Abstract
Background
IL-17 signaling has been implicated in lung and skin fibrosis. Here we examined the role of IL-17 signaling in the pathogenesis of liver fibrosis.
Methods
Using cholestatic and hepatotoxic models of liver injury, the development of liver fibrosis in wild type mice was compared to IL-17RA−/− mice, and to bone marrow chimeric mice devoid of IL-17 signaling in immune cells and Kupffer cells (IL-17RA−/−→wt and IL-17A−/− →wt mice), or in liver resident cells (Wt→ IL-17RA−/− mice).
Results
We determined that IL-17A and its receptor is highly induced in liver injury and has a strong pro-fibrogenic effect on both inflammatory and liver resident cells. IL-17 signaling facilitates production of IL-6, IL-1β, and TNF-α by inflammatory cells, and increases the expression of TGF-β1, the major pro-fibrogenic cytokine. IL-17 directly induces collagen Type I production in hepatic stellate cells (HSCs) via activation of the Stat3 signaling pathway. Mice devoid of Stat3 signaling in HSCs (GFAPStat3−/− mice) are less susceptible to fibrosis. Furthermore, deletion of IL-23 in immune cells results in attenuation of liver fibrosis, while deletion of IL-22 exacerbates fibrosis. Administration of IL-22 and IL-17E (IL-25, a negative regulator of IL-23) protects mice from BDL-induced liver fibrosis.
Conclusions
IL-17 induces liver fibrosis through multiple mechanisms and may serve as an attractive target for anti-fibrotic therapy.
Keywords: IL-17 signaling, liver fibrosis, BM derived and liver resident macrophages (Kupffer cells), activated myofibroblasts, hepatic stellate cells
INTRODUCTION
Fibrosis is the outcome of many chronic liver diseases1, including hepatitis B virus (HBV), hepatitis C virus (HCV), alcoholic liver disease1. It is manifested by accumulation of extracellular matrix (ECM) and scar formation. Several injury-triggered events play a critical role in the pathogenesis of liver fibrosis. Hepatocyte apoptosis causes recruitment of inflammatory cells to the damaged liver and release of pro-fibrogenic cytokines (TGF-β1, IL-6, IL-1β, TNF-α). BM-derived and liver resident macrophages (Kupffer cells, KC) produce TGF-β1 in the fibrotic liver1, 2. TGF-β1 is critical for activation of fibrogenic myofibroblasts, which in response to injury upregulate α-smooth muscle actin (α-SMA) and secrete extracellular matrix proteins, mostly collagen Type I (ColI)2. Hepatic stellate cells (HSCs) are the major source of fibrogenic myofibroblasts in liver injury3. Under physiological conditions, HSCs retain a quiescent phenotype (qHSCs), but following TGF-β1 stimulation activate into ColI+α-SMA+ myofibroblasts (aHSCs)1.
Interleukin-17 (IL-17)-producing effector CD4+ T (Th17) cells have been recently discovered and shown to mediate autoimmunity and immune defense against pathogens4. IL-17A and IL-17F are the members of Th17-family of cytokines which signal through their cognate receptors IL-17RA and IL-17RC5. IL-17A is most abundant in Th17 cells and exhibit higher biological activity than IL-17F5. Therefore, mice deficient in IL-17A (IL-17A−/− mice) exhibit a more pronounced phenotype than IL-17F−/− mice5, 6. The efficiency of IL-17A signaling correlates with the expression level of surface IL-17RA7. IL-17RA is expressed ubiquitously, and is further induced in hematopoietic cells and fibroblasts5. Deletion of IL-17RA results in complete abrogation of both IL-17A and IL-17F effects5. IL-17A is mainly produced by CD4+ Th17 cells, but also by a variety of cells, including γδ T cells, CD8+ T cells, NKT cells, NK cells, innate lymphoid cells, eosinophils, neutrophils, and monocytes5 and signals via TRAF6, Act1, JNK, ERK, NF-κB and Stat35, 8.
Differentiation and expansion of Th17 from Th0 is regulated by TGF-β1, IL-6 and IL-21, and IL-235. In turn, IL-17E (IL-25) and IL-27 negatively regulate Th17 via downregulation of IL-235, 6. Th17 also secrete IL-22, which has been implicated in hepatoprotection9. Recent experimental studies, supported by clinical observations, have implicated IL-17 signaling in the fibrogenesis of lungs, skin, and liver 10, 11.
The aim of our study is to characterize the role of IL-17 signaling and related cytokines in hepatic fibrosis induced by either bile duct ligation (BDL) or carbon tetrachloride (CCl4)1. Here we report that IL-17RA deletion in mice dramatically inhibits both models of liver fibrosis. Using BM chimeric mice, we determined that deletion of IL-17A, IL-23 or IL-17RA in BM-derived cells decreases liver fibrosis by 50–55%, while deletion of IL-22 exacerbates fibrosis. Finally, we demonstrate that IL-17A directly stimulates activation of HSCs in a Stat3-dependent manner.
MATERIALS AND METHODS
Cell Lines and mice
LX-2 cell line (gift of Dr. Friedman)12 and hTERT cell line13, Collagen α1(I)-GFP mice14 were previously described. C57BL/6 mice (8 weeks old) and GFAP-Cre mice were purchased (Jackson Laboratories). We obtained IL-17RA−/− mice15 (gift of Dr. Kolls), IL-17A−/− mice16 (gift of Dr. Iwakura), and STAT3f/f mice17 (gift of Dr. Takeda), IL-22−/− mice and IL-23−/− mice (Genentech). All animal experiments were approved by the UCSD Institutional Animal Care and Use Committee.
Liver injury
Liver injury was induced in mice by intragastric gavage with CCl4 (1:4 dilution in corn oil, 200 μl × 12 injections2) or by BDL (3 weeks)2.
Isolation of hepatocytes and non-parenchymal cell fraction and primary HSCs
Livers are perfused using pronase/collagenase method2. Singe-cell suspensions are centrifuged at 50g for 5 minutes to pellet the hepatocyte fraction. The remaining non-parenchymal cell fraction was collected2. KC and EC were isolated by gradient centrifugation (15% Nycodenz) following by magnetic sorting with anti-CD11b and anti-CD31 antibodies, respectively (Miltenyi Biotec). HSCs were isolated using gradient centrifugation (8.2% Nycodenz, see Suppl. Methods)2.
RT-PCR and real time quantitative PCR
Quiescent HSCs (5 × 105 cells, cultured < 6 h); activated HSCs, KC and hepatocytes (cultured for 48 h) were serum starved (for 24 h) and then stimulated with mIL-17A (10ng/ml) and mIL-17F (10ng/ml) for 2h. LX2 and hTERT cells (5 × 105 cells) were stimulated with hIL-17A (10ng/ml) and hIL-17F (10ng/ml) for 8h. mRNA was isolated and RT-PCR was performed as described in Suppl. Methods.
Immunohistochemistry and immunofluorescent staining
Human tissues18 are gift of Dr. Yanagida. Immunohystochemistry was performed on formalin-fixed livers with anti-α-SMA Ab (Dako Cytomation) using MOM kit (Vector Laboratories) following by DAB staining (Vector), and counterstaining with Haematoxilin. Anti-mouse Stat3 (Cell Signaling Technologies, Inc.), Stat3, NF-κB (p65), and phospho-Stmad2/3 (Santa Cruz) and anti-human IL-17A, Stat3 (Santa Cruz) antibodies were used. The images were taken using confocal microscopy and analyzed by Image J.
Bone marrow transplantation
Recipient mice were intravenously injected with liposomal clodronate (150 μl) to deplete KC2. BMT was performed 24 h later as described19, by transplantation of the whole BM into lethally irradiated (1200 Rad) recipient mice. The efficiency of reconstitution was accessed in mice transplanted with β-actin-GFP BM (See Suppl. Figure 6).
Flow cytometry
Phenotyping was performed on BD LSR II (BD) using anti-mouse antibodies (eBioscience; see Suppl. Materials). Intracellular staining was performed after restimulation of cells with PMA/Ionomycin/Brefeldin A (or IL-23/Brefeldin A) for 5 hours using BD Cytofix/Cytoperm (BD, San Jose, CA).
Protein detection
Western blot analysis was performed using protein lysates from IL-17A-stimulated primary cells or cell lines (see Suppl. Methods). Serum expression of IL-17A, IL-17F, IL-17E (IL-25), IL-22, and IL-6 was detected by ELISA (IL-17A, IL-17E(IL25), IL-22, and IL-6 antibodies from Biolegend; IL-17F antibodies from eBioscience).
Statistical analysis
All data are expressed as the mean ± standard deviation (SD). Differences between experimental and control groups were assessed by two-tailed unpaired Student’s t-tests using Graph Pad Prism 5.0 (GraphPad Software, San Diego, CA). A p-values less than 0.05 was considered statistically significant.
Supplemental material contains Materials, Acknowledgments, References, Eight Figures and Figure legend.
RESULTS
Progression of liver fibrosis correlates with elevated expression of IL-17
Expression of IL-17A and IL-17F and their cognate receptors IL-17RA and IL-17RC was examined in two models of liver fibrosis in mice: BDL and CCl4. We determined that mRNA levels of IL-17A, IL-17F, IL-17RA and IL-17RC in fibrotic livers were strongly upregulated independent of the etiology of fibrosis (Figure 1A). Development of liver fibrosis was also associated high levels of circulating IL-17A (Figure 1A). Moreover, increased expression of IL-17A was detected in livers from patients with liver fibrosis and cirrhosis of different etiology (vs patients with no fibrosis), and correlated with the severity of the disease (Suppl. Figure 1). We conclude that IL-17 signaling may contribute to the pathogenesis of liver fibrosis.
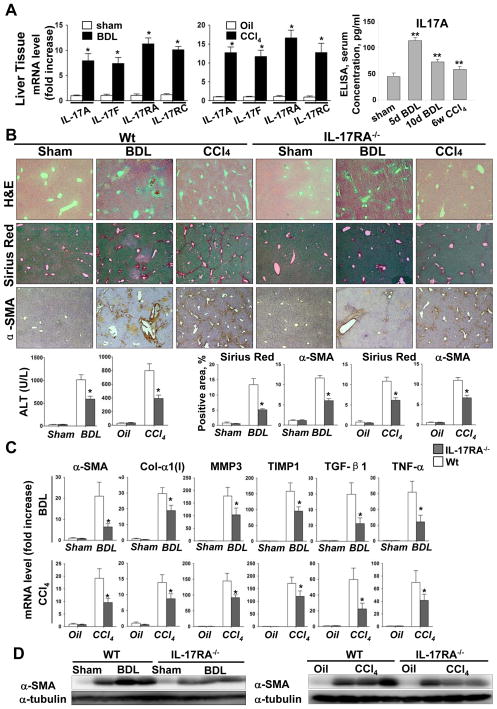
Figure 1. Development of liver fibrosis is strongly inhibited in IL-17RA−/− mice.
(A)Expression of IL-17A and IL-17F, IL-17RA and IL-17C genes is upregulated during liver fibrosis. The data is fold induction of mRNA expression from whole liver of BDL (n=10) and CCl4-treated (n=10) mice compared to control mice, *p<0.01. Fibrogenic liver injury is associated with increased serum IL-17A detected in BDL (5 days, n=6; 10 days n=5) and CCl4-treated (8 weeks, n=5) mice by ELISA, **p<0.05.
(B) Development of BDL- and CCl4-induced liver fibrosis is inhibited in IL-17RA−/− mice. Livers from wild type mice (untreated n=3), BDL (n=10) and CCl4-treated (n=10), and IL-17RA−/− mice (untreated n=3), BDL (n=9) and CCl4-treated (n=10), were analyzed by H&E, Sirius Red and α-SMA staining and quantified (*p<0.01). Bright field micrographs are shown using × 10 objectives. Liver function was assessed by ALT, *p<0.01.
(C) Expression of ibrogenic genes is downregulated in whole livers from BDL and CCl4-treated IL-17RA−/− mice compared to wild type mice. The data are shown as fold mRNA induction compared with untreated mice, *p<0.02.
(D)Reduced expression of α-SMA protein was detected in livers of BDL- and CCl4-treated IL-17RA−/− mice compared to the wild type mice.
IL-17RA−/− mice are resistant to liver fibrosis. The role of IL-17 signaling in hepatic fibrosis was studied in IL-17RA−/− mice, subjected to BDL or CCl4 (Figure 1B-D). BDL-induced liver fibrosis was inhibited in IL-17RA−/− mice, as demonstrated in IL-17RA−/− mice by a decrease of collagen deposition (4 ± 1% positive area) and the number of α-SMA+ myofibroblasts (5 ± 1.5%) compared to wild type mice (13 ± 4% and 12 ± 1%, respectively; Figure 1B). The liver function was also improved in IL-17RA−/− mice (Figure 1B). Reduced mRNA expression of fibrogenic genes (α-SMA, Col-α1(I), MMP3, TIMP1, TGF-β1 and TNF-α, Figure 1C) in livers of BDL-operated IL-17RA−/− mice correlated with low levels of α-SMA protein (vs wild type mice; Figure 1D). Similar results were obtained in CCl4-injured IL-17RA−/− mice (Figure 1B-D), suggesting that ablation of IL-17 signaling significantly attenuates development of liver fibrosis of different etiologies in mice.
CD45+TCRβ+ cells are the major source of IL-17A in fibrotic liver in mice
To identify the source of IL-17 in fibrotic livers, the lymphoid fraction2 was isolated from livers of sham- or BDL-operated wild type mice, in vitro stimulated with known activators of IL-17, phorbol-miristate acetate (PMA) plus Ionomycin or IL-235, and analyzed by flow cytometry for intracellular IL-17A and IL-17F expression. Following PMA/Ionomycin stimulation, the number of IL-17A-expressing cells was 12 fold increased in livers of BDL wild type mice versus sham controls (Figure 2A, Suppl. Figure 2). In comparison, the number of IL-17F+ cells was 2.3 fold increased under similar conditions. Both, PMA/Ionomycin and IL-23 induced mostly production of IL-17A homodimers (over IL-17F homodimers, in ratio 6:1 and 3:1 respectively), suggesting that IL-17A plays a more significant role in liver fibrosis than IL-17F. PMA/Ionomycin-induced IL-17A+ cells expressed predominantly T cell markers CD3 (83 ± 4%), TCRβ (74 ± 2%) and TCRγδ (21 ± 5%). Similar results were obtained in IL-23-stimulated IL-17A+ cells, demonstrating that T cells are the major source of IL-17A in fibrotic livers.
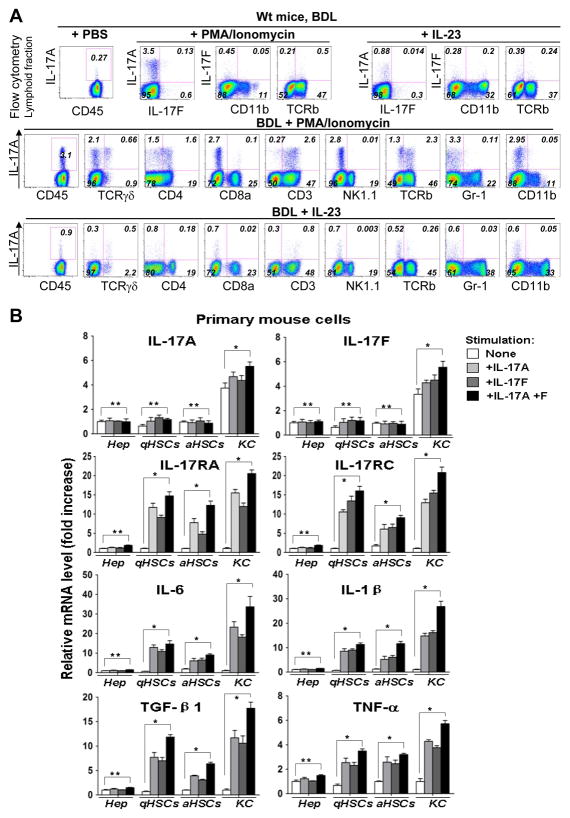
Figure 2. IL-17A/IL-17RA signaling in fibrotic liver is mediated by immune cells, KC and HSCs.
(A)Hepatic T cells are the major source of IL-17A in fibrotic livers. Lymphoid fraction from sham (n=3) and BDL (n=3) wild type mice were in vitro stimulated with PMA (100ng/ml)+Ionomycin (500ng/ml) or IL-23 (20 ng/ml) for 5 hours, and analyzed by flow cytometry for intracellular IL-17A and IL-17F expression. Expression of IL-17A was further analyzed in CD45+, CD3+, CD4+, CD8a+, TCRγδ+, TCRβ+, NK1.1+, CD11b+ cells. Representative dot plots are shown.
(B) Primary wild type hepatocytes, quiescent and in vitro activated HSCs, and KC were stimulated with IL-17A (10ng/ml), IL-17F (10 ng/ml), or a combination of both. mRNA levels of IL-17 cytokine/receptors and inflammatory gene expression are shown as fold induction compared with untreated cells, *p<0.01, ** non significant.
Expression of IL-17 cytokines/receptors by liver resident cells
Hematopoietic cells respond to IL-17A stimulation by upregulation IL-17 production5. We next examined the ability of liver resident cells to produce IL-17 and transmit IL-17 signals. Primary mouse KC, hepatocytes, quiescent and activated HSCs, and endothelial cells (EC) were isolated from wild type mice, and their responses to IL-17 were evaluated in culture (Figure 2B). Only KC express and further upregulate IL-17A, IL-17F and IL-1β in response to stimulation with IL-17 (IL-17A, IL-17F or a combination of both cytokines) as demonstrated by RT-PCR (Figure 2B) and ELISA (Suppl. Figure 3). Although the mRNAs for IL-17RA and IL-17RC were expressed by hepatocytes, KC, HSCs (Figure 2B), and EC (Suppl. Figure 4), only KC and HSCs responded to IL-17 by increasing the mRNAs of IL-17RA and IL-17RC, and expression of fibrogenic cytokines TGF-β1, IL-6, and TNF-α (Figure 2B and Suppl. Figure 5). Thus, in addition to BM-derived T cells, KC may also contribute to local IL-17A production and IL-17-induced secretion of pro-fibrogenic cytokines. Consistent with previous findings, hepatocytes20 and EC21 do not transmit IL-17 signals despite IL-17RA expression.
Deletion of IL-17 signaling in inflammatory and KC results in inhibition of liver fibrosis
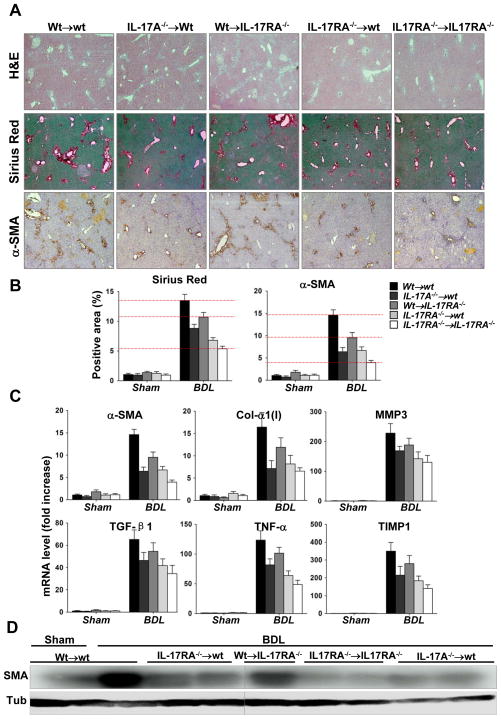
To assess IL-17 signaling in inflammatory + KC, wild type mice were pre-treated with clodronate (to deplete KC)2, lethally irradiated, and reconstituted with IL-17A−/− or IL-17RA−/− BM to generate IL-17A−/− →wt or IL-17RA−/− →wt mice, respectively (Suppl. Figure 6). After a two month recovery, liver fibrosis was induced in these mice by BDL. In comparison with wild type (Wt→wt mice, 100%), deficiency of either IL-17A or IL-17RA in BM cells decreased liver fibrosis by 50 ± 4% or 55 ± 6% (versus 75 ± 5% in IL-17RA−/− →IL-17RA−/− mice) (Figure 3A-B). Inhibition of fibrosis correlated with lower mRNA levels of fibrogenic genes (α-SMA, Col-α1(I), MMP3, TIMP1, TGF-β1 and TNF-α, Figure 3C), and lower α-SMA (Figure 3D), demonstrating that IL-17 signaling in inflammatory cells + KC contribute to the pathogenesis of liver fibrosis. TGF-β1 plays a critical role in development of liver fibrosis, and our data suggest that IL-17 signaling induces ≈30% of TGF-β1 production by BM-derived inflammatory cells + KC in fibrotic livers (Figure 3C).
Figure 3. Selective ablation of IL-17 signaling in inflammatory cells + KC and liver resident cells results in inhibition of BDL-induced liver fibrosis.
(A) Development of liver fibrosis in BM chimeric mice was compared. Livers from BDL-injured Wt→wt (n=7), IL-17A−/− →wt (n=10), Wt→IL-17RA−/− (n=10), IL-17RA−/− →wt (n=10), and IL-17RA−/− → IL-17RA−/− (n=8) mice were analyzed by H&E, Sirius Red staining, and α-SMA immunohistochemistry. Representative bright field micrographs are shown using × 10 objectives.
(B) Quantification of Sirius Red staining and α-SMA immunohistochemistry in BM chimeric mice. Results are shown as fold induction compared with the sham-control Wt→wt mice. (Each group of BMT mice is compared to the BDL-injured wt mice, p<0.01. For Wt→IL-17RA mice, p<0.05).
(C) Fibrogenic mRNAs are downregulated in whole liver from BDL-operated IL-17A−/− →wt, Wt→IL-17RA−/−, or IL-17RA−/− →wt mice, compared to BDL-operated Wt→wt mice (p<0.01). The data are shown as fold induction compared with sham Wt→wt mice.
(D) Expression of α-SMA protein, evaluated by Western blot, was progressively downregulated in Wt→IL-17RA−/− > IL-17A−/− →wt > IL-17RA−/− →wt > IL-17RA−/− →IL-17RA−/− mice. Representative images are shown.
Deletion of IL-17 signaling in non-hematopoietic endogenous liver cells attenuates hepatic fibrosis
The importance of IL-17 signaling in HSCs (and other non-inflammatory liver resident cells) in liver fibrosis was tested in chimeric Wt→IL-17RA−/− mice. In comparison with Wt→wt mice (100%), activation of α-SMA+ myofibroblasts and total collagen deposition was attenuated in Wt→IL-17RA−/− mice (25 ± 7% inhibition vs 69 ± 5% in IL-17RA−/− →IL-17RA−/− mice), suggesting that IL-17 signaling of liver resident (non-inflammatory) cells contributes to fibrosis. Since, hepatocytes20 and EC21 did not respond to IL-17 signaling to facilitate fibrogenesis (Figure 2B, Suppl. Figure 4), we conclude that ablation of IL-17 signaling in HSCs was critical for inhibition of liver fibrosis in Wt→IL-17RA−/− mice.
IL-17RA-deficient KC and HSCs exhibit impaired activation in vivo in response to liver injury
We compared purified KC/macrophages from the fibrotic livers of chimeric IL-17RA−/− →wt mice with wt→wt mice. We detected decreased mRNA levels of TGF-β1 (↓7 fold), IL-6 (↓14 fold), IL-1β (↓4 fold), and TNF-α (↓3 fold) in IL-17RA−/− KC (vs wild type cells, Suppl. Figure 7A). Furthermore, IL-17A stimulates TGF-β1 production in KC/macrophages (Suppl. Figure 7B). In co-culture experiments, IL-17A-stimulated wild type KC activated wild type and IL-17RA-deficient HSCs more effectively than IL-17RA−/− KC (Suppl. Figure 7C).
We next compared expression of fibrogenic genes in in vivo activated primary IL-17RA-deficient and wild type HSCs prepared from Wt→IL-17RA−/− and Wt→Wt mice, respectively (Suppl. Figure 7D). BDL-activated IL-17RA−/− HSCs expressed less α-SMA (↓3 fold), Col-α1(I) (↓5 fold), MMP3 (↓22 fold), TIMP1 (↓24 fold), and TGF-β1(↓3 fold) mRNA (vs wild type HSCs). Similar results were obtained in CCl4-activated HSCs (Suppl. Figure 7D). Thus, despite an intact IL-17 pathway in BM cells in Wt→IL-17RA−/− mice, the in vivo activation of IL-17RA-deficient HSCs into myofibroblasts is impaired. Thus, we hypothesized that IL-17 may directly regulate HSC activation.
Activation of target genes in IL-17A stimulated KC and HSCs
Next, phosphorylation of IL-17 target proteins in KC and HSCs was assessed in vitro (Suppl. Figure 7E). Stimulation of primary wild type KC with IL-17A resulted in phosphorylation (within 30 min) of Stat3, ERK, and JNK. Similarly, phosphorylation of ERK and AKT but not JNK (not shown) was observed in primary wild type HSCs after IL-17A treatment (30 min). In particular, strong and rapid phosphorylation of Stat3 in HSCs was detected 15 min after IL-17A treatment (Suppl. Figure 7E). While the IL-17 signaling pathway in macrophages is characterized8, 22 (and Suppl. Figure 7F), IL-17 signaling in HSCs is unknown. Similar to TNF-α, IL-17A induced nuclear translocation of NFκB p65 in HSCs after 30 min of treatment (Figure 4A). Similar to IL-6, we observed a rapid (within 10–15 min) nuclear translocation of Stat3 in HSCs in response to IL-17A treatment (Figure 4A). Thus, similar to macropahges8, 22, NF-κB and Stat3 are downstream targets of IL-17 signaling in HSCs. While NF-κB signaling is required for survival of activated HSC23, Stat3 may mediate HSC activation by stimulating collagen production.
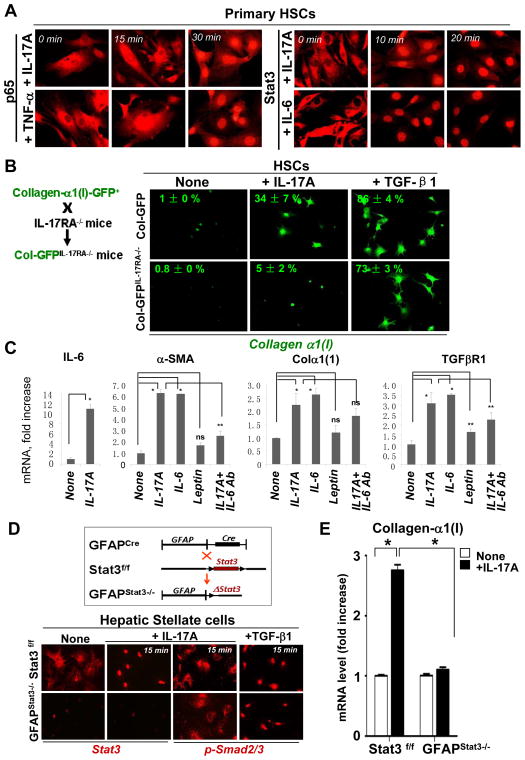
Figure 4. IL-17A facilitates HSC activation into myofibroblasts via Stat3.
(A)IL-17A induced nuclear translocation of p65 and Stat3 in primary murine HSCs. HSCs (5 × 105 cells) were stimulated with IL-17A (10 ng/ml), TNF-α (20 ng/ml) or IL-6 (30 ng/ml) for 0–30 min, translocation of p65 (NF-κB) or Stat3 was analyzed by fluorescent microscopy using × 40 objective.
(B) IL-17A stimulates collagen-α1(I) expression by HSCs. Primary murine qHSCs were isolated from Col-GFP or Col-GFPIL-17RA−/− mice, and stimulated with IL-17A (10 ng/ml) or TGF-β1 (3 ng/ml ) in 1% FSCs for 48 hours. The percent of cells upregulated collagen-α1(I)-GFP was calculated, p<0.05. Representative images are shown using × 20 objective.
(C) Fibrogenic properties of IL-17A, IL-6 and leptin. Fibrogenic mRNA expression was tested in HSCs treated with IL-17A (10 ng/ml), IL-6 (30 ng/ml), or leptin (100 ng/ml) for 4 h. IL-17A induced production of IL-6 in HSCs. The direct effect of IL-17A on collagen-α1(I) mRNA expression was assessed in IL-17A-stimulated HSC cultures depleted for IL-6 (using anti-IL-6 antibody; 0.5 μg/ml). *p<0.01, **p<0.05, ns – non significant.
(D) IL-17A activates Stat3 in HSCs. Deletion of Stat3 in HSCs was achieved by crossing GFAP-Cre mice x floxed Stat3f/f mice = GFAPStat3−/− mice. qHSCs were isolated from GFAPStat3−/− or Stat3f/f wt mice, and stimulated with IL-17A (10 ng/ml) or TGF-β1 (3 ng/ml) for 15 min. Nuclear translocation of Stat3 (but not phosphor-Smad2/3) was detected in wild type HSCs but not in Stat3-deficient HSCs. Representative images are shown using × 20 objective.
(E) IL-17-dependent collagen-α1(I) expression is impaired in Stat3-deficient HSCs. Primary wild type (Stat3f/f) and Stat3-deficient HSCs from GFAPStat3−/− mice were cultured in DMEM + 10% FCS for 18h and stimulated with IL-17A (10 ng/ml in 1% FSCs) for 8h, and mRNA levels of collagen-α1(I) expression were measured, *p<0.01.
IL-17 stimulates collagen production in HSCs via Stat3 activation
The ability of IL-17A to induce HSC activation into myofibroblasts was assessed in vitro. For this purpose, primary HSCs were isolated from Col-GFP reporter mice (expressing GFP under collagen-α1(I) promoter) or Col-GFPIL-17RA−/− mice (generated by crossing IL-17RA−/− mice with Col-GFP mice, Figure 4B). IL-17A stimulation increased expression of GFP in wild type HSCs from a baseline of 1 ± 0 % to 34 ± 7 %, but not in IL-17RA−/− HSCs (5 ± 2 %). As a control, expression of collagen-α1(I)-GFP was strongly induced in both wild type and IL-17RA−/− HSCs by the fibrogenic cytokine TGF-β1 (Figure 4B). Interestingly, the pro-fibrogenic activity of IL-17A on HSCs is comparable to IL-6 (but not leptin), and is only in part depends on autocrine IL-17-induced IL-6 production (Figure 4C). Depletion of IL-6 from IL-17A-stimulated HSCs cultures with anti-IL-6 antibody slightly reduced but did not eliminate HSC activation. Thus, studies in the chimeric mice and in cultured HSCs suggest a direct effect of IL-17A on activation of HSCs.
HSCs are the only liver cell type that expresses GFAP1. The role of Stat3 in IL-17A-mediated collagen-α1(I) expression was tested in primary wild type HSCs and Stat3-deficient HSCs, isolated from GFAPStat3−/− mice (GFAPCre mice x floxed Stat3f/f mice, Figure 4D). As expected, IL-17A triggered nuclear localization of phosphorylated Stat3 in wild type HSCs, but not in Stat3-deleted HSCs (Figure 4D). IL-17A stimulation of wild type HSCs induced collagen-α1(I) expression (Figure 4E). Meanwhile, IL-17A did not induce nuclear localization of p-Smad2/3 (Figure 4D), suggested that compensatory cross-activation of the TGFβ1-Smad2/3 signaling pathway is not responsible for stimulating collagen-α1(I) expression in wild type HSCs. In contrast, IL-17A failed to induce collagen-α1(I) expression in Stat3-deficient HSCs (Figure 4E), demonstrating the requirement of Stat3 in IL-17A-induced fibrogenic activation of HSCs (summarized in Suppl. Figure 8A). Next, the relevance of IL-17 signaling was accessed in human HSC cell lines. The LX-212 cell line responded to IL-17A and IL-17F stimulation by strong induction of fibrogenic genes α-SMA (↑6 fold), Col-α1(I) (↑7 fold) and TGF-β1 (↑3–5 fold) (Suppl. Figure 8B). Similarly, IL-17A and IL-17F mediated activation of the hTERT cell line13, and this effect was associated with nuclear translocation of Stat3 (Suppl. Figure 8C).
Stat3 signaling facilitates fibrogenesis in HSCs
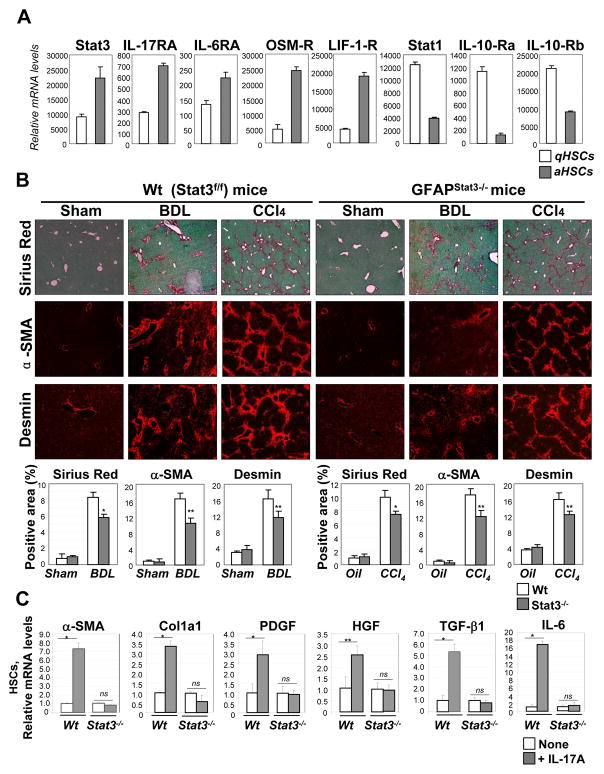
Stat3-signaling cytokines, such as leptin, IL-6, EGF, HGF and PDGF, may have a direct effect on HSC activation, including by increasing collagen production24. Moreover, analysis of the gene expression profile of aHSCs revealed upregulation of Stat3 and Stat3-signaling cytokine receptors (IL-17RA, IL-6RA, OSM-R, LIF-1-R) in comparison with qHSCs (Figure 5A), consistent with a role for Stat3 in HSC activation. In concordance, GFAPStat3−/− mice, that are selectively Stat3 deficient in HSCs, had less BDL- or CCL4-induced liver fibrosis than the wild type Stat3f/f littermates (Figure 5B). Thus, our data demonstrate that activation of the Stat3 signaling pathway in HSCs has a pro-fibrogenic effect. In concordance, not only Stat3−/− HSCs abrogated collagen and aSMA expression in response to IL-17A, but also downregulated expression of pro-fibrogenic cytokines in primary HSCs (Figure 5C).
Figure 5. Stat3-deficient HSCs have decreased activation.
(A)Upregulation of Stat3, IL-17RA, IL-6RA, OSM-R, and LIF-1-R, but not Stat1 or IL-10Ra/b, was detected in aHSCs in comparison with qHSCs using the whole mouse genome microarray (Suppl. Methods). The mRNA level is the average of the multiple probes (p<0.01).
(B) Development of liver fibrosis is attenuated in GFAPStat3−/− mice compared to wild type Stat3f/f mice. Livers from wild type mice (untreated (n=2), BDL (n=4) and CCl4-treated (n=4)), and GFAPStat3−/− mice (untreated (n=2), BDL (n=5) and CCl4-injured (n=6)), were analyzed by Sirius Red staining, and immunostaining for α-SMA and Desmin, and quantified (as percent of positive area, *p<0.01, **p<0.05). Representative bright field micrographs are shown using × 10 objectives.
(C) Stat3-deficient and wild type primary HSCs were in vitro stimulated with IL-17A (10 ng/ml, 4 h). The ability to activate a-SMA and Col1a1 mRNA, and produce cytokines HGF, TGF-β1, PDGF, IL-6 mRNA was impaired in Stat3−/− HSCs compared to wt HSCs. *p<0.001, **p<0.005, ns - non significant.
IL-23 and IL-22 play opposing roles in liver fibrosis
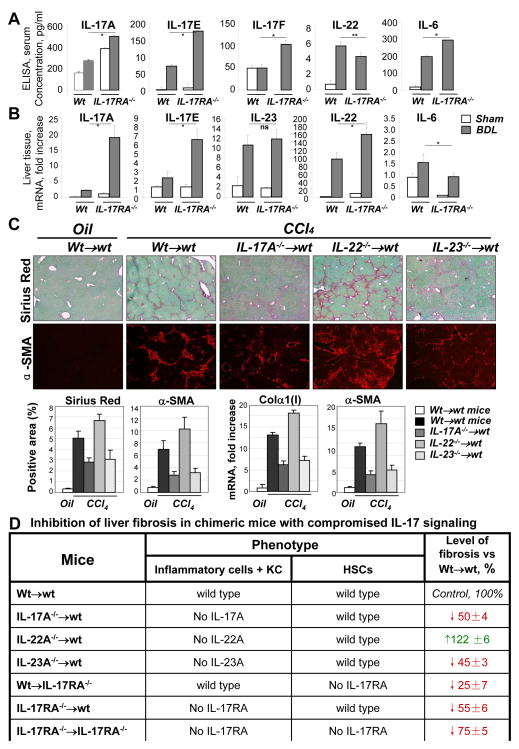
Deletion of IL-17RA in mice results in dysregulation of the IL-17 signaling pathway25. We further evaluated the mechanism of IL-17 signaling in liver fibrosis and compared production of of IL-17A, IL-17F, IL-17E, IL-22 and IL-6 in wild type and IL-17RA−/− mice. Despite elevated levels of circulating IL-17A, IL-17F, IL-6, and IL-17E in the serum of IL-17RA−/− mice (Figure 6A), deletion of IL-17RA resulted in attenuated liver fibrosis (Figure 1B), and was associated with a reduction of the pro-fibrogenic cytokine IL-6 mRNA expression in the liver, and the simultaneous upregulation of hepatoprotective cytokine IL-22 mRNA, and IL-17E mRNA (Figure 6B). In concordance, deletion of IL-23 in the BM of IL-23−/− →wt mice attenuated liver fibrosis to a level similar in IL-17A−/− →wt mice, while deletion of IL-22 significantly exacerbated CCl4-induced liver fibrosis in chimeric BMT IL-22−/− →wt mice, suggesting that IL-23 and IL-22 produce opposing effects on liver fibrosis (Figure 6C). The effect of blocking IL-17 signaling in different chimeric mice on liver fibrosis is summarized in Figure 6D, demonstrating the role of pro-fibrogenic IL-17A and IL-23 cytokines and anti-fibrogenic IL-22 cytokines in liver fibrosis.
Figure 6. IL-23 and IL-22 kave opposing roles in liver fibrosis.
(A) Serum IL-17A, IL-17E (IL-25), IL-17F, IL-6 and IL-22 were measured by ELISA in BDL (10 days) IL-17RA−/− mice versus wild type mice (*p<0.01, **p<0.05).
(B) Hepatic levels of IL-17A, IL-17F, IL-23 and IL-22 mRNA were detected in BDL (10 days) IL-17RA−/− mice versus wild type mice (p<0.05)
(C) Livers from BM chimeric CCl4-treated Wt→wt (n=5), IL-17A−/− →wt (n=6), IL-22−/− →wt (n=6), and IL-23−/− →wt (n=6) mice were analyzed by Sirius Red, α-SMA staining. Bright field micrographs are shown using × 10 objectives. Quantification of Sirius Red and α-SMA staining, mRNA expression is shown (p<0.05, compared to BDL wt mice). mRNA expression is fold induction compared to oil-control Wt→wt mice.
(D) The percent of inhibition of liver fibrosis was calculated for BM chimeric mice in response to liver injury (in comparison Wt→wt mice, 100%, p<0.01) based on Sirius Red and α-SMA expression. The strongest inhibition of liver fibrosis was observed in IL-17RA−/− →IL-17RA−/− mice, the least inhibition of liver fibrosis was observed in Wt→IL-17RA−/− mice. Increased fibrosis was detected in IL-22−/− →wt mice.
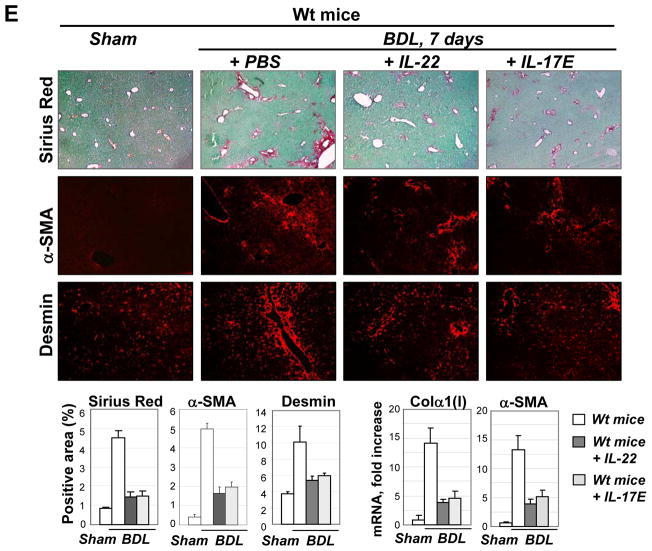
(E) Administration of IL-22 and IL-17E attenuates liver fibrosis. BDL-operated mice were daily treated with IL-22 (0.5 μg/mouse, n=4), IL-17E (0.5 μg/mouse n=5), or vehicle (PBS) for 7 days, tissues were analyzed by Sirius Red, and α-SMA staining; mRNA expression is shown (p<0.05, compared to BDL wt mice + PBS).
Administration of IL-22 and IL-17E decreases hepatic fibrosis in mice
The role of IL-22 and IL-17E in liver fibrosis was examined in BDL-operated mice, injected with IL-22 (0.5 μg/mouse) or IL-17E (0.5 μg/mouse, Figure 6E). Daily administration of IL-22 and IL-17E significantly attenuated development of liver fibrosis, as demonstrated by reduction of Sirius Red staining (↓ 3 fold), mRNA for Col-α1(I) (↓3 fold) and α-SMA (↓2.5 fold). Our data suggest that IL-22 and IL-17E produce anti-fibrogenic effect in mice.
DISCUSSION
Our study demonstrates that IL-17 plays a critical role in the pathogenesis of cholestatic and hepatotoxic liver fibrosis in mice. IL-17 has a strong pro-fibrogenic effect through two independent mechanisms: First, IL-17 stimulates KC to express inflammatory cytokines IL-6, IL-1β, and TNF-α, as well as the major fibrogenic cytokine TGF-β1. Second, IL-17 directly stimulates HSCs to express collagen Type I and promotes their activation into fibrogenic myofibroblasts via Stat3. IL-17 may serve as an attractive target for anti-fibrotic therapy.
Elevated levels of IL-17A were detected in serum of patients with chronic hepatitis B (CHB), and correlated with increased numbers of circulating Th17 cells and histological manifestations of liver fibrosis10, 11. Similar results were obtained in patients with chronic hepatitis C (CHC) in which the development of hepatic fibrosis is accompanied by infiltration of IL-17A+ positive cells in the liver.
We studied role of IL-17 in murine models of liver fibrosis using deletions of different components of the IL-17 signaling pathway (Figure 6D). Complete deletion of IL-17 (IL-17A and F) signaling was achieved in IL-17RA−/− mice, that resulted in ≈75% inhibition of BDL- and CCl4-induced liver fibrosis (vs wild type mice). In turn, deletion of IL-17RA, or IL-17A in BM and KC caused similar inhibition (≈50–55%) of liver fibrosis, indicating that IL-17A (but not other members of IL-17 family of cytokines26) significantly contribute to the pathogenesis of hepatic fibrosis. Our data are in concordance with previous observations suggesting that 1) IL-17A is required for bleomycin-induced pulmonary fibrosis27 and 2) IL-17A and TGF-β play a cooperative roles in the development of fibrosis.
The critical question is how IL-17A signaling stimulates liver fibrosis. We observed suppression of mRNA levels of cytokines (TGF-β1, IL-6, IL-1β, TNF-α) in livers of IL-17RA−/− →wt mice, indicating that IL-17 signaling in BM and KC is required for inflammatory responses in fibrotic liver. Furthermore, TGF-β1 plays a critical role in pathogenesis of liver fibrosis1, and is mainly produced by activated KC/macrophages2. Here we demonstrate that in vivo activated IL-17RA-deficient KC express ≈30% less TGF-β1 than wild type KC. We speculate that TGF-β1 production may be in part regulated by IL-17A. In agreement, IL-17 was recently identified as a regulator of TGF-β1 and IL-22 in allograft fibrosis28.
The contribution of IL-17 signaling to liver fibrosis by endogenous liver cells was examined in Wt→IL-17RA−/− BM chimeric mice. Our data revealed that IL-17 signaling in non-inflammatory liver resident cells contributed ≈25% of the liver fibrosis (Table 1). We determined that IL-17A stimulates multiple changes in HSCs, suggesting that HSCs are the critical hepatic cells that transmit IL-17 signals to become myofibroblasts. Although hepatocytes20 and EC21 did not to respond to IL-17 signaling, their contribution to local cytokine secretion could not be excluded.
How does IL-17 signaling facilitate HSCs activation into myofibroblasts? Our data demonstrate that stimulation of HSCs with IL-17 results in collagen-α1(I) upregulation via IL-17RA. Moreover, Stat3-deficient HSCs do not upregulate collagen-α1(I) in response to IL-17A, indicating that Stat3 is a required target of IL-17 signaling. In support of this notion, mice deficient of Stat3 in HSCs (GFAPStat3−/− mice) are less susceptible to liver fibrosis. For the past decade, it was believed that the role of Stat3 in the pathogenesis of liver fibrosis is confined to inflammatory cells and hepatocytes24, 29. For example, hepatocyte-specific Stat3 knockout mice demonstrate that IL-6, IL-10, and IL-22 produce hepatoprotective and anti-fibrogenic effects20, 24. More recently, Stat3 signaling was linked to activation of HSC/myofibroblasts in response to liver injury30. Thus, leptin promotes the development of liver fibrosis and directly stimulates production of collagen Type I production in HSCs in a Stat3-dependent manner24. Here we demonstrate that IL-17A is another Stat3-signaling cytokine with potent pro-fibrogenic properties, inducing activation of hepatic myofibroblasts directly and via IL-6 secretion.
Deletion of IL-17RA results in dysregulation of a cytokine production in mice, including the upregulatioin of IL-17A25. In concordance, increased levels of circulating IL-17A, IL-17F, and IL-6, and decreased levels of IL-22 were detected in serum of CCl4-injured IL-17RA−/− mice (compared to the wild type controls). Meanwhile, increased IL-22 and IL-17E levels were detected in CCl4-injured livers, suggesting that the local levels of hepatic cytokines (versus serum) are critical for pathogenesis of liver fibrosis. While IL-23 regulates expansion of IL-17A-producing T cells10, 11, IL-22 was implicated in hepatoprotection9. We propose that inhibition of liver fibrosis in IL-17RA−/− mice results from an additive effect of abrogation of IL-17 signals and overproduction of IL-22 (and IL-17E) in the injured liver. In support of this notion, similar to IL-17A→wt mice, deletion of IL-23 in inflammatory cells and KC caused attenuation of liver fibrosis in IL-23→wt mice. In contrast to IL-17A→wt mice, deletion of IL-22 in IL-22→wt mice exacerbated liver fibrosis. Furthermore, administration of IL-22 and IL-17E significantly attenuated development of BDL mice, demonstrating that these cytokines may decrease liver fibrosis.
Here we demonstrate that IL-17 regulates production of TGF-β1 by KC, which in turn, induce activation of HSC into myofibroblasts, and further facilitate differentiation of IL-17 expressing cells. IL-17 directly stimulates collagen expression in HSCs via the Stat3 signaling pathway. In addition, our data suggests that IL-23 and IL-22 have an opposite effect and decrease liver fibrosis.
Supplementary Material
Abbreviations
- HSCs
hepatic stellate cells
- qHSCs
quiescent HSCs
- aHSCs
activated HSCs
- CCl4
carbon tetrachloride
- BDL
bile duct ligation
- α-SMA
α-smooth muscle actin
- Col-GFP mice
Collagen-α1(I)-GFP mice
Footnotes
Conflict of interests: none to declare
Author contribution: FM designed the study, acquired the data, wrote the manuscript; Y-H.P, T.A, S.I.G., M.C., K.I., C.H.O. acquired the data; K.W., S.I.G. and K.L provided support and critical revision of the manuscript, D.A.B. designed the study and provided critical revision of the manuscript, TK designed the study, wrote the manuscript.
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL, Roll FJ, Boyles J, et al. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985;82:8681–5. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 5.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Iwakura Y, Ishigame H, Saijo S, et al. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–62. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subramaniam SV, Cooper RS, Adunyah SE. Evidence for the involvement of JAK/STAT pathway in the signaling mechanism of interleukin-17. Biochem Biophys Res Commun. 1999;262:14–9. doi: 10.1006/bbrc.1999.1156. [DOI] [PubMed] [Google Scholar]
- 9.Radaeva S, Sun R, Pan HN, et al. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–42. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 10.Ge J, Wang K, Meng QH, et al. Implication of Th17 and Th1 cells in patients with chronic active hepatitis B. J Clin Immunol. 2010;30:60–7. doi: 10.1007/s10875-009-9328-2. [DOI] [PubMed] [Google Scholar]
- 11.Lemmers A, Moreno C, Gustot T, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646–57. doi: 10.1002/hep.22680. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Hui AY, Albanis E, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–51. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnabl B, Choi YH, Olsen JC, et al. Immortal activated human hepatic stellate cells generated by ectopic telomerase expression. Lab Invest. 2002;82:323–33. doi: 10.1038/labinvest.3780426. [DOI] [PubMed] [Google Scholar]
- 14.Yata Y, Scanga A, Gillan A, et al. DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology. 2003;37:267–76. doi: 10.1053/jhep.2003.50067. [DOI] [PubMed] [Google Scholar]
- 15.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakae S, Komiyama Y, Nambu A, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–87. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Clausen BE, Kaisho T, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 18.Yanagida A, Iwaisako K, Hatano E, et al. Downregulation of the Wnt antagonist Dkk2 links the loss of Sept4 and myofibroblastic transformation of hepatic stellate cells. Biochim Biophys Acta. 2011;1812:1403–11. doi: 10.1016/j.bbadis.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Kisseleva T, Song L, Vorontchikhina M, et al. NF-kappaB regulation of endothelial cell function during LPS-induced toxemia and cancer. J Clin Invest. 2006;116:2955–63. doi: 10.1172/JCI27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zenewicz LA, Yancopoulos GD, Valenzuela DM, et al. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–59. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang Z, Altuntas CZ, Gulen MF, et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32:414–25. doi: 10.1016/j.immuni.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linden A. A role for the cytoplasmic adaptor protein Act1 in mediating IL-17 signaling. Sci STKE. 2007:re4. doi: 10.1126/stke.3982007re4. [DOI] [PubMed] [Google Scholar]
- 23.Oakley F, Meso M, Iredale JP, et al. Inhibition of inhibitor of kappaB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology. 2005;128:108–20. doi: 10.1053/j.gastro.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Lafdil F, Kong X, et al. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int J Biol Sci. 2011;7:536–50. doi: 10.7150/ijbs.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maitra A, Shen F, Hanel W, et al. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci U S A. 2007;104:7506–11. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson MS, Madala SK, Ramalingam TR, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207:535–52. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faust SM, Lu G, Marini BL, et al. Role of T cell TGFbeta signaling and IL-17 in allograft acceptance and fibrosis associated with chronic rejection. J Immunol. 2009;183:7297–306. doi: 10.4049/jimmunol.0902446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mair M, Zollner G, Schneller D, et al. Signal transducer and activator of transcription 3 protects from liver injury and fibrosis in a mouse model of sclerosing cholangitis. Gastroenterology. 138:2499–508. doi: 10.1053/j.gastro.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 30.Ogata H, Chinen T, Yoshida T, et al. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene. 2006;25:2520–30. doi: 10.1038/sj.onc.1209281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.