Abstract
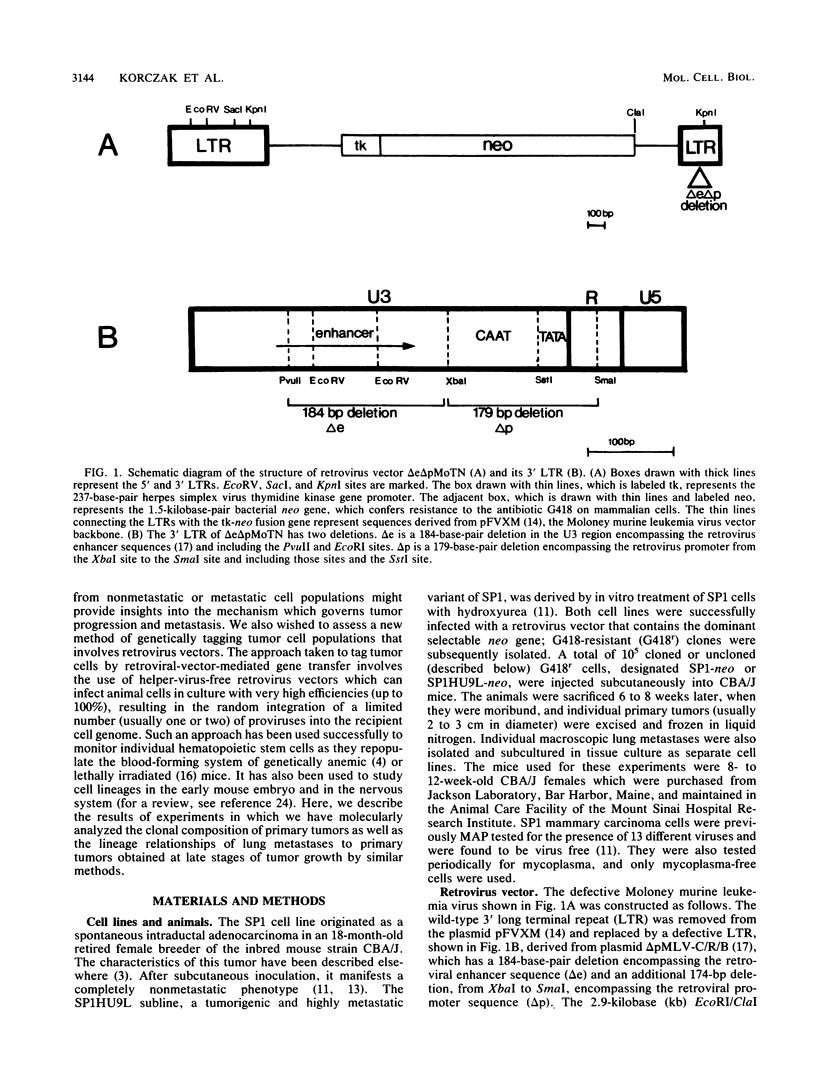
Retrovirus vector infection was used to introduce large numbers of unique genetic markers into tumor cell populations for the purpose of analyzing comparative changes in the clonal composition of metastatic versus that of nonmetastatic tumors during their progressive growth in vivo. The cell lines used were SP1, a nonmetastatic, aneuploid mouse mammary adenocarcinoma, and SP1HU9L, a metastatic variant of SP1. Cells were infected with delta e delta pMoTN, a replication-defective retrovirus vector which possesses the dominant selectable neo gene and crippled long terminal repeats. G418r colonies were obtained at a frequency of 4 x 10(-3). Southern blot analysis of a number of clones provided evidence of random and heritable integration of one or two copies of the proviral DNA. Clonal evolution of primary tumor growth and the nature of lineage relationships among spontaneous metastases and primary tumors were analyzed by subcutaneously injecting 10(5) cells from a pooled mixture of 3.6 x 10(2) G418r SP1HU9L or 10(4) G418r SP1 colonies into syngeneic CBA/J mice. The most striking finding was the relative clonal homogeneity of advanced primary tumors; they invariably consisted of a small number (less than 10) of distinct clones despite the fact that hundreds or thousands of uniquely marked clones had been injected. In the case of the metastatic SP1HU9L cells, the nature of these "dominant" clones varied from one tumor to another. Analysis of a number of lung metastases revealed that a proportion of them were derived from dominant primary tumor clones and were composed of one, and sometimes two, distinct progenitors. In some animals, all the lung metastases were derived from a common progenitor clone, whereas in others, each metastatic nodule had a different progenitor. The results show the following. (i) Retrovirus vector infection can be used to introduce large numbers of unique and stable clonal markers into tumor cell populations. (ii) The progeny of a very limited number of clones dominate in advanced primary tumors. (iii) Mammary carcinoma metastases are of mono- or biclonal origin. The significance of the results is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breitman M. L., Tsui L. C., Buchwald M., Siminovitch L. Introduction and recovery of a selectable bacterial gene from the genome of mammalian cells. Mol Cell Biol. 1982 Aug;2(8):966–976. doi: 10.1128/mcb.2.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlow D. A., Kerbel R. S., Feltis J. T., Elliott B. E. Enhanced expression of class I major histocompatibility complex gene (Dk) products on immunogenic variants of a spontaneous murine carcinoma. J Natl Cancer Inst. 1985 Aug;75(2):291–301. [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Esumi M., Aritaka T., Arii M., Suzuki K., Tanikawa K., Mizuo H., Mima T., Shikata T. Clonal origin of human hepatoma determined by integration of hepatitis B virus DNA. Cancer Res. 1986 Nov;46(11):5767–5771. [PubMed] [Google Scholar]
- FOULDS L. The experimental study of tumor progression: a review. Cancer Res. 1954 Jun;14(5):327–339. [PubMed] [Google Scholar]
- Fearon E. R., Hamilton S. R., Vogelstein B. Clonal analysis of human colorectal tumors. Science. 1987 Oct 9;238(4824):193–197. doi: 10.1126/science.2889267. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. Clonal origin of human tumors. Biochim Biophys Acta. 1976 Oct 12;458(3):283–321. doi: 10.1016/0304-419x(76)90003-2. [DOI] [PubMed] [Google Scholar]
- Frost P., Kerbel R. S., Hunt B., Man S., Pathak S. Selection of metastatic variants with identifiable karyotypic changes from a nonmetastatic murine tumor after treatment with 2'-deoxy-5-azacytidine or hydroxyurea: implications for the mechanisms of tumor progression. Cancer Res. 1987 May 15;47(10):2690–2695. [PubMed] [Google Scholar]
- Hu F. N., Wang R. Y., Hsu T. C. Clonal origin of metastasis in B16 murine melanoma: a cytogenetic study. J Natl Cancer Inst. 1987 Jan;78(1):155–163. doi: 10.1093/jnci/78.1.155. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Waghorne C., Man M. S., Elliott B., Breitman M. L. Alteration of the tumorigenic and metastatic properties of neoplastic cells is associated with the process of calcium phosphate-mediated DNA transfection. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1263–1267. doi: 10.1073/pnas.84.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegler M., Perez C. F., Hardy C., Botchan M. Transformation mediated by the SV40 T antigens: separation of the overlapping SV40 early genes with a retroviral vector. Cell. 1984 Sep;38(2):483–491. doi: 10.1016/0092-8674(84)90503-8. [DOI] [PubMed] [Google Scholar]
- Lagarde A. E., Donaghue T. P., Dennis J. W., Kerbel R. S. Genotypic and phenotypic evolution of a murine tumor during its progression in vivo toward metastasis. J Natl Cancer Inst. 1983 Jul;71(1):183–191. [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Linney E., Davis B., Overhauser J., Chao E., Fan H. Non-function of a Moloney murine leukaemia virus regulatory sequence in F9 embryonal carcinoma cells. 1984 Mar 29-Apr 4Nature. 308(5958):470–472. doi: 10.1038/308470a0. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Miller B. E., Miller F. R., Wilburn D. J., Heppner G. H. Analysis of tumour cell composition in tumours composed of paired mixtures of mammary tumour cell lines. Br J Cancer. 1987 Nov;56(5):561–569. doi: 10.1038/bjc.1987.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C. Mechanisms of tumor progression. Cancer Res. 1986 May;46(5):2203–2207. [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Ootsuyama A., Tanaka K., Tanooka H. Evidence by cellular mosaicism for monoclonal metastasis of spontaneous mouse mammary tumors. J Natl Cancer Inst. 1987 Jun;78(6):1223–1227. [PubMed] [Google Scholar]
- Poste G., Tzeng J., Doll J., Greig R., Rieman D., Zeidman I. Evolution of tumor cell heterogeneity during progressive growth of individual lung metastases. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6574–6578. doi: 10.1073/pnas.79.21.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. Retroviruses and the study of cell lineage. Development. 1987 Nov;101(3):409–419. doi: 10.1242/dev.101.3.409. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Talmadge C., Tanio Y., Meeker A., Talmadge J., Zbar B. Tumor cells transfected with the neomycin resistance gene (neo) contain unique genetic markers useful for identification of tumor recurrence and metastasis. Invasion Metastasis. 1987;7(4):197–207. [PubMed] [Google Scholar]
- Talmadge J. E., Wolman S. R., Fidler I. J. Evidence for the clonal origin of spontaneous metastases. Science. 1982 Jul 23;217(4557):361–363. doi: 10.1126/science.6953592. [DOI] [PubMed] [Google Scholar]
- Talmadge J. E., Zbar B. Clonality of pulmonary metastases from the bladder 6 subline of the B16 melanoma studied by southern hybridization. J Natl Cancer Inst. 1987 Feb;78(2):315–320. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Preisinger A. C., Willard H. F., Michelson A. M., Riggs A. D., Orkin S. H. Clonal analysis using recombinant DNA probes from the X-chromosome. Cancer Res. 1987 Sep 15;47(18):4806–4813. [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Yu S. F., von Rüden T., Kantoff P. W., Garber C., Seiberg M., Rüther U., Anderson W. F., Wagner E. F., Gilboa E. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3194–3198. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]