Abstract
WNT/β– catenin signaling is critical to development of many cancer types. A paper in a recent issue of Cell shows that autocrine CXCL12/CXCR4 chemokine signaling activates β–catenin signaling in a rare peripheral nerve sarcoma. Together with the availability of small molecules targeting CXCR4, this finding suggests new avenues for cancer therapy.
It is exciting to link established signaling pathways. It is especially provocative when compounds designed to target one molecule for a specific disease are shown to have potential in a novel context. In a recent issue of Cell, the laboratories of Luis Parada and Lu Le accomplish just this by showing that a pathway that was first identified as relevant to lymphocyte chemotaxis is also a driver of human Schwann cell tumor progression. The authors go on to find that this pathway acts via another pathway which was initially identified in organismal development and found to be corrupted in many cancers. The paper links the CXCR4 cell surface chemokine receptor, via autocrine CXCL12 ligand production, to the Wnt/β-catenin signaling pathway in malignant peripheral nerve sheath tumors (MPNST) (Mo et al., 2013). CXCR4 antagonists are being intensively investigated because CXCR4 is a co-receptor for human immunodeficiency virus (HIV) on T cells, and early stage clinical studies show that blocking CXCR4 delays the onset of acquired immunodeficiency syndrome (AIDS) in HIV infected individuals (Debnath et al., 2013). The work by Mo et al. suggests that CXCR4 antagonists may be useful to treat MPNST, a peripheral nerve related soft tissue sarcoma with very poor prognosis, especially when it occurs in the context of Neurofibromatosis Type 1 (NF1) disease (Widemann, 2009). In MPNST inactivation of the NF1 gene, which encodes a GTPase activating protein for Ras proteins, increases Ras signaling with activation of the key downstream signaling pathways MEK, AKT and mTOR (DeRaedt et al., 2011; Jessen et al., 2013). The Mo et al. paper also shows expression of CXCR4 and Wnt/β-catenin pathway components in benign neurofibromas, which can be MPNST precursor lesions.
The WNT/β– catenin signaling pathway was identified for its roles in development and controls critical processes in worms to mammals, from the formation of teeth to the control of stem cells in the intestine (reviewed in Clevers and Nusse, 2012). Despite >7,000 papers on WNT/β-catenin signaling listed in the Pubmed database, this pathway had not been directly implicated in MPNSTs. In the cells from which MPNSTs derive, which may be neural crest cells, skin-derived precursors, and/or committed Schwann cells, WNT/β-catenin signaling normally regulates cell fate decisions and transiently suppresses full differentiation (myelination) in Schwann cells, potentially explaining a role in tumor progression by differentiation block (Lewallen et al., 2011; Hari et al., 2012).
Canonical WNT/β-catenin signaling (Figure 1) plays a role in many types of cancer, including colorectal, lung, breast, ovarian, prostate, liver, and brain tumors (Clevers and Nusse, 2012; Saito-Diaz et al., 2013). β-catenin-dependent transcription can promote cell cycle progression, stem cell self-renewal, and epithelial-to-mesenchymal transition. WNT signaling in cancer can be aberrantly activated through activating mutations in β-catenin (CTNNB1), overexpression of WNT ligand genes, inactivating mutations in destruction complex genes (i.e., AXIN1, GSK3B, and APC), or promoter hypermethylation of negative regulators of WNT signaling (Saito-Diaz et al., 2013). Activation of the PI3K/AKT signaling pathway, either by loss of PTEN or through activation of upstream tyrosine kinase receptors, also causes phosphorylation and inactivation of GSK3β, stabilizing β-catenin (Clevers and Nusse, 2012). In the study by Mo et al. (2013), WNT signaling is activated by crosstalk with the CXCL12/CXCR4 signaling, downstream of AKT (Figure 1).
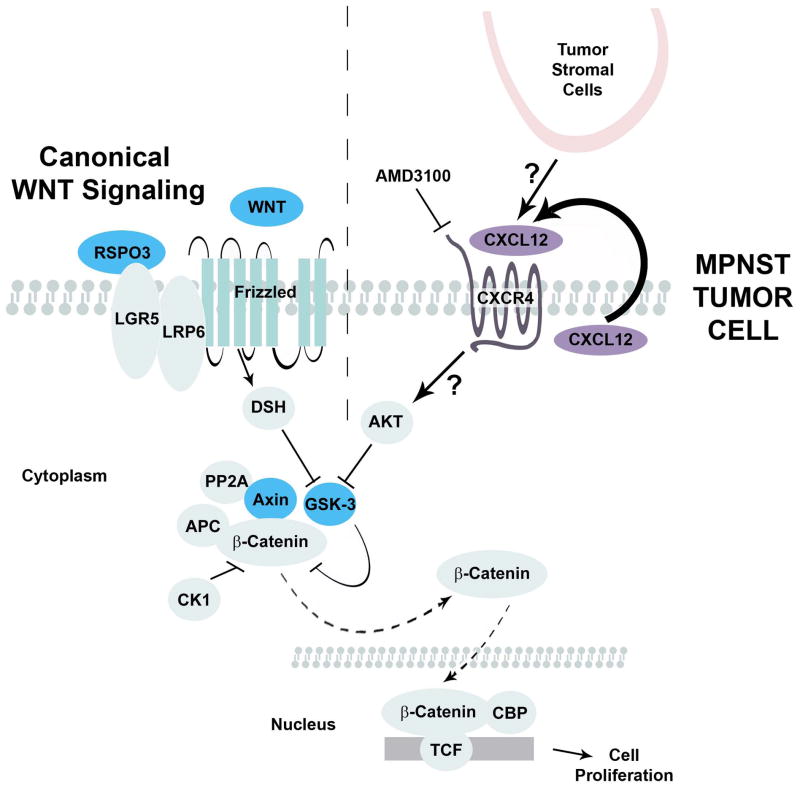
Figure 1. Canonical WNT signaling and CXCR4 activation of β-catenin.
In canonical WNT signaling (left of dotted line), WNT ligands activate FRIZZLED receptors; LRP and LGR are co-receptors. Receptor activation leads, through inactivation of GSK3β to stabilization of β-catenin. Stabilized, β-catenin moves to the nucleus and activates gene transcription. Mo. et al. (right of dotted line) describe a novel mechanism in which MPNST tumor cells secrete CXCL12 ligand, activating CXCR4 receptors and, via AKT, inactivating GSK3β and stabilizing β-catenin.
CXCL12 (SDF-1) is a chemokine. CXCL12 binding to the heterotrimeric G-protein coupled receptor CXCR4 is, like β-catenin signaling, required for normal development. In many situations, stromal cells secrete CXCL12 and attract cells expressing CXCR4 receptors. In this fashion, immune cells are attracted to sites of inflammation and hematopoietic cells home to the bone marrow. This paracrine CXCL12/CXCR4 pathway has been exploited therapeutically; CXCR4 blockade facilitates removal of cells from the bone marrow niche for use in transplantation (reviewed in Domanska et al., 2013). CXCL12/CXCR4 paracrine signaling is also relevant to tumor metastasis, when tumor cells expressing CXCR4 migrate toward distant sites where ligand is produced (Domanska et al., 2013).
The paper by Mo et al. (2013) shows that autocrine, rather than paracrine, CXCL12 promotes tumor cell proliferation. The notion that tumor cells can manufacture, secrete, and respond to their own CXCL12 was discovered in prostate tumors by Sun et al. (2003). Mo et al. use antibodies against CXCL12 and CXCR4 receptor blockade, both of which decrease MPNST cell proliferation. Furthermore, while CXCR4 activation can recruit endothelial cells to promote neoangiogenesis, vessel density remained the same after CXCR4 blockade, providing additional evidence that the tumor cell effects are cell autonomous. CXCL12 produced in a paracrine manner by host tumor stromal cells, as in other forms of cancer, may also contribute to effects on tumor growth.
The authors show that CXCR4 activation in MPNST cells activates β-catenin by AKT-mediated phosphorylation and inactivation of GSK3β, thus stabilizing β-catenin (Figure 1). How CXCR4 activates AKT is not entirely clear. This may occur through the βγ subunits of CXCR4, known to indirectly activate AKT (Domanska et al, 2013). The authors exclude roles for activation of NF-κB, RAS/MAPK, and JAK/STAT3. Additional pathways downstream of CXCR4 might also contribute to β-catenin stabilization. CXCR4 is known to activate SRC family kinases, C-CBL, and RHO GTPases (Domanska et al., 2013), which may be relevant. β-catenin activation likely also requires additional genetic events or activation of signaling pathways in neurofibromas and MPNSTs, particularly in MPNST that develop in the absence of NF1 syndrome. This is because many MPNST express β-catenin but not CXCR4. Also, many neurofibromas express CXCR4 but not β-catenin.
The molecular mechanisms that explain CXCL12 and CXCR4 expression in MPNST cells also remain undefined. One possibility is that CXCR4/CXCL12 expression reflects the embryonic neural crest origin of MPNST cells. The authors demonstrate that CXCR4 expression frequency and intensity are especially pronounced in neurofibromas and MPNSTs associated with NF1 disease. Therefore signaling downstream of NF1 may normally suppress expression of CXCL12/CXCR4.
Despite many efforts to identify drugs to target β-catenin signaling, no inhibitor has to date demonstrated the appropriate pharmacodynamic and pharmacokinetic properties to be used as a drug for treatment of cancer patients. In this light, it is impressive that the CXCR4 inhibitor AMD3100 is shown by the authors to inhibit β-catenin signaling in vitro and in vivo. With AMD3100 (Plerixafor; FDA approved for use in hematopoietic stem cell mobilization) currently in clinical testing in many settings, it should be feasible to test in human MPNST patients. Mo et al. (2013) show a clear delay in MPNST formation in human xenografts and also in a genetically engineered mouse model. Certainly, targeting the CXCR4 receptor alone is insufficient to halt MPNST growth or shrink tumors; the authors show that the main effect is proliferation arrest. Moreover, the authors note that some tumors expressed CXCR4 in a patchy manner, where the cells are not all positive. For both of these reasons, tumor ablation is likely to require co-therapy, perhaps with identified NF1/Ras pathway inhibitors (De Raedt et al., 2011; Jessen et al., 2013). Especially because NF1 mutations have been identified in many types of cancer, it will be exciting to discover those that use the CXCL12/CXCR4 pathway to activate Wnt/β-catenin signaling and may benefit from CXCR4 blockade.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 2.De Raedt T, Walton Z, Yecies JL, Li D, Chen Y, Malone CF, Maertens O, Jeong SM, Bronson RT, Lebleu V, Kalluri R, Normant E, Haigis MC, Manning BD, Wong KK, Macleod KF, Cichowski K. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell. 2011;20(3):400–13. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG, Walenkamp AM. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer. 2013;49(1):219–30. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Hari L, Miescher I, Shakhova O, Suter U, Chin L, Taketo M, Richardson WD, Kessaris N, Sommer L. Temporal control of neural crest lineage generation by Wnt/β-catenin signaling. Development. 2012;139(12):2107–17. doi: 10.1242/dev.073064. [DOI] [PubMed] [Google Scholar]
- 5.Jessen WJ, Miller SJ, Jousma E, Wu J, Rizvi TA, Brundage ME, Eaves D, Widemann B, Kim MO, Dombi E, Sabo J, Hardiman Dudley A, Niwa-Kawakita M, Page GP, Giovannini M, Aronow BJ, Cripe TP, Ratner N. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest. 2013;123(1):340–7. doi: 10.1172/JCI60578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewallen KA, Shen YA, De la Torre AR, Ng BK, Meijer D, Chan JR. Assessing the role of the cadherin/catenin complex at the Schwann cell-axon interface and in the initiation of myelination. J Neurosci. 2011;31(8):3032–43. doi: 10.1523/JNEUROSCI.4345-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mo W, Chen J, Patel A, Zhang L, Chau V, Li Y, Cho W, Lim K, Xu J, Lazar AJ, Creighton CJ, Bolshakov S, McKay RM, Lev D, Le L, Parada LF. CXCR4/CXCL12 mediate autocrine cell cycle progression in NF1-associated malignant peripheral nerve sheath tumors. Cell. 2013;152:1077–1090. doi: 10.1016/j.cell.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito-Diaz K, Chen TW, Wang X, Thorne CA, Wallace HA, Page-McCaw A, Lee E. The way Wnt works: Components and mechanism. Growth Factors. 2013;31(1):1–31. doi: 10.3109/08977194.2012.752737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, Pienta KJ, Taichman RS. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89(3):462–73. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 10.Widemann BC. Current status of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Curr Oncol Rep. 2009;11(4):322–8. doi: 10.1007/s11912-009-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]