Abstract
Recent findings indicate that advanced stage cancers shun the tumor suppressive actions of TGFβ and inexplicably utilize the cytokine as a tumor promoter. We investigated the effect of TGFβ1 on the survival and proliferation of invasive prostate (PC3) and bladder (T24) cancer cells. Our study indicated that TGFβ1 decreased cell viability and induced apoptosis in invasive human PC3 and T24 cells via activation of p38 MAPK-JNK-Caspase9/8/3 pathway. Surprisingly, no change in the phosphorylation of pro-survival Akt kinase was observed. We postulate that TGFβ1 pathway may be utilized for specifically targeting urological cancers without inflicting side effects on normal tissues.
Keywords: Prostate cancer, TGFβ, Apoptosis, P38 MAPK, JNK, Akt
1. Introduction
Transforming growth factor-β (TGFβ) family of pro-fibrotic growth factors control physiological processes such as wound healing as well as clinical conditions such as tissue fibrosis by inducing fibroblast differentiation to myofibroblasts [1]. TGFβ plays fundamental role in many cell functions including cell migration, survival, proliferation, and differentiation [2,3]. The exact role of TGFβ in cancer is highly controversial. TGFβ is a known inhibitor of proliferation in epithelial and lymphoid cells [4], and hence considered to have a tumor suppressive role [5–13]. However, the exact role of TGFβ signaling in tumor development appears to be complex and is context-dependent. On one end, studies have shown that ablation of TGFβ signaling results in enhanced tumor growth [14–16]. On the other end, TGFβ has also been implicated in eliciting tumor promoting effects through its ability to induce epithelial-to-mesenchymal transition (EMT), immunosuppression and metastasis [17–21]. These controversies may also be due to the specific role of TGFβ in cancer cells vs. stromal fibroblasts that influence the tumor growth [22–24]. These discrepancies demand extensive research on TGFβ signaling in cancer with specific effects on the tumor and stromal cells.
Multiple studies have implicated the inter-dependent role of TGFβ-induced canonical (Smad-dependent) and non-canonical (Smad-independent) signaling pathways in both the tumor suppressive and tumor promoting activities [11]. The differential effects of TGFβ in multiple cell types in the regulation of various physiological and pathological processes are attributed mainly to a particular non-canonical pathway activated by this growth factor in a specific cell type. TGFβ stimuli can result in the activation of one or more of the pro-survival pathways such as PI3K/AKT/mTOR and RAF/MEK/ERK1/2 pathways, two major kinases often hyper-activated in cancer cells [25]. Alternatively, pro-apoptotic pathways such as P38 MAPK and JNK pathways are also involved in the regulation of tumor cell apoptosis [26,27].
In light of studies demonstrating both tumor suppressive and tumor promoting effects of TGFβ, we sought to determine the specific effects of TGFβ1, major TGFβ isoform on the survival, proliferation and foci formation of invasive prostate (PC3) and bladder (T24) cancer cell lines in vitro and characterize the underlying mechanisms regulating the process. We report that TGFβ1 inhibits invasive prostate (PC3) and bladder (T24) cancer cell proliferation, survival and foci formation, and induced apoptosis in vitro in a dose- and time-dependent manner via activation of p38 MAP kinase and JNK, subsequently leading to the cleavage of caspases 9, 8 and 3. We also demonstrate the Akt-independent effects of TGFβ on prostate and bladder cancer cell survival and apoptosis.
2. Materials and Methods
2.1. Reagents, Cell Lines and Antibodies
Human PC3 and T24 cell lines were obtained from ATCC (Manassas, VA) and maintained in DMEM-High Glucose (Hyclone, Logan, UT) with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin in 5% CO2 humidified atmosphere at 37 °C. Primary antibodies against p-AKT (S473), p-ERK1/2, p-P38-MAPK, p-SAPK/JNK, cleaved caspase 3, cleaved caspase 9 and cleaved caspase 8 were purchased from Cell Signaling (Boston, MA). Primary antibodies against β-actin were purchased from Sigma (St Louis, MO). Anti-mouse and anti-rabbit HRP conjugated secondary antibodies were obtained from BioRad (Hercules, CA). Recombinant human TGFβ1 was purchased from R&D systems (Minneapolis, MN).
2.2. Cell Proliferation Assay
Proliferation of PC3 and T24 cell lines was determined using the nonradioactive BrdU-based cell proliferation assay (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s protocol. Briefly, PC3 and T24 cells were plated in 96-well flat bottom plates at a density of 5 × 103 cells per 100 μl, and allowed to grow for 24 h. Cells were then treated with (0.1, 0.5, 1, 2.5 and 5 ng/ml TGFβ1) for an additional 24 h in serum free conditions. Cells were then subjected to 5-bromo-2-deoxyuridine assay using the BrdU Labeling and Detection Kit III as done previously [28]. BrdU incorporation into the DNA was determined by measuring the absorbance at both 450 and 690 nm on an ELISA plate reader.
2.3. Cell viability Assay
Cell number was assessed indirectly by means of tetrazolium salt conversion into formazan crystals Briefly, PC3 and T24 cells were plated in 96-well plates at 5000 cells per well in the DMEM containing 10% FBS. 24h after plating, the medium was replaced with the same medium containing (0.1, 0.5, 1, 2.5 and 5 ng/ml TGFβ1), After 24 h treatment, cells viability was measured using the Cell Proliferation Kit I (MTT) (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s protocol. The absorbance at 570 nm was measured using an ELISA reader and used to determine relative cell numbers in each well.
2.4. Apoptosis Assay
Cytoplasmic histone-associated DNA fragments were quantified by using Cell Death Detection ELISAPLUS kit (Roche Applied Science, Indianapolis, IN) according to the manufacture’s protocol. Briefly, PC3 and T24 cells were plated in 96-well plate at a density of either 104 cells/well. After 24 h, the cells were incubated in DMEM containing (0.1, 0.5, 1, 2.5 and 5 ng/ml TGFβ1) for 24 h. Control cells were incubated in DMEM alone. Cells were lysed and centrifuged at 200g for 10 min, and the collected supernatant was subjected to ELISA [29]. The absorbance was measured at 405 nm (reference wavelength, 492 nm).
2.5. Western Blot Analysis
PC3 and T24 cell lines were cultured to reach a monolayer in DMEM in six-well plates. The wells were treated with DMEM containing (0.1, 0.5, 1, 2.5 and 5 ng/ml of TGFβ1). Control cells were grown in DMEM alone. Whole-cell lysates were prepared using lysis buffer [50 mM Tris-HCl, pH 7.4, 1% Triton X-100, 150 mM NaCl, 1 mM EDTA, 2 mM Na3VO4, and 1× complete protease inhibitors (Roche Applied Science, Indianapolis, IN)]. The protein concentration was measured by the DL protein assay (Bio-Rad Laboratories, Hercules, CA). Western analyses were performed using the standard Laemmli’s method as done previously [30].
2.6. Colony/Foci formation assay
Colony formation assay was performed using the standard protocol [31]. In this approach, PC3 cells were cultured on six-well plates until the monolayer was reached. After seven days of culture with TGFβ1 (0.1, 0.5, 1, 2.5 and 5 ng/ml) in serum containing medium, with changing the medium and growth factor every day, each of the wells was counted for the number of colonies and compared with the untreated controls. Plates were fixed using 2% paraformaldehyde, briefly stained with crystal violet, and counted visually or by using ImageJ software.
2.7. Statistical Analysis
All data are presented as mean ± S.D of three to five independent experiments done at least in triplicates. A Student’s two-tailed t test was used to determine significant differences between treatment and control values.
3. Results
3.1. TGFβ1 inhibits proliferation and viability of PC3 and T24 cells
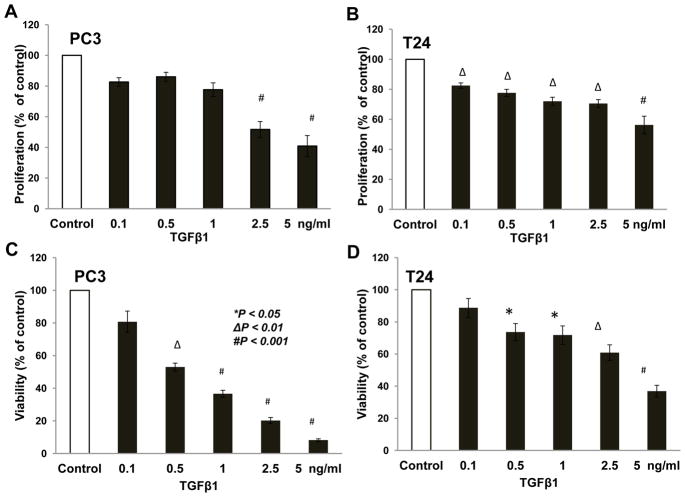
We first determined the direct effect of TGFβ1 on prostate cancer (PC3) and bladder cancer (T24) cell proliferation and viability. Based on the results of a BrdU incorporation assay, our data revealed that treatment with TGFβ1 results in significant inhibition of PC3 cell proliferation by 50 to 60% for 2.5 and 5 ng/ml TGFβ1, respectively, compared control (Fig. 1A). TGFβ1 also inhibited proliferation of T24 cells by 18, 23, 28, 30, and 44% for for doses of 0.1, 0.5, 1.0, 2.5 and 5 ng/ml of TGFβ1, respectively, compared to control (Fig. 1B). Our data from the cell viability assay (MTT assay) revealed that treatment with TGFβ1 resulted in significant inhibition of PC3 cell viability by 48, 64, 80, and 90% for 0.5, 1.0, 2.5 and 5 ng/ml TGFβ1, respectively compared to control (Fig. 1C), and inhibition of T24 cell viability by 27, 29, 40, and 63% for 0.5, 1.0, 2.5 and 5 ng/ml TGFβ1, respectively compared to control (Fig. 1D). Thus, our data indicate that TGFβ1 significantly inhibits PC3 and T24 cell proliferation in vitro.
Figure 1. TGF β1 inhibited proliferation and viability of prostate (PC3) and bladder (T24) cancer cells.
(A and B) Bar graphs showing a reduction in the proliferation (represented as absorbance normalized to control) in PC3 cells and T24 cells, respectively after 24 h treatment with TGFβ1 (0.1, 0.5, 1, 2.5, and 5 ng/ml) compared to control cells. (C and D) Bar graph showing a reduction in the MTT absorbance (represented as percentage change in the MTT absorbance compared to control) in PC3 cells and T24 cells, respectively after 24 h treatment with TGFβ1 compared to control cells.
3.2. TGFβ1 induces apoptosis of PC3 and T24 cells in an Akt-independent manner
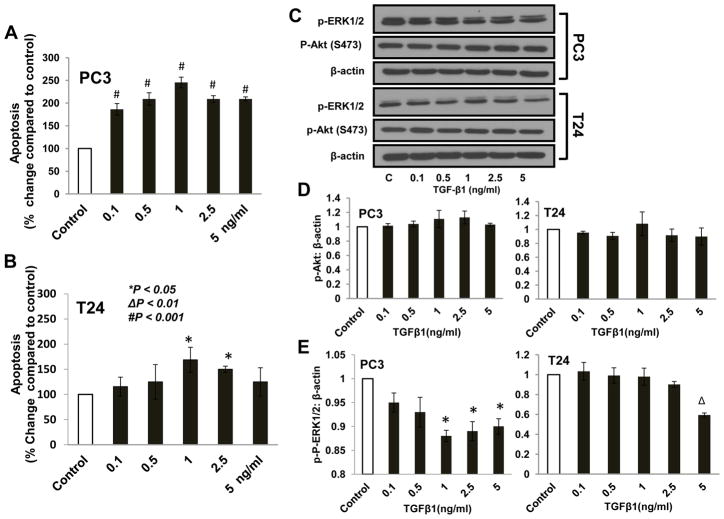
We next determined the effect of TGFβ1 on apoptosis of prostate (PC3) and bladder (T24) cancer cells. Our study indicated that treatment with 0.1 ng/ml TGFβ1 resulted in significant increase in PC3 cell apoptosis by 86% (Fig. 2A). This effect was further enhanced by treatment with 0.5 and 1.0 ng/ml TGFβ1 which exhibited more than a 2 and 2.5 fold increases in cell death. Treatment with 1.0 and 2.5 ng/ml of TGFβ1 resulted in significant increase in T24 cell apoptosis by 68 and 50%, respectively (Fig. 2B). Since Akt is the major cell survival kinase often deregulated in cancers, we sought to determine the effect of TGFβ1 on PC3 and T24 cells. Our data indicated that TGFβ1-induced apoptosis does not involve changes in the phosphorylation of Akt in either of the cancer cells (Fig. 2C and D). However, we observed a significant reduction in the phosphorylation of ERK1/2 in both PC3 and T24 cells, a kinase known to regulate cell proliferation (Fig. 2C and E). Our studies demonstrated that TGFβ1 induces invasive prostate and bladder cancer cell apoptosis in vitro in an Akt-independent manner and that impaired proliferation of PC3 and T24 cells in response to TGFβ1 may be due to reduced phosphorylation of ERK1/2.
Figure 2. TGF β1 induced apoptosis of PC3 and T24 cells in an Akt-independent mechanism.
(A and B) Bar graph showing apoptosis in PC3 and T24 cells treated with control (Serum free DMEM) and TGFβ1 (0.1, 0.5, 1, 2.5, 5 ng/ml) for 24 h. (C) Western blots of pAkt S473 and pERK1/2 expression in PC3 and T24 cells treated with control and TGFβ1 (0.1, 0.5, 1, 2.5, 5 ng/ml) for 24 h. (D and E) Band densitometry analysis of pAkt S473 and pERK1/2 expression in PC3 and T24 cells normalized to β-actin, respectively, treated with control and TGFβ1 (0.1, 0.5, 1, 2.5, 5 ng/ml) for 24 h.
3.3. TGFβ1 increases phosphorylation of p38MAPK and SAPK/JNK pathways in the activation of caspases
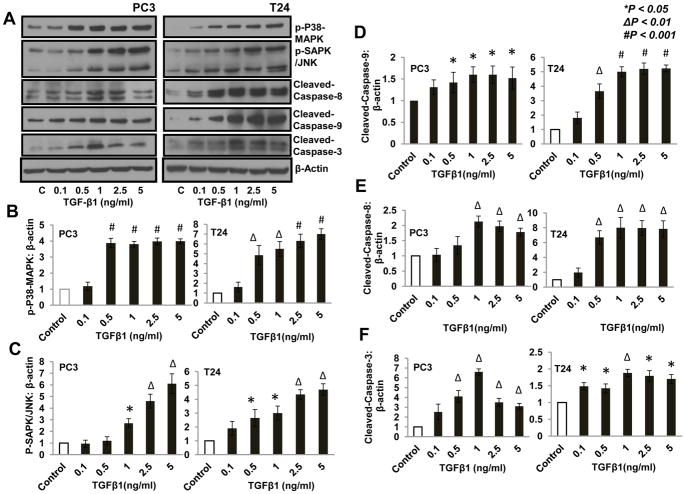
We next sought to identify the signaling pathways responsible for the observed effects of TGFβ1 on the apoptosis and survival of invasive prostate and bladder cancer cells. Our analysis of other major pathways in these cells revealed that treatment with TGFβ1 results in significant increase in the phosphorylation of p38MAPK and JNK (Fig. 3A–C) as well as in the increase in the expression of cleaved caspase-3, cleaved caspase-8 and cleaved caspase-9 in both PC3 and T24 cells, in a dose-dependent manner (Fig. 3A and D–F). Our data indicated that TGFβ1 induced invasive prostate and bladder cancer cell apoptosis in vitro involving p38 MAPK and JNK/SAPK-mediated activation of caspases 9, 8 and 3.
Figure 3. TGF β1 increased phosphorylation of p38 MAPK and SAPK/JNK and enhanced expression of cleaved caspases in PC3 and T24 cells.
(A) Western blots showing increased phosphorylation of p38-MAPK and SAPK/JNK, as well as increased expression of cleaved caspase-9, cleaved caspase-8, and cleaved caspase-3 after 24 h treatment with TGFβ1 (0.1, 0.5, 1, 2.5, and 5 ng/ml) compared to control (DMEM) (B–F) Bar graphs showing band-densitometry analysis of phoshorylations of p38 MAPK, SAPK and expression of cleaved caspase-9, cleaved caspase-8 and cleaved caspase-3, respectively, with TGFβ1 treatment as mentioned above, normalized to β-actin.
3.4. TGFβ1 inhibits colony/ foci formation by PC3 and T24 cells
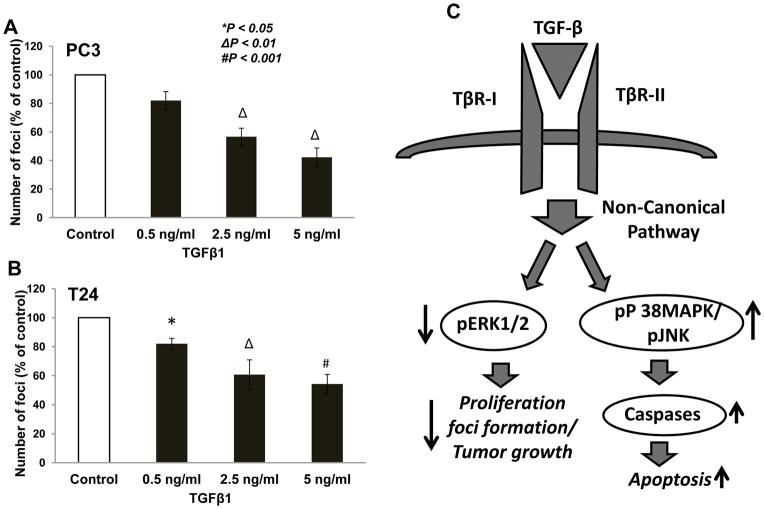
Since the tumor growth mainly relies on the proliferation and apoptosis of cancer cell lines, we next determined the effect of TGFβ1 on the colony/foci formation of PC3 and T24 cells. Our data revealed that treatment with TGFβ1 significantly inhibited colony formation by PC3 cells by 40 to 60% for concentration 2.5 ng/ml and 5 ng/ml of TGFβ1, respectively, compared control (Fig. 4A). TGFβ1 also inhibited colony formation ability of T24 cells by 40 to 45 % for concentrations 2.5 and 5 ng/ml of TGFβ1, respectively, compared to control (Fig. 4B).
Figure 4. TGF β1 inhibited foci formation by PC3 and bladder cancer cells.
(A) Bar graph showing foci formation by the PC3 cells treated with control (Serum free DMEM) and TGFβ1 (0.1, 0.5, 1, 2.5, 5 ng/ml) for 7 days. (B) Bar graph showing foci formation by the T24 cells treated with control (Serum free DMEM) and TGFβ1 (0.1, 0.5, 1, 2.5, 5 ng/ml) for 7 days. (C) Schematic representation of the working hypothesis.
4. Discussion
In the current study, we demonstrated that treatment with TGFβ1, a growth factor that elicits differential effects on various cell types resulted in enhanced apoptosis and reduced proliferation in invasive prostate (PC3) and bladder cancer (T24) cells in vitro in an Akt-independent, but ERK-, p38MAPK- and JNK/SAPK-dependent activation of caspase-9, caspase-8 and caspase-3.
The role of TGFβ signaling in cancer is highly controversial. Recent publications reports both tumor suppressive [14–16] and tumor promoting effects of TGFβ [17–21]. These controversies, by itself speak about the complexity of TGFβ signaling in cancer and demands extensive research on the cell-specific effects of TGFβ on the tumor and stromal cells. Presence of multiple isoforms, differential effects on various cell types, source of cytokine in a specific tumor tissue, stage of tumor growth, varied effects in the presence and absence of other growth factors etc. may influence the total outcome [22–24]. Hence, individual analysis on the specific effects of TGFβ isoforms on tumor and stromal cells is essential to address the existing discrepancies on the role of TGFβ signaling in cancer. Our study demonstrated that even in invasive prostate and bladder cancer cells, TGFβ1 elicited a tumor suppressive role by inducing apoptosis as well as reducing viability and proliferation.
Akt and ERK are two major kinases that are often hyper-activated in most cancer cells and considered central to many signaling pathways [25,28,30]. While Akt is predominantly a cell survival kinase, it also controls other cell functions such as proliferation, migration and integrin activation [32–34]. On the other hand, proliferation and cell growth of cancer cells are more reliant on the activation of ERK kinases [35–37]. In contrast, p38-MAPK and JNK pathways are activated by environmental stress as well as by the growth factors and cytokines that induce apoptosis, and senescence [26,27,38,39]. We have shown previously that TGFβ1 activates Akt in normal fibroblasts and is necessary for the enhanced survival and expression of extracellular matrix (ECM) proteins by the fibroblasts [40]. However, although TGFβ1 treatment resulted in the apoptosis of both PC3 and T24 cells, surprisingly no significant changes in the phosphorylation of Akt was observed, thus indicating the involvement of an Akt-independent pathway in TGFβ1-induced apoptosis in PC3 and T24 cells. Even though we observed a modest, but significant reduction in the phosphorylation of ERK1/2 in PC3 and T24 cells with TGFβ1 treatment, our further analysis indicated that TGFβ1 is involved in the activation of stress activated kinases such as p38 MAPK and JNK/SAPK in PC3 and T24 cells. This, in turn, was associated with enhanced expression of cleaved caspases-8, -9 and -3, which are well known modulators of intrinsic and extrinsic apoptosis pathways [41]. Although physiological and clinical implications of these effects induced by TGFβ1 in prostate and bladder cancer cells is yet to be determined in vivo, our study provides supportive evidence that TGFβ1 plays a direct tumor suppressive role on invasive prostate and bladder cancer cells via inducing apoptosis and inhibiting cell proliferation.
Highlights.
TGFβ induced apoptosis in invasive prostate cancer and bladder cancer cells
TGFβ inhibited prostate/bladder cancer cell proliferation and colony/foci formation
TGFβ induced prostate/bladder cancer cell apoptosis independent of Akt inhibition
TGFβ inhibited ERK1/2 phosphorylation in prostate/bladder cancer cells
TGFβ induced p38 MAPK and JNK-mediated activation of caspases-9, -8 and -3
Acknowledgments
Funds were provided by the University of Georgia Research Foundation, Wilson Pharmacy Foundation and UGA College of Pharmacy through intramural grants to PRS and in part by the National Institutes of Health grant (R01HL103952) and American Heart Association Scientist Development Grant (0830326N) to PRS. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- TGFβ
Transforming growth factor-β
- p38 MAPK
p38 mitogen activated protein kinase
- JNK
c-jun N-terminal kinase
- EMT
epithelial-to-mesenchymal transition
- ECM
extracellular matrix
Footnotes
Conflicts of interest: The authors have declared that no conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- 4.Moses HL, Yang EY, Pietenpol JA. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990;63:245–7. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- 5.Wakefield LM, Sporn MB. Suppression of carcinogenesis: a role for TGF-beta and related molecules in prevention of cancer. Immunol Ser. 1990;51:217–43. [PubMed] [Google Scholar]
- 6.Pierce DF, Jr, et al. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-beta 1. Genes Dev. 1993;7:2308–17. doi: 10.1101/gad.7.12a.2308. [DOI] [PubMed] [Google Scholar]
- 7.Liu F, Matsuura I. Inhibition of Smad antiproliferative function by CDK phosphorylation. Cell Cycle. 2005;4:63–6. doi: 10.4161/cc.4.1.1366. [DOI] [PubMed] [Google Scholar]
- 8.Donovan J, Slingerland J. Transforming growth factor-beta and breast cancer: Cell cycle arrest by transforming growth factor-beta and its disruption in cancer. Breast Cancer Res. 2000;2:116–24. doi: 10.1186/bcr43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laiho M, DeCaprio JA, Ludlow JW, Livingston DM, Massague J. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990;62:175–85. doi: 10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- 10.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 11.Pardali K, Moustakas A. Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007;1775:21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Chen CR, Kang Y, Massague J. Defective repression of c-myc in breast cancer cells: A loss at the core of the transforming growth factor beta growth arrest program. Proc Natl Acad Sci U S A. 2001;98:992–9. doi: 10.1073/pnas.98.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valderrama-Carvajal H, Cocolakis E, Lacerte A, Lee EH, Krystal G, Ali S, Lebrun JJ. Activin/TGF-beta induce apoptosis through Smad-dependent expression of the lipid phosphatase SHIP. Nat Cell Biol. 2002;4:963–9. doi: 10.1038/ncb885. [DOI] [PubMed] [Google Scholar]
- 14.Novitskiy SV, et al. TGF-beta receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov. 2011;1:430–41. doi: 10.1158/2159-8290.CD-11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matise LA, et al. Lack of transforming growth factor-beta signaling promotes collective cancer cell invasion through tumor-stromal crosstalk. Breast Cancer Res. 2012;14:R98. doi: 10.1186/bcr3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borczuk AC, Sole M, Lu P, Chen J, Wilgus ML, Friedman RA, Albelda SM, Powell CA. Progression of human bronchioloalveolar carcinoma to invasive adenocarcinoma is modeled in a transgenic mouse model of K-ras-induced lung cancer by loss of the TGF-beta type II receptor. Cancer Res. 2011;71:6665–75. doi: 10.1158/0008-5472.CAN-11-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amatangelo MD, Goodyear SM, Varma D, Stearns ME. c-myc expression and MEK1 induced Erk2 nuclear localization are required for TGF-beta induced epithelial-mesenchymal transition and invasion in prostate cancer. Carcinogenesis. 2012 doi: 10.1093/carcin/bgs227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy L, Hill CS. Alterations in components of the TGF-beta superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 2006;17:41–58. doi: 10.1016/j.cytogfr.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Shiota M, Zardan A, Takeuchi A, Kumano M, Beraldi E, Naito S, Zoubeidi A, Gleave ME. Clusterin mediates TGF-beta-induced epithelial-mesenchymal transition and metastasis via Twist1 in prostate cancer cells. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-0254. [DOI] [PubMed] [Google Scholar]
- 20.Vo BT, Cody B, Cao Y, Khan SA. Differential Role of Sloan-Kettering Institute (Ski) Protein in Nodal and Transforming Growth Factor-Beta (TGF-beta) Induced Smad Signaling in Prostate Cancer Cells. Carcinogenesis. 2012 doi: 10.1093/carcin/bgs252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Coulson-Thomas VJ, Gesteira TF, Coulson-Thomas YM, Vicente CM, Tersariol IL, Nader HB, Toma L. Fibroblast and prostate tumor cell cross-talk: fibroblast differentiation, TGF-beta, and extracellular matrix down-regulation. Exp Cell Res. 2010;316:3207–26. doi: 10.1016/j.yexcr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Franco OE, et al. Altered TGF-beta signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;71:1272–81. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiskowski MA, et al. Role for stromal heterogeneity in prostate tumorigenesis. Cancer Res. 2011;71:3459–70. doi: 10.1158/0008-5472.CAN-10-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somanath PR, Vijai J, Kichina JV, Byzova T, Kandel ES. The role of PAK-1 in activation of MAP kinase cascade and oncogenic transformation by Akt. Oncogene. 2009;28:2365–9. doi: 10.1038/onc.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey RS, Mulder KM. Involvement of extracellular signal-regulated kinase 2 and stress-activated protein kinase/Jun N-terminal kinase activation by transforming growth factor beta in the negative growth control of breast cancer cells. Cancer Res. 1997;57:628–33. [PubMed] [Google Scholar]
- 27.Bakkebo M, Huse K, Hilden VI, Smeland EB, Oksvold MP. TGF-beta-induced growth inhibition in B-cell lymphoma correlates with Smad1/5 signalling and constitutively active p38 MAPK. BMC Immunol. 2010;11:57. doi: 10.1186/1471-2172-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goc A, Al-Husein B, Kochuparambil ST, Liu J, Heston WW, Somanath PR. PI3 kinase integrates Akt and MAP kinase signaling pathways in the regulation of prostate cancer. Int J Oncol. 2011;38:267–77. [PubMed] [Google Scholar]
- 29.Goc A, Abdalla M, Al-Azayzih A, Somanath PR. Rac1 activation driven by 14-3-3zeta dimerization promotes prostate cancer cell-matrix interactions, motility and transendothelial migration. PLoS One. 2012;7:e40594. doi: 10.1371/journal.pone.0040594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goc A, Liu J, Byzova TV, Somanath PR. Akt1 mediates prostate cancer cell microinvasion and chemotaxis to metastatic stimuli via integrin beta(3) affinity modulation. Br J Cancer. 2012;107:713–23. doi: 10.1038/bjc.2012.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kochuparambil ST, Al-Husein B, Goc A, Soliman S, Somanath PR. Anticancer efficacy of simvastatin on prostate cancer cells and tumor xenografts is associated with inhibition of Akt and reduced prostate-specific antigen expression. J Pharmacol Exp Ther. 2011;336:496–505. doi: 10.1124/jpet.110.174870. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–96. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somanath PR, Razorenova OV, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5:512–8. doi: 10.4161/cc.5.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somanath PR, Kandel ES, Hay N, Byzova TV. Akt1 signaling regulates integrin activation, matrix recognition, and fibronectin assembly. J Biol Chem. 2007;282:22964–76. doi: 10.1074/jbc.M700241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCubrey JA, et al. Interactions between the PI3K and Raf signaling pathways can result in the transformation of hematopoietic cells. Cancer Detect Prev. 2001;25:375–93. [PubMed] [Google Scholar]
- 36.McCubrey JA, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–79. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 38.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–9. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21:3749–59. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goc A, Choudhary M, Byzova TV, Somanath PR. TGFbeta- and bleomycin-induced extracellular matrix synthesis is mediated through Akt and mammalian target of rapamycin (mTOR) J Cell Physiol. 2011;226:3004–13. doi: 10.1002/jcp.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]