Abstract
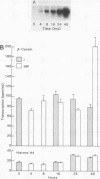
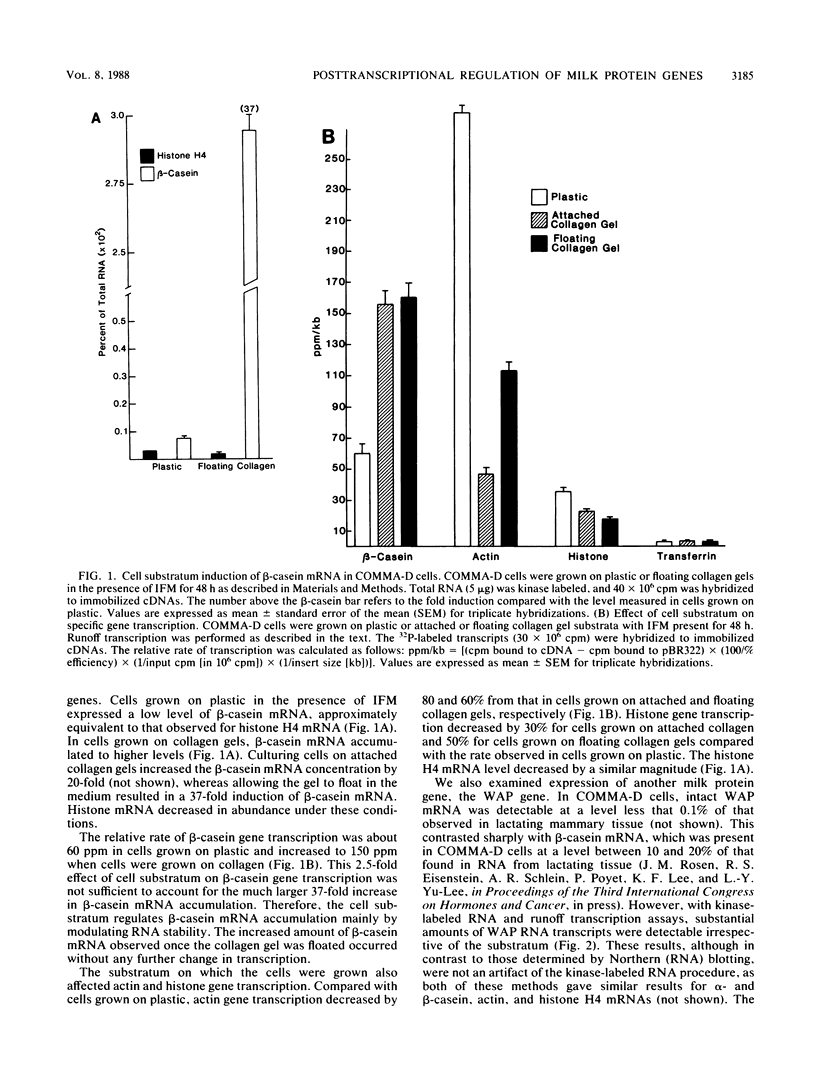
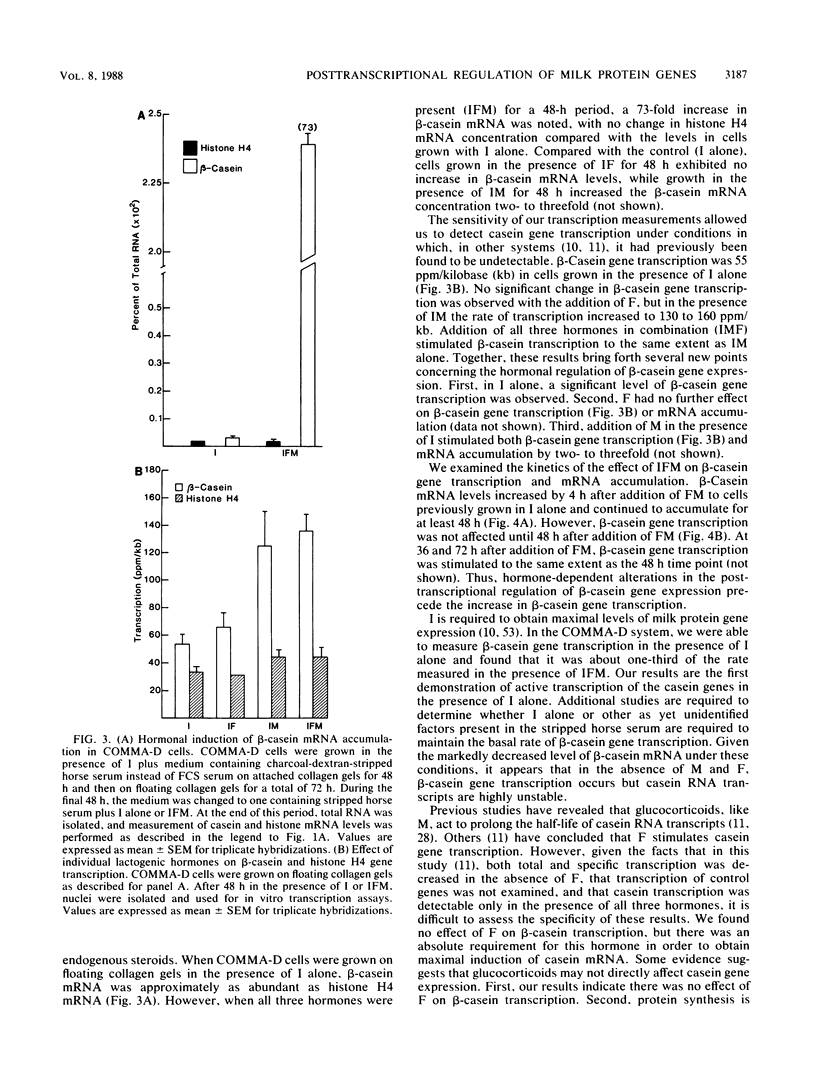
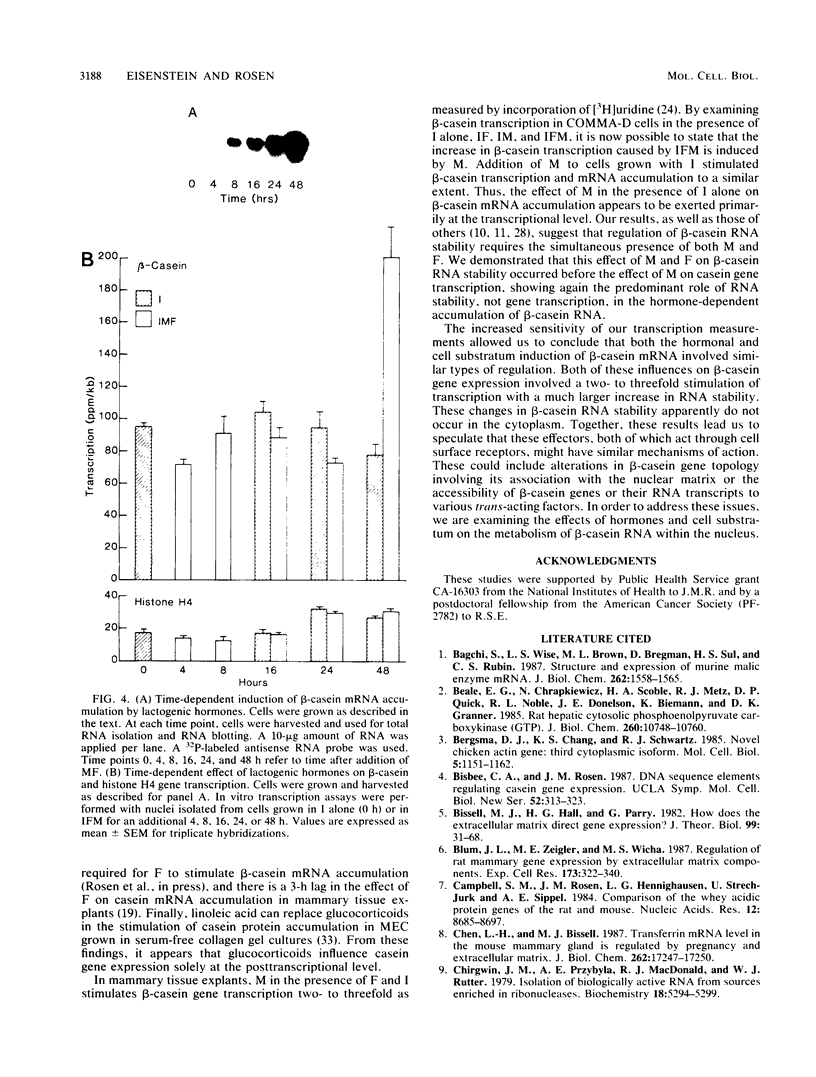
The mechanism by which individual peptide and steroid hormones and cell-substratum interactions regulate milk protein gene expression has been studied in the COMMA-D mammary epithelial cell line. In the presence of insulin, hydrocortisone, and prolactin, growth of COMMA-D cells on floating collagen gels in comparison with that on a plastic substratum resulted in a 2.5- to 3-fold increase in the relative rate of beta-casein gene transcription but a 37-fold increase in beta-casein mRNA accumulation. In contrast, whey acidic protein gene transcription was constitutive in COMMA-D cells grown on either substratum, but its mRNA was unstable and little intact mature mRNA was detected. Culturing COMMA-D cells on collagen also promoted increased expression of other genes expressed in differentiated mammary epithelial cells, including those encoding alpha- and gamma-casein, transferrin, malic enzyme, and phosphoenolpyruvate carboxykinase but decreased the expression of actin and histone genes. Using COMMA-D cells, we defined further the role of individual hormones in influencing beta-casein gene transcription. With insulin alone, a basal level of beta-casein gene transcription was detected in COMMA-D cells grown on floating collagen gels. Addition of prolactin but not hydrocortisone resulted in a 2.5- to 3.0-fold increase in beta-casein gene transcription, but both hormones were required to elicit the maximal 73-fold induction in mRNA accumulation. This posttranscriptional effect of hormones on casein mRNA accumulation preceded any detectable changes in the relative rate of transcription. Thus, regulation by both hormones and cell substratum of casein gene expression is exerted primarily at the post transcriptional level.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi S., Wise L. S., Brown M. L., Bregman D., Sul H. S., Rubin C. S. Structure and expression of murine malic enzyme mRNA. Differentiation-dependent accumulation of two forms of malic enzyme mRNA in 3T3-L1 cells. J Biol Chem. 1987 Feb 5;262(4):1558–1565. [PubMed] [Google Scholar]
- Beale E. G., Chrapkiewicz N. B., Scoble H. A., Metz R. J., Quick D. P., Noble R. L., Donelson J. E., Biemann K., Granner D. K. Rat hepatic cytosolic phosphoenolpyruvate carboxykinase (GTP). Structures of the protein, messenger RNA, and gene. J Biol Chem. 1985 Sep 5;260(19):10748–10760. [PubMed] [Google Scholar]
- Bergsma D. J., Chang K. S., Schwartz R. J. Novel chicken actin gene: third cytoplasmic isoform. Mol Cell Biol. 1985 May;5(5):1151–1162. doi: 10.1128/mcb.5.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Blum J. L., Zeigler M. E., Wicha M. S. Regulation of rat mammary gene expression by extracellular matrix components. Exp Cell Res. 1987 Dec;173(2):322–340. doi: 10.1016/0014-4827(87)90274-6. [DOI] [PubMed] [Google Scholar]
- Campbell S. M., Rosen J. M., Hennighausen L. G., Strech-Jurk U., Sippel A. E. Comparison of the whey acidic protein genes of the rat and mouse. Nucleic Acids Res. 1984 Nov 26;12(22):8685–8697. doi: 10.1093/nar/12.22.8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. H., Bissell M. J. Transferrin mRNA level in the mouse mammary gland is regulated by pregnancy and extracellular matrix. J Biol Chem. 1987 Dec 25;262(36):17247–17250. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Qasba P., Topper Y. J. Essential role of insulin in transcription of the rat 25,000 molecular weight casein gene. Science. 1984 Dec 14;226(4680):1326–1328. doi: 10.1126/science.6390680. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Qasba P., Topper Y. J. Transcriptional and post-transcriptional roles of glucocorticoid in the expression of the rat 25,000 molecular weight casein gene. Biochem Biophys Res Commun. 1986 Jan 29;134(2):812–818. doi: 10.1016/s0006-291x(86)80493-4. [DOI] [PubMed] [Google Scholar]
- Danielson K. G., Oborn C. J., Durban E. M., Butel J. S., Medina D. Epithelial mouse mammary cell line exhibiting normal morphogenesis in vivo and functional differentiation in vitro. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3756–3760. doi: 10.1073/pnas.81.12.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinoy E., Houdebine L. M., Delouis C. Role of prolactin and glucocorticoids in the expression of casein genes in rabbit mammary gland organ culture. Quantification of casein mRNA. Biochim Biophys Acta. 1978 Feb 16;517(2):360–366. doi: 10.1016/0005-2787(78)90202-2. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Bartley J. C., Bissell M. J. Glucose metabolite patterns as markers of functional differentiation in freshly isolated and cultured mouse mammary epithelial cells. Exp Cell Res. 1981 Jul;134(1):241–250. doi: 10.1016/0014-4827(81)90481-x. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Burwen S. J., Pitelka D. R. Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue Cell. 1979;11(1):109–119. doi: 10.1016/0040-8166(79)90011-9. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Pitelka D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977 May;13(5):316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Babiss L. E., Weiss M., Darnell J. E., Jr Hepatoma variants (C2) are defective for transcriptional and post-transcriptional actions from both endogenous and viral genomes. EMBO J. 1987 Jun;6(6):1727–1731. doi: 10.1002/j.1460-2075.1987.tb02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Spray D. C., Choi H., Saez J., Jefferson D. M., Hertzberg E., Rosenberg L. C., Reid L. M. Extracellular matrix regulation of cell-cell communication and tissue-specific gene expression in primary liver cultures. Prog Clin Biol Res. 1986;226:333–360. [PubMed] [Google Scholar]
- Ganguly R., Ganguly N., Mehta N. M., Banerjee M. R. Absolute requirement of glucocorticoid for expression of the casein gene in the presence of prolactin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6003–6006. doi: 10.1073/pnas.77.10.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Gupta P., Rosen J. M., D'Eustachio P., Ruddle F. H. Localization of the casein gene family to a single mouse chromosome. J Cell Biol. 1982 Apr;93(1):199–204. doi: 10.1083/jcb.93.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A. A., Richards D. A., Kessler D. J., Rosen J. M. Complex hormonal regulation of rat casein gene expression. J Biol Chem. 1982 Apr 10;257(7):3598–3605. [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Houdebine L. M., Devinoy E., Delouis C. Stabilization of casein mRNA by prolactin and glucocorticoids. Biochimie. 1978;60(1):57–63. doi: 10.1016/s0300-9084(78)80198-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Israel D. I., Whitlock J. P., Jr Regulation of cytochrome P1-450 gene transcription by 2,3,7, 8-tetrachlorodibenzo-p-dioxin in wild type and variant mouse hepatoma cells. J Biol Chem. 1984 May 10;259(9):5400–5402. [PubMed] [Google Scholar]
- Kleinman H. K., Luckenbill-Edds L., Cannon F. W., Sephel G. C. Use of extracellular matrix components for cell culture. Anal Biochem. 1987 Oct;166(1):1–13. doi: 10.1016/0003-2697(87)90538-0. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Parry G., Bissell M. J. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984 Jan;98(1):146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levay-Young B. K., Bandyopadhyay G. K., Nandi S. Linoleic acid, but not cortisol, stimulates accumulation of casein by mouse mammary epithelial cells in serum-free collagen gel culture. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8448–8452. doi: 10.1073/pnas.84.23.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. F., Stockdale F. E. Cell-cell interactions promote mammary epithelial cell differentiation. J Cell Biol. 1985 May;100(5):1415–1422. doi: 10.1083/jcb.100.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. L., Aggeler J., Farson D. A., Hatier C., Hassell J., Bissell M. J. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato M. F., Careche M., Ros M., Moreno F. J., García-Ruíz J. P. Effect of prolactin and glucocorticoids on P-enolpyruvate carboxykinase activity in liver and mammary gland from diabetic and lactating rats. Mol Cell Biochem. 1985 May;67(1):19–23. doi: 10.1007/BF00220981. [DOI] [PubMed] [Google Scholar]
- Lobato M. F., Ros M., Moreno F. J., García-Ruíz J. P. Nutritional and hormonal regulation of malic enzyme synthesis in rat mammary gland. Biochem J. 1986 Jun 1;236(2):441–445. doi: 10.1042/bj2360441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina D., Li M. L., Oborn C. J., Bissell M. J. Casein gene expression in mouse mammary epithelial cell lines: dependence upon extracellular matrix and cell type. Exp Cell Res. 1987 Sep;172(1):192–203. doi: 10.1016/0014-4827(87)90105-4. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko A. S., Boettiger D. Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell. 1987 Oct 9;51(1):51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Murdoch G. H., Potter E., Nicolaisen A. K., Evans R. M., Rosenfeld M. G. Epidermal growth factor rapidly stimulates prolactin gene transcription. Nature. 1982 Nov 11;300(5888):192–194. doi: 10.1038/300192a0. [DOI] [PubMed] [Google Scholar]
- Muschel R., Khoury G., Reid L. M. Regulation of insulin mRNA abundance and adenylation: dependence on hormones and matrix substrata. Mol Cell Biol. 1986 Jan;6(1):337–341. doi: 10.1128/mcb.6.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G., Cullen B., Kaetzel C. S., Kramer R., Moss L. Regulation of differentiation and polarized secretion in mammary epithelial cells maintained in culture: extracellular matrix and membrane polarity influences. J Cell Biol. 1987 Nov;105(5):2043–2051. doi: 10.1083/jcb.105.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G., Lee E. Y., Farson D., Koval M., Bissell M. J. Collagenous substrata regulate the nature and distribution of glycosaminoglycans produced by differentiated cultures of mouse mammary epithelial cells. Exp Cell Res. 1985 Feb;156(2):487–499. doi: 10.1016/0014-4827(85)90556-7. [DOI] [PubMed] [Google Scholar]
- Rodgers J. R., Johnson M. L., Rosen J. M. Measurement of mRNA concentration and mRNA half-life as a function of hormonal treatment. Methods Enzymol. 1985;109:572–592. doi: 10.1016/0076-6879(85)09116-9. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Rodgers J. R., Couch C. H., Bisbee C. A., David-Inouye Y., Campbell S. M., Yu-Lee L. Y. Multihormonal regulation of milk protein gene expression. Ann N Y Acad Sci. 1986;478:63–76. doi: 10.1111/j.1749-6632.1986.tb15521.x. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Sklar V. E., Jaehning J. A., Weinmann R., Roeder R. G. Isolation and partial characterization of the multiple forms of deoxyribonucleic acid-dependent ribonucleic acid polymerase in the mouse myeloma, MOPC 315. J Biol Chem. 1974 Sep 25;249(18):5889–5897. [PubMed] [Google Scholar]
- Shannon J. M., Pitelka D. R. The influence of cell shape on the induction of functional differentiation in mouse mammary cells in vitro. In Vitro. 1981 Nov;17(11):1016–1028. doi: 10.1007/BF02618428. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Farmer S. R. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982 May;29(1):53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Ginty C. A. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983 Dec;35(3 Pt 2):657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- Suard Y. M., Haeuptle M. T., Farinon E., Kraehenbuhl J. P. Cell proliferation and milk protein gene expression in rabbit mammary cell cultures. J Cell Biol. 1983 May;96(5):1435–1442. doi: 10.1083/jcb.96.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper Y. J., Freeman C. S. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980 Oct;60(4):1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- Ucker D. S., Yamamoto K. R. Early events in the stimulation of mammary tumor virus RNA synthesis by glucocorticoids. Novel assays of transcription rates. J Biol Chem. 1984 Jun 25;259(12):7416–7420. [PubMed] [Google Scholar]