Abstract
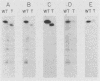
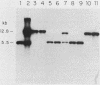
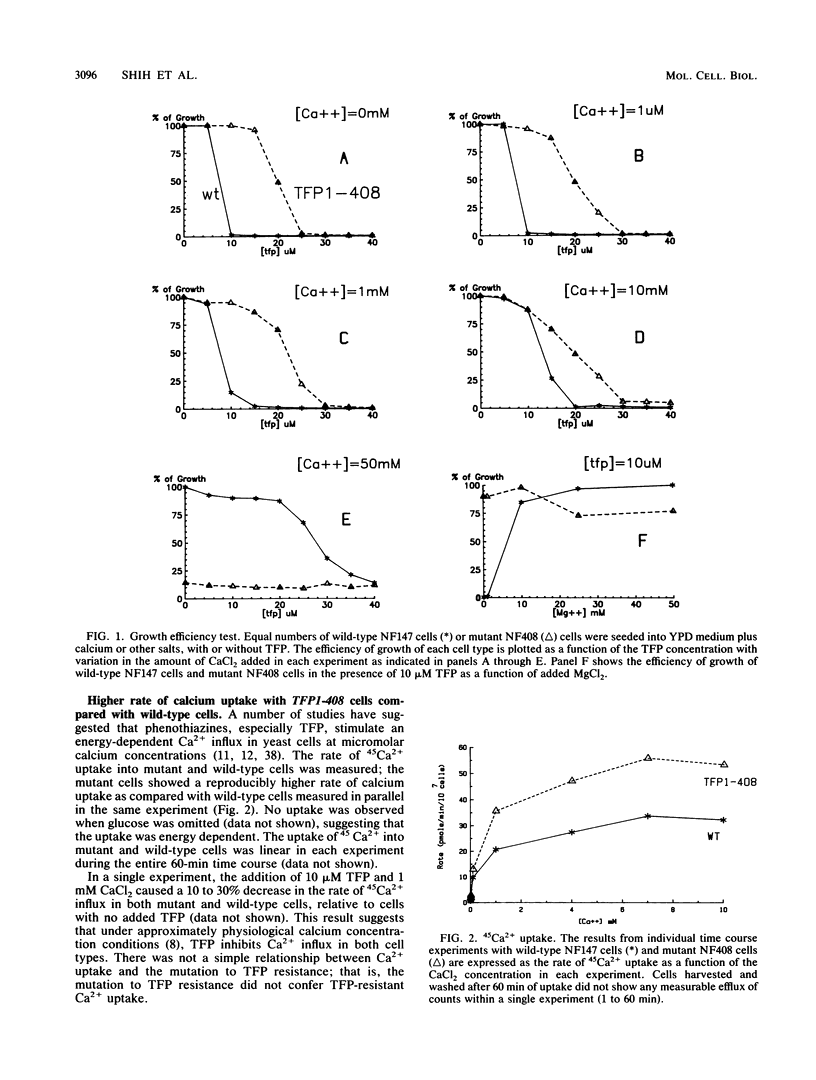
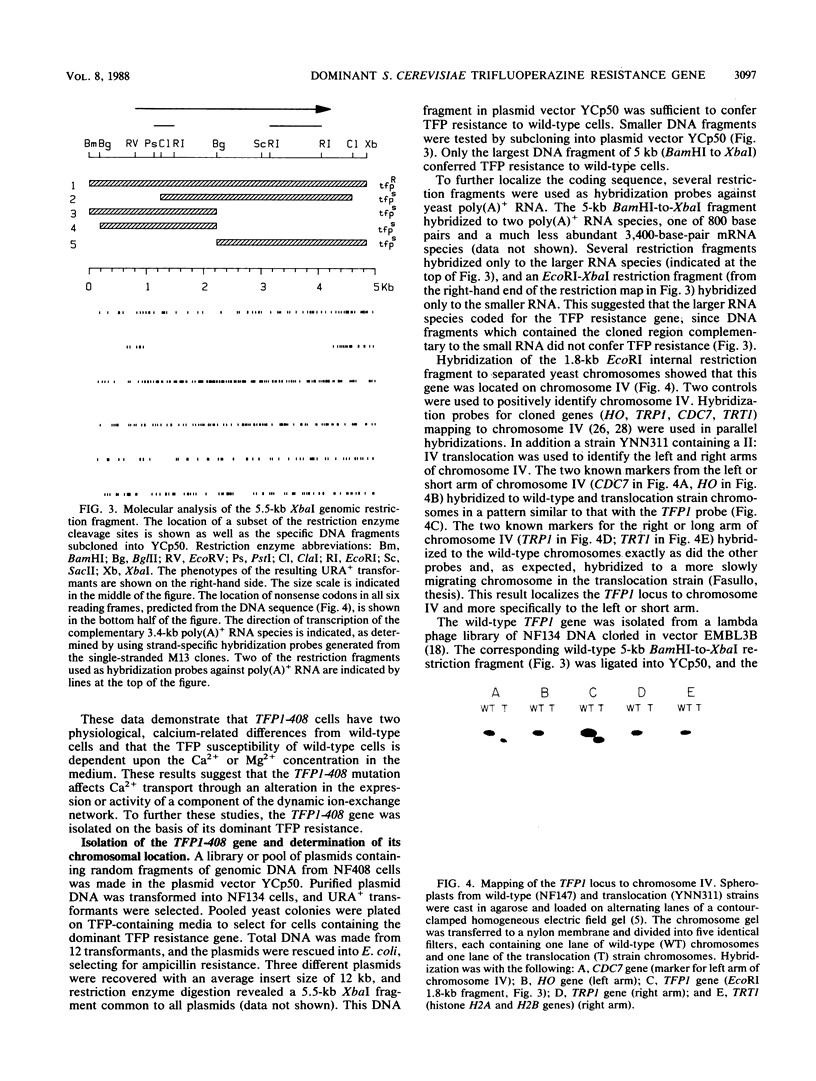
The antipsychotic drug trifluoperazine has been long considered a calmodulin inhibitor from in vitro studies but may function in vivo as a more general inhibitor by disturbing ion fluxes and altering the membrane potential. Resistance to trifluoperazine can arise in Saccharomyces cerevisiae cells by alterations in at least three distinct genetic loci. One locus, defined by a spontaneous dominant trifluoperazine resistance mutation (TFP1-408), was isolated and sequenced. The sequence of the TFP1-408 gene revealed a large open reading frame coding for a large protein of 1,031 amino acids with predicted hydrophobic transmembrane domains. A search of existing amino acid sequences revealed a significant homology with F0F1 ATP synthase. Mutant TFP1-408 cells did not grow efficiently in the presence of 50 mM CaCl2, whereas wild-type cells did. Wild-type cells became resistant to trifluoperazine in the presence of 50 mM CaCl2 or 50 mM MgCl2. Mutant cells showed a higher rate of calcium transport relative to wild-type cells. These data suggest that the TFP1 gene product codes for a transmembrane ATPase-like enzyme possibly involved in Ca2+ transport or in generating a transmembrane ion gradient between two cellular compartments.
Full text
PDF


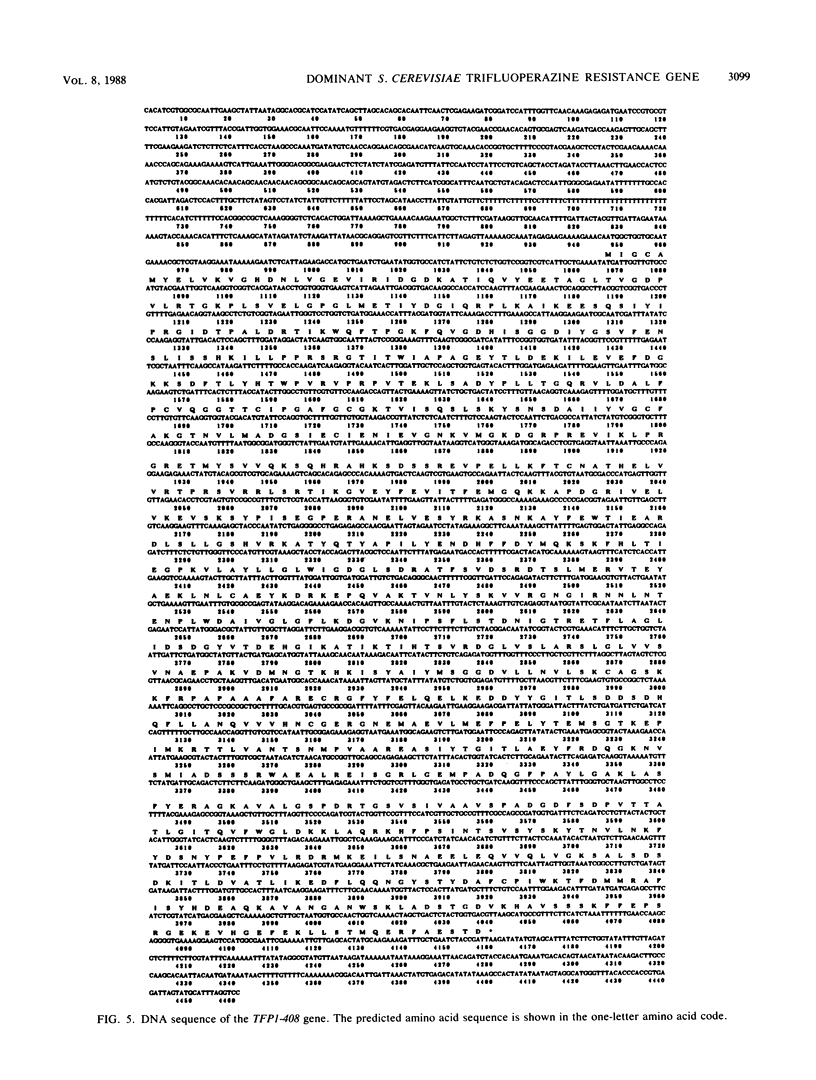
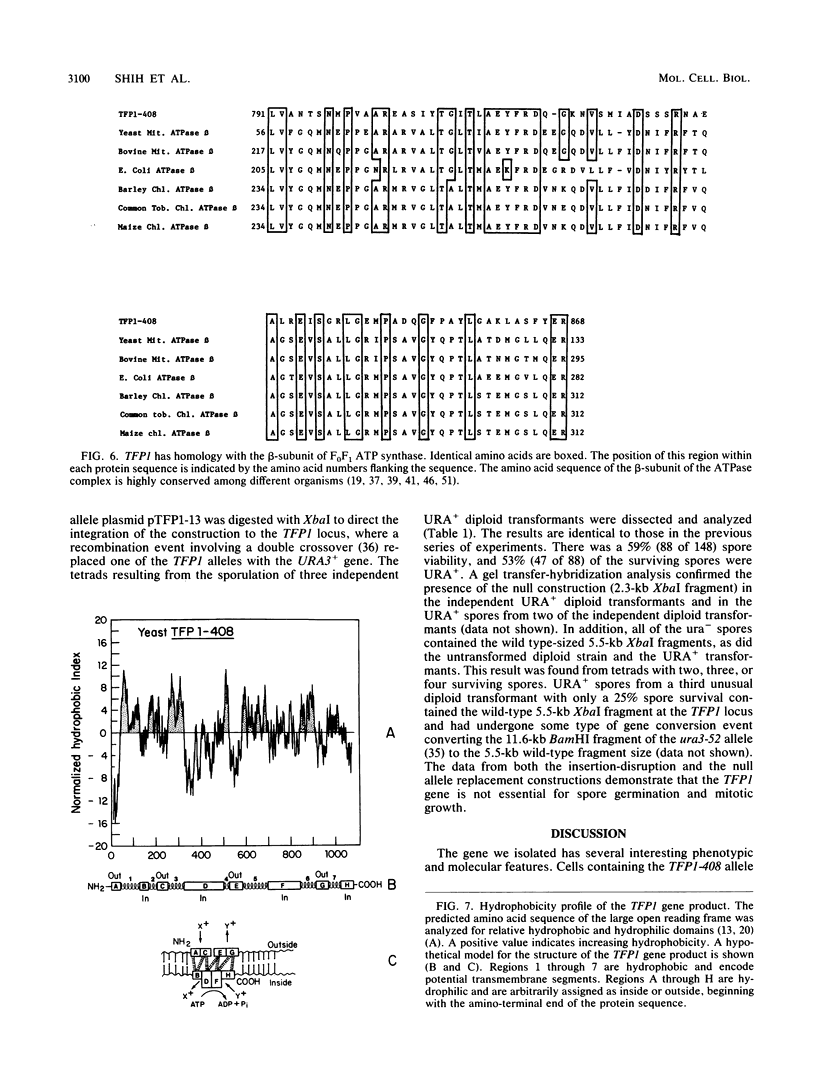



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Awqati Q. Proton-translocating ATPases. Annu Rev Cell Biol. 1986;2:179–199. doi: 10.1146/annurev.cb.02.110186.001143. [DOI] [PubMed] [Google Scholar]
- Barnes W. M., Bevan M., Son P. H. Kilo-sequencing: creation of an ordered nest of asymmetric deletions across a large target sequence carried on phage M13. Methods Enzymol. 1983;101:98–122. doi: 10.1016/0076-6879(83)01008-3. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Calcium-transporting systems of plasma membranes, with special attention to their regulation. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:543–549. [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. An electrophoretic karyotype for yeast. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Eilam Y. Effects of phenothiazines on inhibition of plasma membrane ATPase and hyperpolarization of cell membranes in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Feb 15;769(3):601–610. doi: 10.1016/0005-2736(84)90059-2. [DOI] [PubMed] [Google Scholar]
- Eilam Y. Membrane effects of phenothiazines in yeasts. I. Stimulation of calcium and potassium fluxes. Biochim Biophys Acta. 1983 Sep 7;733(2):242–248. doi: 10.1016/0005-2736(83)90528-x. [DOI] [PubMed] [Google Scholar]
- Eilam Y. The effect of monovalent cations on calcium efflux in yeasts. Biochim Biophys Acta. 1982 Apr 23;687(1):8–16. doi: 10.1016/0005-2736(82)90164-x. [DOI] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Futai M., Kanazawa H. Structure and function of proton-translocating adenosine triphosphatase (F0F1): biochemical and molecular biological approaches. Microbiol Rev. 1983 Sep;47(3):285–312. doi: 10.1128/mr.47.3.285-312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz D. Construction of a yeast actin gene intron deletion mutant that is defective in splicing and leads to the accumulation of precursor RNA in transformed yeast cells. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3493–3497. doi: 10.1073/pnas.79.11.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm C., Meeks-Wagner D. W., Fangman W. L., Botstein D. A rapid, efficient method for isolating DNA from yeast. Gene. 1986;42(2):169–173. doi: 10.1016/0378-1119(86)90293-3. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebbers E. T., Larrinua I. M., McIntosh L., Bogorad L. The maize chloroplast genes for the beta and epsilon subunits of the photosynthetic coupling factor CF1 are fused. Nucleic Acids Res. 1982 Aug 25;10(16):4985–5002. doi: 10.1093/nar/10.16.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J., Hiley C. R. Anti-muscarinic properties of neuroleptics and drug-induced Parkinsonism. Nature. 1974 Apr 12;248(449):596–597. doi: 10.1038/248596a0. [DOI] [PubMed] [Google Scholar]
- Mori T., Takai Y., Minakuchi R., Yu B., Nishizuka Y. Inhibitory action of chlorpromazine, dibucaine, and other phospholipid-interacting drugs on calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1980 Sep 25;255(18):8378–8380. [PubMed] [Google Scholar]
- Mortimer R. K., Schild D. Genetic map of Saccharomyces cerevisiae, edition 9. Microbiol Rev. 1985 Sep;49(3):181–213. doi: 10.1128/mr.49.3.181-213.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff N. F., Thomas J. H., Grisafi P., Botstein D. Isolation of the beta-tubulin gene from yeast and demonstration of its essential function in vivo. Cell. 1983 May;33(1):211–219. doi: 10.1016/0092-8674(83)90350-1. [DOI] [PubMed] [Google Scholar]
- Norris D., Osley M. A. The two gene pairs encoding H2A and H2B play different roles in the Saccharomyces cerevisiae life cycle. Mol Cell Biol. 1987 Oct;7(10):3473–3481. doi: 10.1128/mcb.7.10.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1983 May 10;258(9):5614–5617. [PubMed] [Google Scholar]
- Ohya Y., Miyamoto S., Ohsumi Y., Anraku Y. Calcium-sensitive cls4 mutant of Saccharomyces cerevisiae with a defect in bud formation. J Bacteriol. 1986 Jan;165(1):28–33. doi: 10.1128/jb.165.1.28-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Ohsumi Y., Anraku Y. Genetic study of the role of calcium ions in the cell division cycle of Saccharomyces cerevisiae: a calcium-dependent mutant and its trifluoperazine-dependent pseudorevertants. Mol Gen Genet. 1984;193(3):389–394. doi: 10.1007/BF00382073. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Ohsumi Y., Anraku Y. Isolation and characterization of Ca2+-sensitive mutants of Saccharomyces cerevisiae. J Gen Microbiol. 1986 Apr;132(4):979–988. doi: 10.1099/00221287-132-4-979. [DOI] [PubMed] [Google Scholar]
- Rose M., Winston F. Identification of a Ty insertion within the coding sequence of the S. cerevisiae URA3 gene. Mol Gen Genet. 1984;193(3):557–560. doi: 10.1007/BF00382100. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Runswick M. J., Walker J. E. The amino acid sequence of the beta-subunit of ATP synthase from bovine heart mitochondria. J Biol Chem. 1983 Mar 10;258(5):3081–3089. [PubMed] [Google Scholar]
- Saavedra-Molina A., Villalobos R., Borbolla M. Calcium uptake during the cell cycle of Saccharomyces cerevisiae. FEBS Lett. 1983 Aug 22;160(1-2):195–197. doi: 10.1016/0014-5793(83)80965-x. [DOI] [PubMed] [Google Scholar]
- Saltzgaber-Muller J., Kunapuli S. P., Douglas M. G. Nuclear genes coding the yeast mitochondrial adenosine triphosphatase complex. Isolation of ATP2 coding the F1-ATPase beta subunit. J Biol Chem. 1983 Oct 10;258(19):11465–11470. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Gay N. J., Eberle A., Runswick M. J., Walker J. E. The atp operon: nucleotide sequence of the genes for the gamma, beta, and epsilon subunits of Escherichia coli ATP synthase. Nucleic Acids Res. 1981 Oct 24;9(20):5287–5296. doi: 10.1093/nar/9.20.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. Anti-schizophrenic drugs--membrane receptor sites of action. Biochem Pharmacol. 1977 Oct 1;26(19):1741–1748. doi: 10.1016/0006-2952(77)90340-9. [DOI] [PubMed] [Google Scholar]
- Segev N., Botstein D. The ras-like yeast YPT1 gene is itself essential for growth, sporulation, and starvation response. Mol Cell Biol. 1987 Jul;7(7):2367–2377. doi: 10.1128/mcb.7.7.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R., Kielland-Brandt M. C., Fink G. R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature. 1986 Feb 20;319(6055):689–693. doi: 10.1038/319689a0. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Deno H., Kato A., Sugiura M. Overlap and cotranscription of the genes for the beta and epsilon subunits of tobacco chloroplast ATPase. Gene. 1983 Oct;24(2-3):147–155. doi: 10.1016/0378-1119(83)90074-4. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Greenberg D., Yamumura H. I. Antischizophrenic drugs: affinity for muscarinic cholinergic receptor sites in the brain predicts extrapyramidal effects. J Psychiatr Res. 1974;11:91–95. doi: 10.1016/0022-3956(74)90078-8. [DOI] [PubMed] [Google Scholar]
- Speaker M. G., Orlow S. J., Sturgill T. W., Rosen O. M. Characterization of a calmodulin-binding protein that is deficient in trifluoperazine-resistant variants of the macrophage-like cell line J774. Proc Natl Acad Sci U S A. 1983 Jan;80(2):329–333. doi: 10.1073/pnas.80.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. Mechanism of free energy coupling in active transport. Annu Rev Biochem. 1983;52:379–409. doi: 10.1146/annurev.bi.52.070183.002115. [DOI] [PubMed] [Google Scholar]
- Weiss B., Levin R. M. Mechanism for selectively inhibiting the activation of cyclic nucleotide phosphodiesterase and adenylate cyclase by antipsychotic agents. Adv Cyclic Nucleotide Res. 1978;9:285–303. [PubMed] [Google Scholar]
- Zurawski G., Clegg M. T. The barley chloroplast DNA atpBE, trnM2, and trnV1 loci. Nucleic Acids Res. 1984 Mar 12;12(5):2549–2559. doi: 10.1093/nar/12.5.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Meis L., Vianna A. L. Energy interconversion by the Ca2+-dependent ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 1979;48:275–292. doi: 10.1146/annurev.bi.48.070179.001423. [DOI] [PubMed] [Google Scholar]