Abstract
Purpose
Despite recent advances in the management of high-grade and recurrent gliomas, survival remains poor. Antiangiogenic therapy has been shown to be efficacious in the treatment of high-grade gliomas both in preclinical models and in clinical trials. We sought to determine the safety and maximum tolerated dose of sorafenib when combined with both radiation and temozolomide in the primary setting or radiation alone in the recurrent setting.
Methods and Materials
This was a preclinical study and an open-label phase I dose escalation trial. Multiple glioma cell lines were analyzed for viability after treatment with radiation, temozolomide, or sorafenib or combinations of them. For patients with primary disease, sorafenib was given concurrently with temozolomide (75 mg/m2) and 60 Gy radiation, for 30 days after completion of radiation. For patients with recurrent disease, sorafenib was combined with a hypofractionated course of radiation (35 Gy in 10 fractions).
Results
Cell viability was significantly reduced with the combination of radiation, temozolomide, and sorafenib or radiation and sorafenib. Eighteen patients (11 in the primary cohort, 7 in the recurrent cohort) were enrolled onto this trial approved by the institutional review board. All patients completed the planned course of radiation therapy. The most common toxicities were hematologic, fatigue, and rash. There were 18 grade 3 or higher toxicities and no grade 5 events. The median overall survival was 18 months for the entire population.
Conclusions
Sorafenib can be safely combined with radiation and temozolomide in patients with high-grade glioma and with radiation alone in patients with recurrent glioma. The recommended phase II dose of sorafenib is 200 mg twice daily when combined with temozolomide and radiation and 400 mg with radiation alone. To our knowledge, this is the first publication of concurrent sorafenib with radiation monotherapy or combined with radiation and temozolomide.
Introduction
The standard of care for patients with diagnoses of high-grade glioma (HGG) is maximal safe surgical resection followed by concurrent chemotherapy with temozolomide (TMZ) and radiation therapy (RT) (1). For patients with recurrent HGG there is no standard of care; treatment options include reresection, chemotherapy alone (2), 3-dimensional conformal radiation therapy (3), and fractionated stereotactic RT (4). Despite aggressive management, overall survival (OS) remains poor. Understanding of the molecular pathways involved in disease progression and therapeutic resistance has led to the development of targeted biologic agents.
Sorafenib (Nexavar, BAY43-9006) is a multikinase inhibitor that inhibits vascular endothelial growth factor receptor (VEGFR)-2, VEGFR-3, platelet-derived growth factor receptor (PDGFR)β, c-Kit, and RAF. Sorafenib is approved by the US Food and Drug Administration for the treatment of renal cell carcinoma (5) and hepatocellular carcinoma (6). The drug has demonstrated antiglioma activity in both in vitro and in vivo models, inhibiting cell proliferation, inducing tumor cell apoptosis and autophagy, and inhibiting angiogenesis. Preclinical data demonstrate that the combination of sorafenib and RT results in significant in vivo growth delays (7). The combination of sorafenib and TMZ results in inhibition of tumor growth in both in vitro and in vivo (8) models of melanoma.
Clinically, sorafenib has been evaluated with TMZ, both in patients with recurrent glioblastoma (9) and with adjuvant TMZ after completion of RT and TMZ for frontline management of glioblastoma (10). The phase II trial in patients with recurrent disease found that although sorafenib could be safely administered with TMZ, there was limited activity (9). In the adjuvant setting, sorafenib did not improve the efficacy of treatment in comparison with TMZ alone; however, more than 40% of patients did not receive any maintenance sorafenib because of disease progression (10). Not addressed was whether concurrent treatment of sorafenib with TMZ and RT would improve the outcome.
Thus, we evaluated the activity of RT combined with TMZ and sorafenib preclinically and determined the safety profile and efficacy of sorafenib in combination with TMZ and RT in the setting of primary HGG and the addition of sorafenib to hypo-fractionated stereotactic RT in recurrent gliomas.
Methods and Materials
Cell viability assays
Cell viability assays were performed essentially as previously described (11) with minor modifications. Six glioblastoma multiforme cell lines (U87MG, T98G, U251, SNB19, SNB75, and SF295) were obtained from ATCC (American Type Culture Collection) Manassas, VA and maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 50 μg/mL penicillin and streptomycin. Cells (3 × 104) were plated in 6-well plates, cultured overnight, and then irradiated with 6 Gy or left untreated. Immediately after irradiation, cells were treated with a combination of 5 μM sorafenib and/or 200 μM TMZ, concentrations chosen on the basis of a previous study (11). Seven days later, the cells were trypsinized, a proportion of cells (200 μL out of 5 mL) were replated in a 96-well plate, and cell viability was assayed by use of MTT : (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (12).
Patient eligibility
Eligibility criteria included a histologically or cytologically confirmed high-grade glioma (World Health Organization [WHO] grade 3, grade 4, or gliosarcoma) and required at least 2 weeks of RT, WHO performance status ≤2, life expectancy >3 months, adequate hematologic reserve (defined as white blood cell count > 3 109/L, absolute neutrophil count >1.5 109/L, hemoglobin >9.0 g/dL, and platelet count >100 109/L), adequate renal and liver function (defined as total bilirubin <1.5 times the upper limit of normal [ULN], aspartate aminotransferase and alanine aminotransferase <2.5 times the ULN, alkaline phosphatase <2.5 times the ULN, and serum creatinine <1.5 times the ULN), and age > 18 years.
Exclusion criteria included major surgery or radiation therapy within 1 week of enrollment in the study, receipt of chemotherapy or other investigational drugs within 14 days before enrollment, New York Hospital Association class 3 or 4 heart failure, active or suspected acute or chronic uncontrolled infection, human immunodeficiency virus positivity, uncontrolled hypertension defined as systolic blood pressure above 150 mm Hg or diastolic pressure above 90 mm Hg despite optimal medical management, National Cancer Institute Common Toxicity Criteria Adverse Events grade 3 hemorrhage or grade 2 pulmonary hemorrhage within 4 weeks of enrollment, known or suspected allergy to sorafenib or TMZ, and inability to swallow whole pills.
Our institutional parameters for reirradiation with hypo-fractionated stereotactic RT (4) include a Karnofsky performance score above 60, the ability to encompass the tumor within a 10- × 10-cm field, and the patient’s ability to tolerate treatment delivery.
Study design
The present study was designed as an open-label, 3+3 drug dose escalation, phase I clinical study to establish the maximal tolerated dose (MTD) when oral sorafenib is combined with RT and TMZ for primary high-grade gliomas and RT alone for recurrent high-grade gliomas. Patients with either primary or recurrent disease were enrolled to the same dose level cohort.
The study consisted of a screening and baseline assessment period, a treatment period, and an observation period. A complete clinical evaluation including medical history, physical and neurologic examination, and WHO performance score assessment, and a laboratory evaluation consisting of baseline complete blood count plus differential, complete metabolic profile, urinalysis, and pregnancy test for female patients was completed during the assessment period. Electrocardiograms and baseline brain imaging using either computed tomography (CT) or magnetic resonance imaging (MRI) was also performed during the assessment period.
During the treatment period, patients with WHO grade 4 tumors received 60 Gy with concurrent TMZ (75 mg/m2) and sorafenib, and those with WHO grade 3 tumors received 59.4 Gy with concurrent TMZ and sorafenib. The 3-dimensional conformal RT was delivered in accordance with Radiation Therapy Oncology Group guidelines and constraints. Standard dose constraints were used for normal structures.
During the treatment period, for patients with recurrent glioma, RT consisted of 35 Gy delivered over 10 treatment days by use of fractionated stereotactic RT (4). All patients were fitted with the Gill-Thomas-Cosman relocatable frame and were taken to the MRI and/or CT scanning suite, where the Brown-Roberts-Well fiducial cage was placed on the frame and axial images were obtained. Treatment planning was carried out with Brain Lab (Novalis, Westchester, IL) by use of CT/MRI fusion and delivered stereotactically with a dedicated 600SR linear accelerator (Varian, Palo Alto, CA) by use of minimultileaf collimator leaves and an Exac Trac feature. The gross tumor volume was defined by the contrast-enhanced tumor edge using T1-weighted gadolinium MRI imaging and coapproved by a neurosurgeon and a radiation oncologist. The planning target volume was considered equivalent to the gross tumor volume, and edema was not included in the treatment volume. Tumors were treated to the 85%-90% isodose line, and coverage of the target volume was reviewed on the planning system.
The starting dose level of sorafenib was 200 mg orally twice daily, beginning at bedtime the night before the first radiation fraction and continuing until the night before the last RT treatment (7 days/week). The second dose level was 400 mg twice daily. After completion of RT, sorafenib was continued for an additional 30 days at the physician’s discretion. Weekly assessment of hematology and chemistry panels was performed during active treatment.
For patients with primary high-grade gliomas, TMZ was administered continuously at a daily dose of 75 mg/m2 concurrently with RT and resumed 4 weeks after RT at a dose of 150–200 mg/m2 on days 1–5 for 6 cycles (1).
Toxicity and response evaluation
Toxicity was defined in accordance with the National Cancer Institute Common Toxicity Criteria, version 3.0. Dose-limiting toxicity (DLT) was defined as any grade 4 or 5 toxicity or any grade 3 toxicity directly attributable to the protocol treatment and requiring hospitalization and/or an interruption of RT. Patients were evaluated for potential DLTs weekly during treatment and for 4 additional weeks after the completion of treatment. The study was designed to be terminated early if the actual DLT rate was 50% or greater. A provision for dose deescalation of sorafenib was provided if more than a third of the initial patients enrolled experienced a DLT.
Steroids were prescribed at the discretion of the treating physician. The tumor response was measured using brain MRI scans obtained approximately 1 month after treatment completion and at regular follow-up appointments thereafter. The tumor response was determined according to the Response Evaluation Criteria in Solid Tumors criteria.
Correlative studies: VEGF measurements
Urine was collected from all study patients at baseline, at the completion of RT, and 1 month after treatment. Urine was stored at −20°C before evaluation, then centrifuged at 3000 rpm for 10 minutes at 4°C. The supernatant was used for subsequent assays. Urinary creatinine levels were obtained on the Bayer DCA 2000+ Analyzer (Bayer HealthCare, Elkhart, IN) according to the manufacturer’s protocol. All samples were normalized by creatinine level. Urine samples were assayed for vascular endothelial growth factor (VEGF).
The VEGF was measured in urine specimens via electrochemiluminescent immunoassay (Meso Scale Discovery, Gaithersburg, MD). All samples were thawed at room temperature for 4 hours before evaluation. The VEGF assay was performed according to the manufacturer’s protocol.
Statistical methods
The median OS rate was calculated from the time of enrollment with the Kaplan-Meier method. Actuarial OS rates were calculated by the life-tables method. Fisher’s exact test was used to test for an association between relative baseline VEGF levels and treatment response. Median urinary VEGF levels over time were compared with Friedman’s χ2 test based on ranks. All statistical tests were 2-sided, and P values less than or equal to .05 were considered significant. All analyses were performed with SAS v 9.2 (SAS Institute Inc, Cary, NC) and R v2.11.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
In vitro study
To investigate the synergistic effect of combination treatments in vitro, we treated 6 human glioblastoma cell lines, all of which have a methylated MGMT promoter (U87MG, T98G, U251, SNB19, SNB75, and SF295) with a combination of sorafenib, TMZ, and/or irradiation, and monitored cell viability. In all 6 cell lines, the coadministration of sorafenib, TMZ, and irradiation greatly reduced cell viability (Fig. 1). These results indicate that a combination of sorafenib, TMZ, and irradiation may substantially increase the sensitivity of glioblastoma cells to these treatments (P<.05).
Fig. 1.
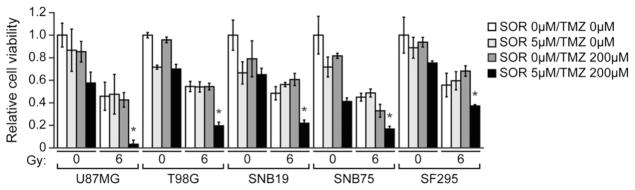
Cell viability analysis in human glioblastoma cell lines treated with a combination of sorafenib (SOR), temozolomide (TMZ), and/or irradiation (0 or 6 Gy), as indicated. Cell viability was measured using MTT, and normalized to that observed in the absence of treatment, which was set to 1. Error bars represent standard deviation of 3 independent experiments. *P<.05
Patient characteristics
Eighteen patients (7 men, 11 women) were enrolled between March 2008 and July 2009 with histologically confirmed primary frontline high-grade glioma (11 patients) and recurrent glioma (7 patients). All patients were included in the safety analysis. Two dose levels were studied: 200 mg twice daily (BID) (n=9), and 400 mg BID (n=9). Patient characteristics are shown in Table 1. The median age was 53 years (range, 31–69 years), and the median Karnofsky performance score was 90 (range, 70–100). All patients completed a full course of RT.
Table 1.
Patient characteristics
| Characteristic | Primary setting | Recurrent setting |
|---|---|---|
| No. of patients | 11 | 7 |
| Median age, y (range) | 51 (31–67) | 55 (38–69) |
| Sex | ||
| M | 4 | 3 |
| F | 7 | 4 |
| Race | ||
| Caucasian | 9 | 5 |
| Hispanic | 0 | 1 |
| African American | 2 | 0 |
| Asian American | 0 | 1 |
| KPS | ||
| 90–100 | 7 | 1 |
| 70–80 | 4 | 7 |
| Histology | ||
| Glioblastoma | 7 | 5 |
| Gliosarcoma | 1 | 0 |
| Anaplastic astrocytoma | 2 | 2 |
| Anaplastic oligodendroglioma | 1 | 0 |
| RT dose (median, Gy) | 60 Gy | 35 Gy |
| Sorafenib Dose | ||
| 200 mg BID | 4 | 5 |
| 400 mg BID | 7 | 2 |
| Median total dose of sorafenib (range) | 20,000 mg (8000–37,600) | 12,000 mg (6000–28,000) |
Abbreviations: BID = twice a day; KPS = Karnofsky performance score; RT = radiation therapy.
Toxicity and compliance
The most common toxicities were hematologic, fatigue, and rash. Grade 3 and 4 toxicity is shown in Table 2; hematologic toxicity was transient, and 3 patients experienced grade 3/4 rash. There was 1 Grade 5 event in the frontline cohort at 200 mg BID; the patient halted sorafenib and bactrim after developing a rash, and subsequently developed pancytopenia and bacteremia. The patient had progressive multifocal disease on post-treatment MRI. Overall, 8 patients (3 at the 200-mg level and 5 at the 400-mg level) experienced any grade of rash. In all, 50 and 43 any-grade toxicities were reported in the 200-mg dose cohort and the 400-mg dose cohort, respectively. However, there were 7 grade 3/4 toxicities in the 200-mg dose cohort and 13 grade 3/4 toxicities in the 400-mg dose cohort.
Table 2.
Grade 3/4 toxicity and adverse events
| Toxicity | No. of patients (%)
|
|||||
|---|---|---|---|---|---|---|
| Total cohort
|
200 mg
|
400 mg
|
||||
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Anemia | 1 (5.5%) | 1 (5.5%) | 1 | 0 | 0 | 1 |
| Leukopenia | 0 | 1 (5.5%) | 0 | 0 | 0 | 1 |
| Lymphopenia | 2 (11%) | 2 (11%) | 0 | 2 | 2 | 0 |
| Febrile neutropenia | 0 | 1 (5.5%) | 0 | 0 | 0 | 1 |
| Thrombocytopenia | 1 (5.5%) | 1 (5.5%) | 0 | 1 | 0 | 1 |
| Hypoglycemia | 1 (5.5%) | 1 (5.5%) | 1 | 0 | 0 | 1 |
| Hypokalemia | 0 | 1 (5.5%) | 0 | 0 | 0 | 1 |
| Rash | 1 (5.5%) | 2 (11%) | 1 | 0 | 0 | 2 |
| Photosensitivity | 0 | 1 (5.5%) | 0 | 0 | 0 | 1 |
| Neuropathy | 1 (5.5%) | 0 | 1 | 0 | 0 | 0 |
| Myelopathy | 0 | 1 (5.5%) | 0 | 0 | 0 | 1 |
| Palpitations | 0 | 1 (5.5%) | 0 | 0 | 0 | 1 |
In the 200-mg dose cohort, 2 (22%) patients discontinued sorafenib during RT; both had primary high-grade gliomas. One patient experienced a grade 3 rash and another experienced grade 3 anemia and grade 4 thrombocytopenia. In the 400-mg cohort, 5 (55%) patients discontinued sorafenib during RT; all had primary high-grade gliomas. One patient discontinued treatment because of grade 2 rash and grade 4 febrile neutropenia, 2 discontinued secondary to a grade 3 rash despite dose reduction, 1 patient declined to continue the drug for unspecified reasons, and another experienced elevated liver function test results and hyperglycemia.
Patients were allowed to continue sorafenib for 30 days after completion of RT. In the 200-mg and 400-mg cohorts, 4 of 7 patients and 2 of 4 patients, respectively, completed sorafenib for the additional 30 days. The reasons for discontinuation during the optional period included grade 2/3 rash, grade 4 lymphopenia, and physician preference. Overall, 4 patients (44%) and 2 patients (22%) in the 200-mg BID and 400-mg BID cohorts completed sorafenib for the entire treatment course.
Antitumor activity
All patients underwent an MRI 1-month after RT. We observed 1 complete response, 4 partial responses, and 3 patients with stable disease (Table 3 and Fig. 2). The objective radiographic response was 28%. However, the majority of patients had imaging findings consistent with progressive disease.
Table 3.
Treatment efficacy as determined by gadolinium-enhanced MRI 1 month after completion of treatment
| Response | All patients | Primary glioma | Recurrent glioma |
|---|---|---|---|
| Complete response | 1 | 1 | 0 |
| Partial response | 4 | 2 | 2 |
| Stable disease | 3 | 2 | 1 |
| Progressive disease | 10 | 6 | 4 |
Abbreviations: MRI = magnetic resonance imaging.
Fig. 2.
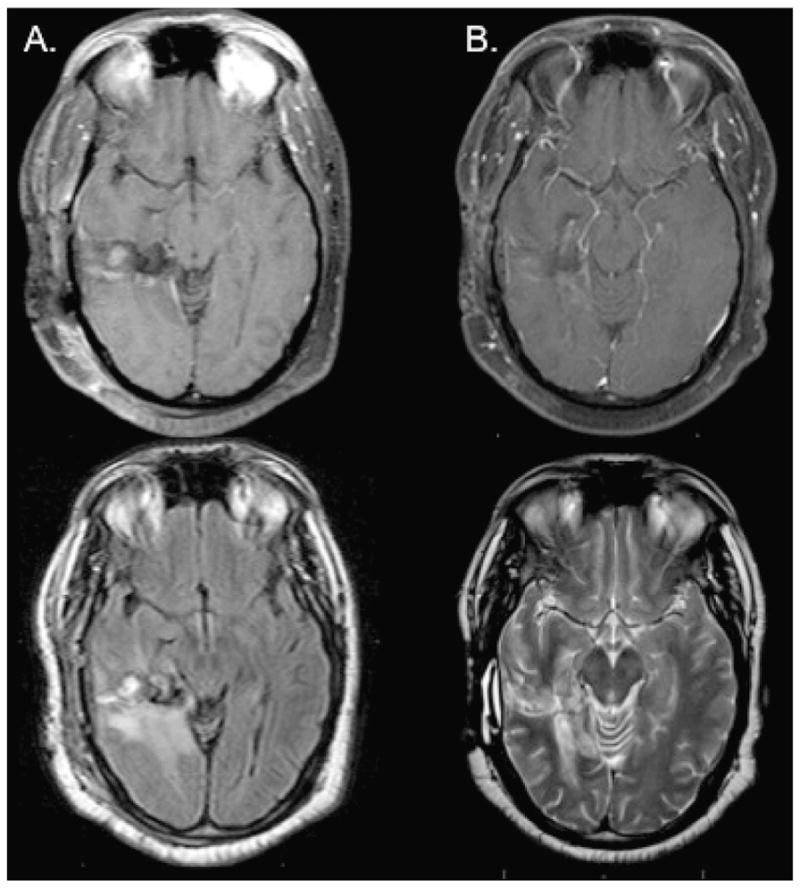
Magnetic resonance images (MRI). (A) Pretreatment T1 after gadolinium weighted MRI and T2 weighted MRI. (B) Post-treatment T1 after gadolinium weighted MRI and T2 weighted MRI from patient with complete response to combination radiation, temozolomide, and sorafenib.
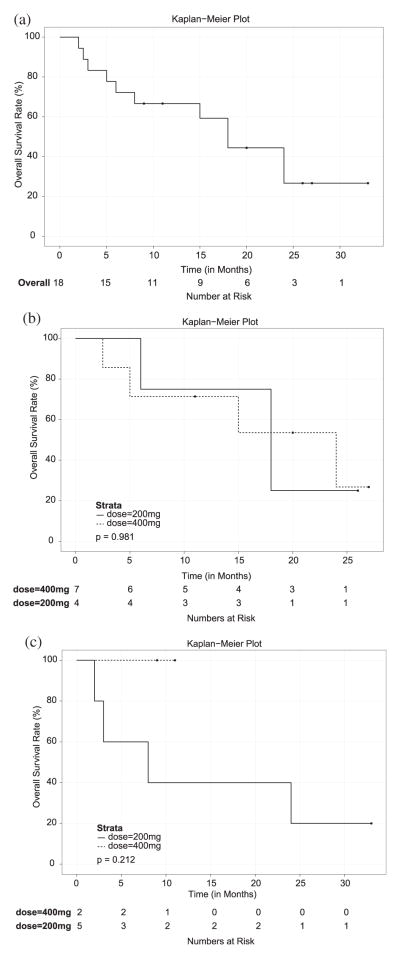
There have been 11 deaths, all resulting from disease progression. The median follow-up period for all patients was 13 months (range, 2–33 months) and 20 months for surviving patients (range, 9–33 months). The 1-year actuarial OS rate is 67% (95% CI, 40%-83%), and the 2-year is 44% (95% CI, 20%-67%). The median OS for the entire cohort was 18 months (95% CI, [6, UND]) (Fig. 3A). For the primary high-grade gliomas (Fig. 3B) and recurrent gliomas (Fig. 3C), the median OS was 18 months (95% CI, [5, UND]) and 24 months (95% CI, [2,UND]), respectively. For the 200-mg dose cohort, the median OS was 18 months (95% CI, [2, UND]), and for the 400-mg dose cohort, the median OS was 24 months (95% CI, [2.5, UND]). The median OS for each group was 18 (95% CI, [6, UND]), 24 (95% CI, [2.5, UND]), 8 (95% CI, [2, UND]), and undefined for the high-grade glioma 200-mg and 400-mg cohorts and for the recurrent glioma 200-mg and 400-mg cohorts.
Fig. 3.
(a) Kaplan-Meier survival curve, entire study population (n=18). Median survival time for all patients treated was 18 months. (b) Kaplan-Meier survival curve for primary high-grade glioma patients. Median survival was 18 months: 18 months for cohort taking 200 mg twice daily (n=4) and 24 months for cohort taking 400 mg twice daily (n=7). (c) Kaplan-Meier survival curve for patients with recurrent glioma. Median survival was 24 months: 8 months for cohort taking 200 mg twice daily (n=5) and undefined for cohort taking 400 mg twice daily (n=2).
VEGF levels
Baseline urine VEGF levels were measured for 17 of the 18 patients in the study. The VEGF levels varied, with a median value of 220 pg/mg (range, 26–604 pg/mg). Sixteen patients had VEGF levels measured at the end of RT (median, 259 pg/mg; range, 42–1784 pg/mg), and 7 patients had VEGF levels measured 1 month after RT (median, 208 pg/mg; range, 16–853 pg/mg). Six of 9 patients with disease progression based on MRI performed 1 month after RT had VEGF levels higher than the median at baseline, whereas 2 of 8 patients with stable disease, partial response, or complete response had levels higher than the median (P=.153). The VEGF levels at baseline, at the end of RT, and 1 month after RT were not significantly different between time points for the entire cohort of patients (P=.545) or when VEGF levels were stratified by treatment response (complete response, P=.368; progressive disease, P=.926; partial response, P=.245; stable disease, P=.244). However, the 1 patient with a complete response had a 5.9-fold increase in VEGF levels between baseline and 1 month after RT. This was the largest increase noted for all 7 patients with available data for this comparison. Patients with progressive disease tended to have a decrease in VEGF at 1 month after RT compared with baseline, whereas those with stable disease or partial response or complete response tended to have an increase.
Discussion
This study demonstrated the efficacy and feasibility of adding sorafenib to RT in the treatment of high-grade and recurrent gliomas. The preclinical and clinical results suggest that this treatment has merit to be further evaluated. Antiangiogenic therapy is currently being evaluated in both the phase II and phase III setting within the Radiation Therapy Oncology Group for high-grade gliomas. Recent clinical trials using bevacizumab (13) and cediranib (14) have shown promising results as monotherapy, in combination with chemotherapy, and in combination with RT (15). However, a third antiangiogenic agent, vandetanib, although shown to be safe, did not demonstrate an improvement in median OS (16).
The main toxicities in this study were hematologic and rash. Hematologic toxicity (leukopenia, neutropenia, and thrombocytopenia) was comparable to that in published randomized trials of TMZ and radiation (1) and in sorafenib monotherapy (5, 6). However, there was a higher incidence of grade 3/4 anemia (11% vs 1% vs 3%). The incidence of grade 3/4 rash in this study was 16%, whereas only a 1% incidence of grade 3/4 rash was reported in prior randomized trials. Most of the rashes occurred outside the radiation fields, and it is unclear why a higher incidence was noted, given that the maximum dose of sorafenib used in this study was the current dose approved by the US Food and Drug Administration.
Patients receiving reirradiation did not experience higher levels of toxicity. This suggests that a third agent may be added to this regimen to further augment the therapeutic effect of RT in the setting of recurrent glioma. One of the limitations of this particular trial design was that the primary and recurrent cohorts were not enrolled independently. Thus, there is limited information regarding toxicity in the setting of 400-mg BID recurrent glioma.
Although OS was promising in this phase I trial, compliance was suboptimal, inasmuch as 7 patients (63%) in the primary high-grade glioma cohort discontinued sorafenib during concurrent chemoradiation. Variations in the delivery of sorafenib, perhaps administration over a shorter time such as 2 weeks, or intermittent usage, may lead to fewer toxicities and hence increased compliance. Other researchers have found sorafenib to be well tolerated both in the maintenance phase of TMZ after completion of chemoradiation (10) and in the recurrent setting with TMZ (9). Efficacy in the latter trial (which did not include reirradiation) was not as robust as in the current study.
Conclusion
The results of our study demonstrate that sorafenib can statistically significantly reduce glioblastoma multiforme cell viability when it is combined with either RT and TMZ or RT alone. Sorafenib can be safely combined with RT and TMZ in patients with high-grade glioma and with RT alone in patients with recurrent glioma. To our knowledge, this is the first publication of concurrent sorafenib with radiation monotherapy or combined radiation and TMZ. The MTD and recommended phase II dose of sorafenib is 200 mg BID when combined with TMZ and RT and 400 mg with RT alone.
Summary.
Patients with high-grade and recurrent gliomas have poor overall survival. Multiple glioma cell lines have demonstrated statistically significant reduction in cell viability with (1) radiation (RT), temozolomide (TMZ), and sorafenib or (2) radiation and sorafenib. Based on this preclinical work, a phase I clinical trial was conducted. The maximum tolerated doses of sorafenib were 200 mg and 400 mg daily in the frontline (RT/TMZ/sorafenib) and recurrent settings (RT/sorafenib) respectively, with a median overall survival of 18 months.
Acknowledgments
Research in this publication includes work carried out by the Kimmel Cancer Center, which is supported in part by NCI Cancer Center Support Grant P30 CA56036. This study was supported in part by a grant from Bayer-Onyx to A.P.D.
Footnotes
Conflict of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 3.Chan JL, Lee SW, Fraass BA, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20:1635–1642. doi: 10.1200/JCO.2002.20.6.1635. [DOI] [PubMed] [Google Scholar]
- 4.Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28:3048–3053. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Suen AW, Galoforo S, Marples B, et al. Sorafenib and radiation: a promising combination in colorectal cancer. Int J Radiat Oncol Biol Phys. 2010;78:213–220. doi: 10.1016/j.ijrobp.2010.02.064. [DOI] [PubMed] [Google Scholar]
- 8.Ma XH, Piao S, Wang D, et al. Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin Cancer Res. 2011;17:3478–3489. doi: 10.1158/1078-0432.CCR-10-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reardon DA, Vredenburgh JJ, Desjardins A, et al. Effect of CYP3A-inducing anti-epileptics on sorafenib exposure: results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastoma. J Neurooncol. 2011;101:57–66. doi: 10.1007/s11060-010-0217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hainsworth JD, Ervin T, Friedman E, et al. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer. 2010;116:3663–3669. doi: 10.1002/cncr.25275. [DOI] [PubMed] [Google Scholar]
- 11.Sheng Z, Li L, Zhu LJ, et al. A genome-wide RNA interference screen reveals an essential CREB3L2-ATF5-MCL1 survival pathway in malignant glioma with therapeutic implications. Nat Med. 2010;16:671–677. doi: 10.1038/nm.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheng Z, Wang SZ, Green MR. Transcription and signalling pathways involved in BCR-ABL-mediated misregulation of 24p3 and 24p3R. EMBO J. 2009;28:866–876. doi: 10.1038/emboj.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 14.Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28:2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayana A, Golfinos JG, Fischer I, et al. Feasibility of using bevacizumab with radiation therapy and temozolomide in newly diagnosed high-grade glioma. Int J Radiat Oncol Biol Phys. 2008;72:383–389. doi: 10.1016/j.ijrobp.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 16.Drappatz J, Norden AD, Wong ET, et al. Phase I study of vandetanib with radiotherapy and temozolomide for newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys. 2010;78:85–90. doi: 10.1016/j.ijrobp.2009.07.1741. [DOI] [PubMed] [Google Scholar]