5;261(19):8744–8749. [PubMed] [Google Scholar]
- Robinson L. C., Gibbs J. B., Marshall M. S., Sigal I. S., Tatchell K. CDC25: a component of the RAS-adenylate cyclase pathway in Saccharomyces cerevisiae. Science. 1987 Mar 6;235(4793):1218–1221. doi: 10.1126/science.3547648. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Tatchell K. RAS genes and growth control in Saccharomyces cerevisiae. J Bacteriol. 1986 May;166(2):364–367. doi: 10.1128/jb.166.2.364-367.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M., Beullens M. Cyclic AMP and the stimulation of trehalase activity in the yeast Saccharomyces cerevisiae by carbon sources, nitrogen sources and inhibitors of protein synthesis. J Gen Microbiol. 1985 Dec;131(12):3199–3209. doi: 10.1099/00221287-131-12-3199. [DOI] [PubMed] [Google Scholar]
- Thevelein J. M., Beullens M., Honshoven F., Hoebeeck G., Detremerie K., Griewel B., den Hollander J. A., Jans A. W. Regulation of the cAMP level in the yeast Saccharomyces cerevisiae: the glucose-induced cAMP signal is not mediated by a transient drop in the intracellular pH. J Gen Microbiol. 1987 Aug;133(8):2197–2205. doi: 10.1099/00221287-133-8-2197. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Trahey M., Milley R. J., Cole G. E., Innis M., Paterson H., Marshall C. J., Hall A., McCormick F. Biochemical and biological properties of the human N-ras p21 protein. Mol Cell Biol. 1987 Jan;7(1):541–544. doi: 10.1128/mcb.7.1.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevillyan J. M., Pall M. L. Control of cyclic adenosine 3',5'-monophosphate levels by depolarizing agents in fungi. J Bacteriol. 1979 May;138(2):397–403. doi: 10.1128/jb.138.2.397-403.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle E., Bergillos L., Gascón S., Parra F., Ramos S. Trehalase activation in yeasts is mediated by an internal acidification. Eur J Biochem. 1986 Jan 15;154(2):247–251. doi: 10.1111/j.1432-1033.1986.tb09389.x. [DOI] [PubMed] [Google Scholar]



