Abstract
Apolipoprotein E (apoE) synthesized in liver and brain plays a key role in both cholesterol transport and Alzheimer's disease (AD): apoE-knockout mice develop hypercholesterolemia and atherosclerosis and cannot support AD amyloid deposition. The ApoE4 allele is the strongest genetic risk factor for late-onset AD, and apoE4 protein preferentially catalyzes amyloid-beta (Aβ) peptide fibrillization in vitro and amyloid plaque deposition in vivo. Circulating apoE may also have the potential to draw Aβ from the brain and reduce amyloid deposition. We used parabiosis to determine how circulating apoE impacts brain amyloid deposition and blood cholesterol levels in transgenic mice carrying AD-promoting APP and PS1 human transgenes—either with or without the endogenous mouse apoE gene. ApoE transferred through the joined circulations from WT to parabiosed APP+/+,PS1+/−,apoE-KO mice prevented hypercholesterolemia and reduced already low brain amyloid deposition. The findings indicate that apoE synthesis in the brain itself is necessary for amyloid accumulation. Furthermore, plasma apoE can both normalize cholesterol levels in apoE-KO mice and act as a peripheral sink to induce net efflux of Aβ peptide from the brain. The therapeutic implication is that inhibiting Alzheimer's disease neuropathology may be accomplished by either reducing apoE in the brain or increasing apoE in the blood.
Keywords: Apolipoprotein E (apoE), Parabiosis, Amyloid, Alzheimer's disease, Atherosclerosis, Blood
INTRODUCTION
Amyloid deposits composed primarily of amyloid-beta (Aβ) peptide are a key feature of Alzheimer's disease (AD) and are associated with the neuronal degeneration that underlies clinical dementia. Aβ's central role in the Alzheimer's pathogenic pathway was demonstrated by the identification of familial AD-causing mutations in the amyloid precursor protein (APP) gene, which affect the production and/or structure of Aβ (15).
Several other proteins are also found in AD amyloid deposits, some of which have been dem onstrated to bind to Aβ and promote amyloid filament formation. The best characterized of these amyloid promoters (pathological chaperones) are α1-antichymotrypsin (ACT) and apolipoprotein E (apoE). We and others have shown that both of these proteins promote efficient Aβ polymerization in vitro and in transgenic mouse models of AD (1,2,6,12,16,18,21–23,27,28,30,31,34,37,38). Of particular importance is that the apoE4 isoform shows greater amyloid-promoting activity than apoE3, and apoE2 is protective. Thus, the amyloid-promoting abilities of different isoforms of apoE can explain why human apoE4 and E2 carriers are at three times higher and two times lower risk, respectively, of developing AD symptoms and amyloid pathology (17,29,35,36).
Both ApoE and ACT levels are increased in the blood of AD patients (3,19,20), and apoE has been shown to shuttle Aβ across the blood–brain barrier in both directions (7,24). Therefore, apoE in the circulation may influence amyloid deposition and associated AD pathology in the brain. In this article, we used the technique of parabiosis to determine whether apoE in the peripheral circulation plays a pathogenic or protective role in AD amyloid deposition.
Surgical parabiosis can be used to fuse the peripheral circulations of two animals and allow the exchange of circulating cells and molecules (13,25). We employed this technique to investigate the effect of circulating apoE on two processes—brain amyloid deposition and blood cholesterol homeostasis—in a transgenic model of Alzheimer's disease. Each parabiosed mouse pair consisted of one that was transgenic for two Alzheimer's-promoting human genes (APPV717F and PS1M146L) and lacked the mouse apoE gene. The other mouse had the same two FAD transgenes but retained the mouse apoE gene.
Comparison of parabiosed and control mice allowed us to determine whether the pathological chaperone apoE can be derived from the blood or must be synthesized within the brain itself to promote amyloid deposition—or alternatively, whether circulating apoE can act as a peripheral sink, drawing Aβ from the brain and thereby reducing amyloid accumulation.
MATERIALS AND METHODS
Procedures were approved by the Institutional Animal Care and Use Committee in compliance with the Guide for the Care and Use of Laboratory Animals. Animals were housed in shoe box cages with static microisolator tops under climate-controlled conditions on a 12-h light/12-h dark cycle, fed Harlan Teklad Global Diet #2018 and tap water ad libitum. The animal facility maintains a specific pathogen-free status based on a sentinel system.
Parabiosis
Six-week-old siblings of the same sex were selected for parabiosis. Both animals were transgenic for APP (PDGF-hAPPV717F, human mutant amyloid precursor protein) and PS1 (PDGF-hPS1M146L, human mutant presenilin-1); one parabiont was apoE+/−, the other was apoE−/−. Animals were anesthetized with ketamine 100 mg/kg, xylazine 20 mg/kg, and acepromazine 3 mg/kg, placed in a parallel orientation, and a left lateral incision was made on one mouse while a right one was made on the partner mouse, extending from the base of the ear toward the middle of the femur of the extended pelvic extremity. The incision included skin and muscle along thorax and abdomen. Starting at the last rib, the opening was extended into the abdominal cavities to accomplish convergence. The peritonea and muscle layers of the two animals were joined by simple interrupted suture with 4–0 PDS® (polydioxanone) and the skin closed via stainless steel clips. The animals were allowed to recover in a warm, clean environment before being transferred into the husbandry area. Prophylactic antibiotic treatment (enrofloxacin, 5 mg/kg) was started 1 day prior to surgery and continued for 3 days. All animals received analgesic/anti-inflammatory treatment (acetyl salicylic acid 5 mg/kg) for 14 days.
Immunohistochemical Procedures
At 7 months, parabiosed mice were fasted overnight, anesthetized with Nembutal (0.1 mg/g body weight) and blood was collected by cardiac puncture, immediately supplemented with 0.1% (w/v) EDTA, and centrifuged (2000 × g, 15 min). Total plasma cholesterol was measured with a colorimetric assay (Infinity Cholesterol Reagent procedure 401, Sigma). The animals were intracardially per-fused with 0.9% NaCl (25 ml) followed by 50 ml 4% paraformaldehyde in 1× Sorenson's phosphate buffer. Brains were sequential immersed in cryo-protecting sucrose (10%, 20%, and 30%) and sectioned (25 μm) by sledge microtome. The mounted brain sections were processed through antigen retrieval in prewarmed 25 mM citrate buffer (pH 7.3) at +82°C for 5 min and further processed as previously described (28). Sections were incubated with primary antibodies against Aβ (6E10, diluted 1:5000, Signet and rAβ40, diluted 1:3000, QCB) and apoE (AB947, diluted 1:5000, Chemicon) overnight at +4°C. Immunostaining was visualized with anti-rabbit IgG or anti-mouse IgG (1:300) and a NovaRED substrate kit (Vector). For each mouse, data were collected from three equally spaced coronal tissue sections for both dorsal hippocampus and overlying parietal cortex (bregma −1.30 to −2.30 mm) using a Nikon Eclipse E1000 microscope with a Retiga 1300 CCD (Qimaging) with a Qimaging RGB LCD-slider. Thioflavine S-staining was performed and visualized with a Nikon BV-2B fluorescence filter cube. Customized software, written in Visual Basic 6.0 (Microsoft) utilizing Auto-Pro function calls (Image Pro Plus, Media Cybernetics) was used to segment and quantify images (5). Aβ deposition was calculated as percent area of interest (=area stainedtot/area measuredtot) from no less than seven microscope fields. Results were analyzed using a two-tailed, unpaired Student's t-test with Welch's correction.
RESULTS
Apolipoprotein E Is Transferred to the Blood of Parabiosed apoE-KO Mice But Does Not Enter the Brain
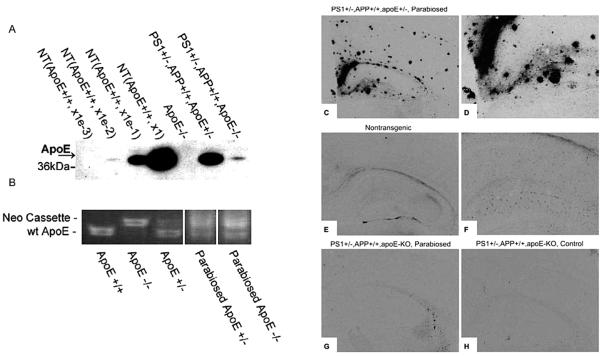
Plasma apoE quantitation by western blot analysis verified that apoE from the APP+/+,PS1+/−, apoE+/− donor parabionts entered the APP+/+,PS1+/−,apoE-KO parabionts and reached a level of 5% of that found in nontransgenic mice (Fig. 1). This rather low steady-state level of apoE protein in the blood of the recipient mice is likely due to the incoming apoE being quickly sequestered by lipoprotein particles from the blood in the apoE-KO mice, leading to lower steady-state levels of apoE. This conclusion is supported by PCR analysis of lymphocyte DNA, which showed nearly equal amounts of apoE DNA in the blood of the parabiosed apoE-KO mice compared to their apoE-containing “donor” partners (Fig. 1B).
Figure 1.
Apolipoprotein E transfer in parabiosed mice. (A) Apolipoprotein E can be detected in plasma of parabiosed apoE-KO (lane 7) but not in a apoE-KO mouse that has not been parabiosed (lane 5). Lane 6 shows a parabiosed partner that is heterozygous for the apoE gene. Lanes 1–4 are 10-fold dilutions of plasma from a nontransgenic mouse. (B) PCR analysis of apoE DNA in blood. Parabiosed APP+/+,PS1+/−,apoE−/− and APP+/+,PS1+/−,apoE+/− mice show an equal extent of murine apoE and neo DNA in blood cells, indicating successful and efficient white blood cell transfer between parabiosed partners. Immunocyto-chemistry shows that apoE is present in amyloid plaques of parabiosed APP+/+,APP+/+,apoE+/− mice (C and high power D) as well in astrocytes of nontransgenic control animals (E, F). In APP+/+,PS1+/−,apoE−/− control mice, no apoE immunoreactivity is detected (H), but parabiosed mice of the same genotypes present with a small amount of staining in choroid plexus, but none in the brain parenchyma (G).
Further analysis showed that circulating apoE does not easily cross the blood–brain barrier to reach the brain parenchyma, thereby limiting its potential to directly impact AD neuropathology. As expected from previous studies, apoE immunoreactivity was detected in virtually all amyloid plaques of parabiosed APP+/+,PS1+/−,apoE+/− (Fig. 1C, D) and in astrocytes even in both transgenic and nontransgenic mice (Fig. 1E, F). However, despite the transfer of parabiosed apoE through the blood, the brains of recipient APP+/+,PS1+/−,apoE-KO mice showed only minor apoE-immunoreactive staining in the choroid plexus (compare Fig. 1G to control Fig. 1H) and none in the parenchyma (Fig. 1G).
Apolipoprotein E Transferred Through Parabiosis Prevents Hypercholesterolemia in apoE-KO Mice
While parabiotically transferred apoE failed to reach the brain, it did have a pronounced effect in the circulation. Mice with targeted disruption of the mouse-apoE gene develop severe hypercholesterolemia and atherosclerosis (25,32). Accordingly, the unoperated APP+/+,PS1+/−,apoE-KO mice used in these experiments showed elevated levels of serum cholesterol compared to nontransgenic, wild-type mice and PS1+/−,APP+/+ mice with the endogenous murine apoE gene (Table 1). Nontransgenic mice had 105 ± 6 mg/dl (n = 19) of total cholesterol in their plasma, while APP+/+,PS1+/− transgenic mice with only one copy of apoE (APP+/+,PS1+/−,apoE+/−) had 79 ± 6 mg/dl (n = 8). In contrast, total cholesterol was approximately four times higher in APP+/+,PS1+/−,apoE-KO mice (392 ± 121 mg/dl, n = 3) and five times higher in apoE-KO mice lacking APP expression (501 ± 39mg/dl, n = 13). In APP+/+,PS1+/−,apoE-KO mice that had been parabiosed with a partner harboring even one copy of the murine apoE gene, cholesterol levels in the apoE knockout mice were reduced almost to normal (125 mg/dl for a 5-month APP+/+,PS1+/−,apoE-KO mouse and 87 mg/dl for a 7-month APP+/+,PS1+/−,apoE-KO mouse, Table 1). This result dramatically underscores the role of apoE in preventing hypercholesterolemia and, of course, confirms that the parabiosis had indeed transferred physiologically functional apoE to the apoE-KO recipient mice.
Table 1.
Apolipoprotein E (apoE) Derived From the Blood Through Parabiosis Restores Hypercholesterolemia in Aβ-Producing apoE Knockout Mice
| Genotype | Age | Total Cholesterol (mg/dl) | n |
|---|---|---|---|
| Control | |||
| Nontransgenic | 7 months | 105 ± 6 | 15 |
| PS1−/−,APP+/+,apoE+/− | 7 months | 73 ± 8 | 7 |
| PS1+/−,APP+/+,apoE+/− | 7 months | 79 ± 6 | 8 |
| PS1+/−,APP+/+,apoE−/− | 7 months | 392 ± 121 | 3 |
| ApoE−/− | 7 months | 501 ± 39 | 13 |
| Parabiosed pairs | |||
| 1a. PS1+/−,APP+/+,apoE+/− | 5 months | 118 | 1 |
| 1b. PS1+/−,APP+/+,apoE−/− | 5 months | 125 | 1 |
| 2a. PS1+/−,APP+/+,apoE+/− | 7 months | 87 | 1 |
| 2b. PS1+/−,APP+/+,apoE−/− | 7 months | 86 | 1 |
| 3a. PS1+/−,APP+/+,apoE+/− | 7 months | 106 | 1 |
| 3b. PS1+/−,APP+/+,apoE−/− | 7 months | 90 | 1 |
| 4a. PS1+/−,APP+/+,apoE+/− | 5 months | 89 | 1 |
| 4b. PS1+/−,APP+/+,apoE−/− | 5 months | 90 | 1 |
Apolipoprotein E That Is Exclusively Present in the Peripheral Circulation Is Unable to Promote Brain Amyloid Deposition
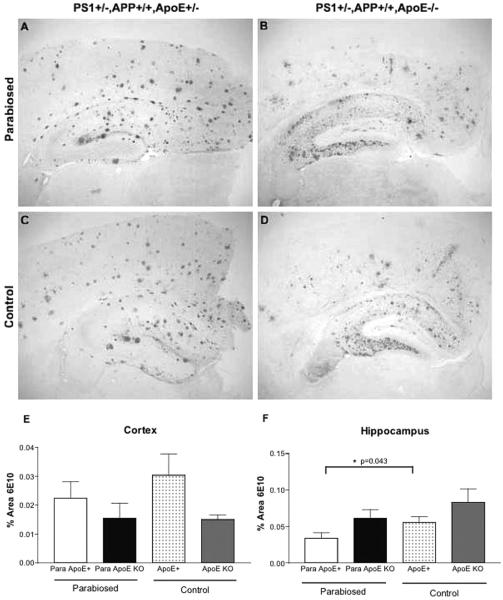
Immunohistochemical analysis of total Aβ immunoreactivity in brain sections after 7 months of parabiosis revealed only minor differences between the parabiosed apoE-KO partners and their genetically identical nonparabiosed controls in either the cortex or the hippocampus (Fig. 2). This result indicates that apoE present in the peripheral circulation is not sufficient to promote an increase in total brain Aβ deposition in the apoE-KO mice.
Figure 2.
Aβ-immunoreactive (6E10) staining in parabiosed or control APP+/+,PS1+/−,apoE−/− and APP+/+,PS1+/−,apoE+/− mice. Both parabiosed and nonparabiosed APP+/+,PS1+/−,apoE+/− mice show an abundance of amyloid plaques particularly in the cortex (A, C). The Aβ staining in APP+/+,PS1+/−,apoE−/− mice shows essentially only diffuse deposition and is extensive in the hippocampus (B, D). The total Aβ immunostaining is unaffected by parabiosis (2.2 ± 0.5; n = 6) in the cerebral cortex (E), but modestly reduced in parabiosed APP+/+,PS1+/−,apoE+/− mice in the hippocampus, compared to unoperated mice (n = 3) (F).
There also was no statistically significant difference in Aβ burden in cerebral cortex of the parabiosed “donor” apoE+/− mice compared to their nonparabiosed controls and only a slightly reduced level (p = 0.043) of Aβ deposition in the hippocampus of the parabiosed apoE+/− mice—as though transferring apoE to the KO recipient diminished the effective level of this pathological chaperone in the donor. This finding also serves as an essential internal control, indicating that the parabiosis procedure itself was not responsible for the large reduction in amyloid deposition in the apoE “recipient” animal.
Amyloid Plaque Number Is Reduced in Parabiosed Mice Lacking apoE
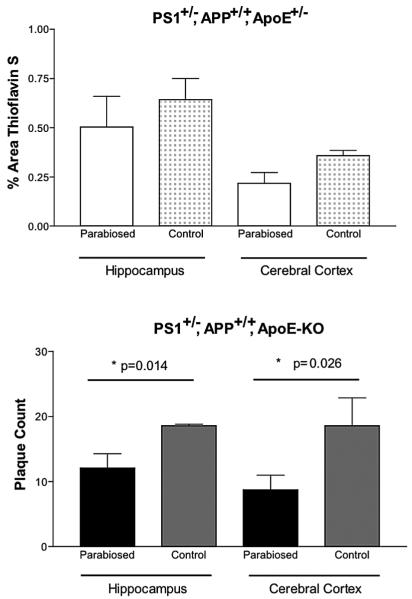
There is far more β-sheet structure in the amyloid deposits in APP+/+,PS1+/− mice expressing endogenous apoE compared to their apoE-KO counterparts (5), indicating that filamentous amyloid formation requires apoE. However, this structural difference is not associated with a quantitative difference in the overall Aβ-immunoreactive burden in old mice, which is equal in AD mice with and without apoE. To compare the levels of mature, compact, amyloid in our parabiosed mice pairs and their respective nonparabiosed controls, thioflavin S staining was performed. No statistically significant difference in the percent area of thioflavin S staining was found between parabiosed APP+/+,PS1+/−,apoE+/− “donor” mice and nonparabiosed control mice of the same genotype (Fig. 3, top). In contrast, only small amounts of compact amyloid were found in apoE-KO mice, confirming that the deposition of compact amyloid requires apoE. The few detectable plaques were counted at 400× magnification. Remarkably, even though apoE-KO mice develop few amyloid plaques, this number was further reduced in APP+/+,PS1+/−,apoE-KO mice that had been parabiosed. Both the hippocampal and cortical regions showed a significant difference between parabiosed APP+/+,PS1+/−,apoE-KO mice (hippocampus 17.2 ± 5.4, n = 6; cortex 13.0 ± 3.0, n = 6) and nonparabiosed APP+/+,PS1+/−, apoE-KO mice (hippocampus 28.3 + 3.0, n = 3; cortex 20.7 ± 5.2, n = 3) in the number of plaques per brain section (Fig. 3, bottom). Thus, the amyloid plaque numbers were 53% and 112% higher for the hippocampus and the cerebral cortex, respectively, in nonparabiosed apoE-KO mice versus parabiosed apoE-KO mice. apoE in the parabiosed circulation appears to reduce plaque numbers, perhaps by a sequestration process.
Figure 3.
Thioflavine S tissue staining of filamentous Aβ. Top: There is no statistically significant difference in thioflavine S staining, by densitometric analysis, in APP+/+,PS1+/−, apoE+/− between parabiosed (n = 6) and control (n = 3) mice in either the hippocampus or cerebral cortex. Bottom: The number of plaques counted per brain section in parabiosed APP+/+,PS1+/−,apoE−/− (n = 6) mice is significantly smaller than that of nonparabiosed control (n = 3) mice of the same genotype in both hippocampus and cerebral cortex.
DISCUSSION
It has long been debated to what extent blood-derived proteins contribute to AD pathology. Many experiments have shown that, in the brain, expression of Aβ and the amyloid-promoting proteins apoE and ACT facilitate plaque deposition in transgenic mouse models of AD. However, these proteins are also produced outside the brain and some are present in large concentrations in the blood, implicating a possible hematogenous origin for amyloidogenic proteins in AD. The frequent presence of cerebral amyloid angiopathy in AD brain is also consistent with vascular involvement in the disease process, and it has been suggested that altered permeability of the blood–brain barrier may increase influx and efflux of amyloidogenic proteins such as apoE and Aβ. It is therefore important to determine if amyloid deposits in the brain are derived, at least in part, from circulating proteins or solely from proteins produced locally in the brain. Moreover, determining the contribution of peripheral proteins to AD pathology might aid the development of therapeutic agents.
Here we show that parabiosis is a viable method for determining whether a given protein in the blood, in this case apoE, is involved in AD amyloidosis. The free exchange of blood between parabiosed animals was demonstrated by PCR analysis. The “recipient” apoE-KO mouse contained two classes of lymphocytes in approximately equal numbers—lymphocytes with intact apoE genes from the “donor” and lymphocytes with the apoE-disrupting neo cassette from the “recipient.” Although the steady-state levels of apoE protein in the recipient mice was relatively low, the transferred apoE was easily sufficient to clear lipoprotein particles and reverse the hypercholesterolemia that is characteristic of these mice. In sum, physiologically functional apoE was transferred to the recipient mouse by parabiosis.
In AD, the onset and extent of amyloid deposition is strongly influenced by apoE gene dosage. Knocking out both mouse apoE genes greatly slows and anatomically redistributes total Aβ deposition in the brain; knocking out a single apoE gene has an intermediate effect (1,2,16,28). Furthermore, almost no filamentous amyloid, characteristic of the cores of mature plaques, is detectable in the absence of apoE (5,16,34,37). Collectively, the data show that apoE is a “pathological chaperone” that is essential for β-sheet amyloid deposition. It was therefore reasonable to anticipate that even a small amount of apoE transferred by parabiosis to an apoE-KO mouse could, if it entered the CNS, significantly promote amyloid pathology. The present experiments confirm the requirement for apoE in amyloid deposition and extend this result by showing that the apoE must be synthesized locally in the brain. ApoE introduced into the circulation of APP-PS-apoE-knockout mice during 7 months of parabiosis failed to enter the brain and promote amyloid deposition.
While brain apoE clearly serves as a promoter of Aβ fibrillization, there is also evidence that peripheral apoE may promote Aβ clearance in certain situations. Such a “peripheral sink hypothesis” was initially introduced to explain the ability of intravenous injections of high-affinity Aβ antibody (passive vaccination against Aβ) to sequester soluble Aβ in the periphery, thereby draining Aβ from the brain and reducing amyloid load in APP transgenic mice (8,9,11). A similar role of apoE as an Aβ-binding, a peripheral sink has been proposed and might apply in the parabiosis experiment. For example, intravenous injections of Aβ, which would normally be quickly cleared by the liver, were not detectably cleared in apoE-KO mice (17), while both anti-Aβ antibodies and apoE were found to sequester Aβ in a dialysis system (10). Furthermore, transgenic mice experiments suggest that human apoE can shift the equilibrium of Aβ between different compartments and favor transport of Aβ out of the brain (7). With respect to the results presented here, the transfer of apoE via parabiosis could similarly shift the Aβ equilibrium towards efflux from the brain, thereby reduced the already very low level of filamentous amyloid in the recipient apoE-KO mice by 50%, as we observed.
The ability of circulating apoE to reduce AD amyloid deposition may be due to its ability to directly bind Aβ (as discussed above), but apoE may also work indirectly by influencing cholesterol metabolism. The hypercholesterolemia characteristic of apoE-knockout mice is clearly not, by itself, sufficient to promote amyloid deposition, as evidenced by the lack of amyloid in the brains of these mice (18,40). However, hypercholesterolemia is a definite risk factor the developing AD (18,31), increasing plasma cholesterol by diet in APP/apoE+ mice increases amyloid deposition, while decreasing plasma cholesterol by statins reduces amyloid deposition (4). Thus, the amyloid-reducing effect of circulating apoE may arise from its dual role as both an Aβ-binding and a cholesterol-transport protein.
Here, we have shown that parabiosis can be successfully employed as a means to investigate the role of circulating proteins in the pathogenesis of a neurodegenerative disease. Our major findings confirm that apoE is a potent amyloid-promoting protein (there are almost no amyloid plaques in the apoE-knockout AD mice) and, most important, show that the apoE must be synthesized in the brain to promote amyloid pathology. That is, the apoE transferred by parabiosis proved unable to enter the brain and promote plaque development. However, the data also demonstrate that apoE in the blood may act as a peripheral sink that can indirectly reduce filamentous amyloid levels in the brain by either favoring the efflux of Aβ into the periphery or possibly by reducing the levels of amyloid-promoting cholesterol. These findings reinforce the idea that agents that are able to either strongly sequester and clear soluble Aβ monomers in the periphery, such as apoE, Aβ antibodies, or other high-affinity Aβ binders could become useful AD therapeutics (26).
Alternatively, agents that decrease amyloid-promoting apoE in the brain without reducing plasma apoE, or that block the interaction between apoE and Aβ, could be very effective inhibitors of amyloid filament formation and deposition, without inducing hypercholesterolemia as a side effect. For example, the 12–28 fragment of Aβ corresponding to the amino acid sequences to which apoE binds can serve as a decoy peptide that prevents the binding of apoE to Aβ and its catalysis of Aβ into neurotoxic species (14,21,22,23). This approach has been successfully extended in vivo by preparing a version of Aβ 12–28 that has a better plasma half-life and is nonfibrillogenic/nontoxic. This therapeutic peptide could be peripherally introduced into a transgenic APP mouse, where it effectively entered the brain and prevented/reversed oligomer formation, amyloid deposition, and cognitive decline (32,33,39).
In sum, apoE is both an essential catalyst of Aβ polymerization and deposition in the brain and a peripheral sink, able to sequester Aβ and aid its removal from the blood. Promising therapies for AD are being developed that take advantage of these dual roles of apoE.
ACKNOWLEDGMENTS
Funding provided by Alzheimer's Association grant IIRG-013111 the Eric Pfeiffer Chair for Research on Alzheimer's Disease and National Institute on Aging grants AG09665 and AG037942.
Footnotes
The authors have no conflicts of interest.
REFERENCES
- 1.Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM. Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat. Genet. 1997;17(3):254–256. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 2.Bales KR, Liu F, Wu S, Lin S, Koger D, DeLong C, Hansen JC, Sullivan PM, Paul SM. Human APOE isoform-dependent effects on brain b-amyloid lev els in PDAPP transgenic mice. J. Neurosci. 2009;29(21):6771–6779. doi: 10.1523/JNEUROSCI.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blain H, Jeandel C, Merched A, Visvikis S, Siest G. Apolipoprotein E level in cerebrospinal fluid increases with aging. J. Am. Geriatr. Soc. 1997;45(12):1536. doi: 10.1111/j.1532-5415.1997.tb03211.x. [DOI] [PubMed] [Google Scholar]
- 4.Burns M, Duff K. Use of in vivo models to study the role of cholesterol in the etiology of Alzheimer's disease. Neurochem. Res. 2003;28(7):979–986. doi: 10.1023/a:1023294820218. [DOI] [PubMed] [Google Scholar]
- 5.Costa DA, Nilsson LN, Bales KR, Paul SM, Potter H. Apolipoprotein is required for the formation of filamentous amyloid, but not for amorphous Abeta deposition, in an AbetaPP/PS double transgenic mouse model of Alzheimer's disease. J. Alzheimers Dis. 2004;6(5):509–514. doi: 10.3233/jad-2004-6508. [DOI] [PubMed] [Google Scholar]
- 6.Christie RH, Bacskai BJ, Zipfel WR, Williams RM, Kajdasz ST, Webb WW, Hyman BT. Growth arrest of individual senile plaques in a model of Alzheimer's disease observed by in vivo multiphoton microscopy. J. Neurosci. 2001;21(3):858–864. doi: 10.1523/JNEUROSCI.21-03-00858.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE iso-form-specific disruption of amyloid beta peptide clearance from mouse brain. J. Clin. Invest. 2008;118(12):4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2001;98(15):8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: A measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science. 2002a;295(5563):2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 10.DeMattos RB, Bales KR, Parsadanian M, O'Dell MA, Foss EM, Paul SM, Holtzman DM. Plaque-associated disruption of CSF and plasma amyloid-beta (Abeta) equilibrium in a mouse model of Alzheimer's disease. J. Neurochem. 2002b;81(2):229–236. doi: 10.1046/j.1471-4159.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- 11.Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat. Neurosci. 2002;5(5):452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 12.Dodart JC, Marr RA, Koistinaho M, Gregersen BM, Malkani S, Verma IM, Paul SM. Gene delivery of human apolipoprotein E alters brain Abeta burden in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2005;102(4):1211–1216. doi: 10.1073/pnas.0409072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finerty JC. Parabiosis in physiological studies. Physiol. Rev. 1952;32:277–302. doi: 10.1152/physrev.1952.32.3.277. [DOI] [PubMed] [Google Scholar]
- 14.Hao J, Zhang W, Zhang P, Liu R, Liu L, Lei G, Su C, Miao J, Li Z. Abeta20–29 peptide blocking apoE/Abeta interaction reduces full-length Abeta42/40 fibril formation and cytotoxicity in vitro. Neuropeptides. 2010;44(4):305–313. doi: 10.1016/j.npep.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 16.Holtzman DM, Fagan AM, Mackey B, Tenkova T, Sartorius L, Paul SM, Bales K, Ashe KH, Irizarry MC, Hyman BT. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer's disease model. Ann. Neurol. 2000;47(6):739–747. [PubMed] [Google Scholar]
- 17.Hone E, Martins IJ, Fonte J, Martins RN. Apolipo-protein E influences amyloid-beta clearance from the murine periphery. J. Alzheimers Dis. 2003;5(1):1–8. doi: 10.3233/jad-2003-5101. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63(3):287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Licastro F, Parnetti L, Morini MC, Davis LJ, Cucinotta D, Gaiti A, Senin U. Acute phase reactant alpha 1-antichymotrypsin is increased in cerebrospinal fluid and serum of patients with probable Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1995;9(2):112–118. doi: 10.1097/00002093-199509020-00009. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman J, Schleissner L, Tachiki KH, Kling AS. Serum alpha 1-antichymotrypsin level as a marker for Alzheimer-type dementia. Neurobiol. Aging. 1995;16(5):747–753. doi: 10.1016/0197-4580(95)00056-k. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q, Wu WH, Fang CL, Li RW, Liu P, Lei P, Hu J, Sun X, Zheng YZ, Zhao YF, Li YM. Mapping ApoE/Abeta binding regions to guide inhibitor discovery. Mol. Biosyst. 2011;7(5):1693–1700. doi: 10.1039/c1mb05019b. [DOI] [PubMed] [Google Scholar]
- 22.Ma J, Yee A, Brewer HB, Jr., Das S, Potter H. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994;372(6501):92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- 23.Ma J, Brewer HB, Jr., Potter H. Alzheimer A-beta neurotoxicity: Promotion by antichymotrypsin, ApoE4; inhibition by A beta-related peptides. Neurobiol. Aging. 1996;17(5):773–780. doi: 10.1016/0197-4580(96)00112-1. [DOI] [PubMed] [Google Scholar]
- 24.Martel CL, Mackic JB, Matsubara E, Governale S, Miguel C, Miao W, McComb JG, Frangione B, Ghiso J, Zlokovic BV. Isoform-specific effects of apolipoproteins E2, E3, and E4 on cerebral capillary sequestration and blood–brain barrier transport of circulating Alzheimer's amyloid beta. J. Neurochem. 1997;69(5):1995–2004. doi: 10.1046/j.1471-4159.1997.69051995.x. [DOI] [PubMed] [Google Scholar]
- 25.Martinez C, Aust JB, Smith JM, Mariani A, Good RA. Acquired tolerance to homologous transplantation of normal and neoplastic tissues in inbred mice. Acta Unio Int. Contra Cancrum. 1959;15:960–970. [PubMed] [Google Scholar]
- 26.Matsuoka Y, Saito M, LaFrancois J, Saito M, Gaynor K, Olm V, Wang L, Casey E, Lu Y, Shiratori C, Lemere C, Duff K. Novel therapeutic approach for the treatment of Alzheimer's disease by peripheral administration of agents with an affinity to beta-amyloid. J. Neurosci. 2003;23(1):29–33. doi: 10.1523/JNEUROSCI.23-01-00029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson LN, Bales KR, DiCarlo G, Gordon MN, Morgan D, Paul SM, Potter H. Alpha-1-antichymotrypsin promotes beta-sheet amyloid plaque deposition in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 2001;21(5):1444–1451. doi: 10.1523/JNEUROSCI.21-05-01444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson LN, Arendash GW, Leighty RE, Costa DA, Low MA, Garcia MF, Cracciolo JR, Rojian A, Wu X, Bales KR, Paul SM, Potter H. Cognitive impairment in PDAPP mice depends on ApoE and ACT-catalyzed amyloid formation. Neurobiol. Aging. 2004;25(9):1153–1167. doi: 10.1016/j.neurobiolaging.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Plump AS, Smith JD, Hayek T, Aalto-Set?l? K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 30.Potter H, Wefes IM, Nilsson LN. The inflammation-induced pathological chaperones ACT and apoE are necessary catalysts of Alzheimer amyloid formation. Neurobiol. Aging. 2001;22(6):923–930. doi: 10.1016/s0197-4580(01)00308-6. [DOI] [PubMed] [Google Scholar]
- 31.Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer's disease: Allelic variation and receptor interactions. Neuron. 1993;11(4):575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 32.Sadowski M, Pankiewicz J, Scholtzova H, Ripellino JA, Li Y, Schmidt SD, Mathews PM, Fryer JD, Holtzman DM, Sigurdsson EM, Wisniewski TA. Synthetic peptide blocking the apolipoprotein E/beta-amyloid binding mitigates beta-amyloid toxicity and fibril formation in vitro and reduces beta-amyloid plaques in transgenic mice. Am. J. Pathol. 2004;165(3):937–948. doi: 10.1016/s0002-9440(10)63355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadowski M, Pankiewicz J, Scholtzova H, Mehta PD, Prelli F, Quartermain D, Wisniewski T. Blocking the Apolipoprotein E/Amyloid-b interaction as a potential therapeutic approach for Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2006;103(49):18787–18792. doi: 10.1073/pnas.0604011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanan DA, Weisgraber KH, Russell SJ, Mahley RW, Huang D, Saunders A, Schmechel D, Wisniewski T, Frangione B, Roses AD, Strittmatter WJ. Apolip oprotein E associates with beta amyloid peptide of Alzheimer's disease to form novel monofibrils. Isoform apoE4 associates more efficiently than apoE3. J. Clin. Invest. 1994;94(2):860–869. doi: 10.1172/JCI117407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a conse quence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1993;90(20):9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1993;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wisniewski T, Castano EM, Golabek A, Vogel T, Frangione B. Acceleration of Alzheimer's fibril formation by apolipoprotein E in vitro. Am. J. Pathol. 1994;145(5):1030–1035. [PMC free article] [PubMed] [Google Scholar]
- 38.Wisniewski T, Lalowski M, Golabek AA, Vogel T, Frangione B. Is Alzheimer's disease an apolipoprotein E amyloidosis? Lancet. 1995;345(8955):956–958. doi: 10.1016/s0140-6736(95)90701-7. [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Ji Y, Mehta P, Bates KA, Sun Y, Wisniewski T. Blocking the apolipoprotein E/amyloid-β interaction reduces fibrillar vascular amyloid deposition and cerebral microhemorrhages in TgSwDI mice. J. Alzheimers Dis. 2011;24(2):269–285. doi: 10.3233/JAD-2011-101401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]