Abstract
Background
Protein carbamylation, a post-translational modification promoted during uremia and catalyzed by myeloperoxidase (MPO) at sites of inflammation, is linked to altered protein structure, vascular dysfunction, and poor prognosis. We examine the relationship between plasma protein-bound homocitrulline (PBHCit) levels, a marker of protein lysine residue carbamylation, with cardio-renal function and long-term outcomes in chronic systolic heart failure.
Methods and Results
In 115 patients with chronic systolic HF (LVEF≤35%), we measured plasma PBHCit by quantitative mass spectrometry and performed comprehensive echocardiography with assessment of cardiac structure and performance. Adverse long-term events (death, cardiac transplant) were tracked for 5 years. In our study cohort, the median PBHCit level was 87 [IQR: 59, 128] μmol/mol Lysine. Higher plasma PBHcit levels were associated with poorer renal function (eGFR Spearman’s r= −0.37, p<0.001); cystatin C (r=0.31, p=0.001), and elevated plasma NT-proBNP levels (r= 0.26, 0.006), but not with markers of systemic inflammation or oxidant stress (hsCRP and MPO, p>0.10 for each). Furthermore, elevated plasma PBHCit levels were not related to indices of cardiac structure or function (p>0.10 for all examined) except modestly with increased right atrial volume index (RAVi; r=0.31, p=0.002). PBHCit levels predicted adverse long-term events (Hazard ratio [HR]: 1.8, 95% CI 1.3– 2.6, p<0.001), including following adjustment for age, eGFR, MPO and NT-proBNP (HR: 1.9, 95% CI: 1.2–3.1, p=0.006).
Conclusions
In chronic systolic HF, protein carbamylation is associated with poorer renal but not cardiac function, and portends poorer long-term adverse clinical outcomes even when adjusted for cardio-renal indices of adverse prognosis.
Keywords: Protein-bound homocitrulline, carbamylation, heart failure, kidney, cardio-renal syndrome, oxidative stress
INTRODUCTION
Urea is in equilibrium with a reactive electrophilic species, cyanate, which can post-translationally modify proteins through a process called “carbamylation”1, 2. Protein carbamylation has been linked to altered protein structure, vascular dysfunction, altered drug binding, and poor prognosis3–7. The major target for protein carbamylation is lysine residues, forming N-ε-carbamyllysine (also termed homocitrulline5). Myeloperoxidase (MPO)-catalyzed oxidation of thiocyanate, a relatively abundant plasma anion influenced by dietary intake of foods high in thiocyanate and smoking, has been shown to be an important additional mechanism for cyanate formation and protein carbamylation at sites of inflammation5. Plasma levels of protein-bound homocitrulline (PBHCit) serve as a measure of protein carbamylation and have been shown to predict risk for major adverse cardiac events in patients with relatively preserved renal function5.
Our group and others have previously demonstrated that chronic systolic heart failure is associated with increased circulating MPO levels8–10. We also identified elevated MPO as an important mediator for future development of heart failure11. However in chronic systolic heart failure where cardio-renal compromise commonly occurs, the relationship between plasma PBHCit levels (an MPO-mediated product of protein carbamylation) with cardio-renal function and long-term outcomes has not been examined. Herein, the objective of this study is to examine the relationship between plasma PBHCit levels, as a marker of degree of protein carbamylation, and the development and progression of cardio-renal function and adverse long-term clinical outcomes in chronic systolic heart failure.
METHODS
Study Population
This study was approved by the Cleveland Clinic Institutional Review Board, and all subjects gave informed consent as part of the neurohormonal sub-study of the Assessment of Doppler Echocardiography Prognosis and Therapy (ADEPT) study, a single-center, prospective cohort study examining the natural history of stable but symptomatic chronic systolic heart failure. Subjects enrolled in ADEPT were 18 to 75 years of age, had a diagnosis of heart failure for at least 3 months, a left ventricular (LV) ejection fraction ≤35% at the time of enrollment, New York Heart Association (NYHA) functional class I–IV symptoms, and were free of significant renal, hepatic, and valvular diseases. Estimated glomerular filtration rate (eGFR) was calculated using the standard 4-variable Modification of Diet in Renal Disease equation11. The composite endpoint of adverse clinical events (all-cause mortality and cardiac transplantation) was prospectively tracked for 5 years by prospective telephone follow-up and medical chart review
Comprehensive transthoracic echocardiography was performed as previously described using commercially available HDI 5000 (Phillips Medical Systems, N.A., Bothell, Washington) and Acuson Sequoia (Siemens Medical Solutions USA Inc., Malvern, Pennsylvania) machines. Two-dimensional and color Doppler imaging was performed in standard parasternal and apical views. Diastolic indices (including pulse-wave Doppler, color M-mode [CMM], and tissue Doppler imaging) were acquired over ten consecutive beats using sweep speeds of 50 and 100 cm/s using previously described techniques and diastolic staging. The LV ejection fraction and cardiac volumes were measured using Simpson’s biplane method. LV mass was calculated according to previously published recommendations. All ventricular volume and mass measurements were indexed to body surface area (BSA). Measurements were averaged over three cycles (five cycles for atrial fibrillation).
Assay Measurements
Quantification of PBHcit in plasma was performed using stable isotope dilution high performance liquid chromatography (HPLC) with online electrospray ionization tandem mass spectrometry on an AB SCIEX QTRAP 5500 mass spectrometer, as previously described 5. Briefly, plasma proteins were desalted, delipidated, [13C615N2] Lys and synthetic [13C6] homocitrulline added as internal standards, and proteins hydrolyzed over night with HCl , prior to HPLC MS/MS analysis. Results are expressed as a ratio of μmol protein bound homocitrulline to moles of the precursor, lysine (μmol/mol Lys) 5. In a cohort of self-reported healthy subjects (n=90), the median value of PBHcit was 109 μmol/mol Lys (interquartile range 93–138 μmol/mol Lys).
Amino-terminal pro-B-type natriuretic peptide (NT-proBNP) was determined by a commercially available assay (Roche Elecsys® proBNP assay, Roche Diagnostics, Indianapolis IN). Inter-assay coefficients of variation were 8–15%; intra-assay coefficients of variation were 6–8%. Plasma MPO levels were determined by CardioMPO II™ test (Cleveland Heart Lab, Inc, Cleveland, OH), with a minimum limit of quantification of 30 pM, intra-assay and inter-assay CVs of < 5%. In a cohort of self-reported healthy subjects (n=294), the median of MPO was 193 pM (interquartile range of 138 to 288 pM). All laboratory analyses were performed with investigators blinded to clinical outcomes. Plasma cystatin C (CysC) levels were determined by the N Latex cystatin C assay (Dade-Behring, Deerfield IL), a latex-enhanced nephelmetric immunoassay using rabbit polyclonal antibodies Intra-assay and inter-assay CVs were < 1.8%.
Statistical Analyses
Continuous variables were summarized as mean ± standard deviation if normally distributed and median and interquartile ranges (IQR) if non-normally distributed. Spearman’s rank correlation was used as non-parametric measure of association between PBHCit levels and clinical and echocardiographic indices. PBHCit levels were compared across categorical variables using the Wilcoxon rank-sum or Kruskal-Wallis test. Kaplan-Meier survival plots and Cox proportional hazards analysis were used to assess the clinical risks associated with higher PBHCit levels (in tertiles or in standard deviation increments, respectively). The optimal cut-off value of 107 μmol/mol Lys was determined by receiver operator characteristic (ROC) curve analysis for composite endpoint. A p-value <0.05 was considered statistically significant. Statistical analyses were performed using JMP 9.0 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Table 1 illustrates the baseline characteristics of the 115 patients in this analysis, with the median PBHCit level being 87 μmol/mol Lys (IQR 59–128 μmol/mol Lys). These values were similar in comparison with that measured in self-reported healthy controls (p=NS). There were also no significant differences in PBHCit levels according to heart failure etiology or neurohormonal antagonist use.
Table 1.
Baseline characteristics (n=115)
| Variable | Value |
|---|---|
|
| |
| Mean Age (years) | 57 ± 14 |
|
| |
| Male (n/%) | 86 (75%) |
|
| |
| NYHA Class ≥ III (n/%) | 38 (33%) |
|
| |
| Ischemic etiology (n/%) | 48 (42%) |
|
| |
| Diabetes mellitus (n/%) | 33 (29%) |
|
| |
| Echocardiographic indices: | |
| LVEDVi (mL/m2) | 110 ± 36 |
| LV ejection fraction (%-units) | 26 ± 6 |
| Mitral E/septal Ea ratio | 16 [12, 22] |
| RA volume index (mL/m2) | 26 [17, 38] |
| Diastolic stage III (n/%) | 43 (38%) |
|
| |
| Baseline medications: | |
| ACE inhibitors or ARBs (n/%) | 105 (95%) |
| Beta-blockers (n/%) | 69 (61%) |
| Spironolactone (n/%) | 33 (31%) |
| Loop diuretics (n/%) | 89 (78%) |
|
| |
| Laboratory data: | |
| eGFR (mL/min/1.73 m2) | 69 ± 26 |
| NT-proBNP (pg/mL) | 1,252 [529, 3,431] |
| Blood urea nitrogen (mg/dL) | 20 [16, 19] |
| Myeloperoxidase (pM) | 305 [260, 425] |
| Protein Bound Homocitrulline (μmol/mol Lys) | 87 [59, 128] |
|
| |
| Cystatin C (mg/L) | 1.22 [0.99, 1.65] |
Abbreviation: LVEDVi, left ventricular end-diastolic volume indexed; LV = left ventricular, RA = right atrial, NT-proBNP = amino-terminal pro-B-type natriuretic peptide; eGFR = estimated glomerular filtration rate
Protein-Bound Homocitrulline and Cardio-Renal and Inflammatory Biomarkers
Increasing levels of PBHCit correlated with measures of myocardial stress including elevated NT-proBNP, as well as with indices of renal dysfunction, including increasing cystatin C and reduced eGFR levels (Table 2). However, there were no statistically significant associations between PBHcit and inflammatory biomarkers such as MPO or hsCRP (Table 2). Elevated plasma PBHCit levels were not related to indices of cardiac structure or function (p>0.10 for all), except modestly with increased right atrial volume index (RAVi; r=0.31, p=0.002) including after adjustment for eGFR (Standardized β = 0.26, p=0.027). Subjects with right ventricular systolic pressure (RVSP) ≥32 mmHg (median cut-off) demonstrated higher PBHcit levels (104 [64, 154] vs 79 [59, 111] μmol/mol Lys, p=0.035), and PBHcit levels directly correlated with RVSP (r= 0.26, p=0.017). In comparison, subjects with larger inferior vena cava diameter >2.1 cm or IVC collapse <50% with sniff test only trended towards higher PBHcit levels (p=0.10 and p=0.06, respectively).
Table 2.
Correlations between Plasma Protein-Bound Homocitrulline Levels and Cardio-Renal Indices and Inflammatory Biomarkers.
| Variable | Spearman’s r | P value |
|---|---|---|
|
| ||
| Age (years) | 0.13 | 0.18 |
|
| ||
| Systolic blood pressure (mmHg) | − 0.18 | 0.08 |
|
| ||
| NYHA class | 0.19 | 0.04 |
|
| ||
| Echocardiographic indices: | ||
| LV ejection fraction | − 0.15 | 0.12 |
| Mitral E/septal Ea | 0.08 | 0.41 |
| LVEDVi | 0.10 | 0.34 |
| RA volume index | 0.31 | 0.002 |
|
| ||
| Cardiac biomarkers: | ||
| NT-proBNP (pg/mL) | 0.26 | 0.006 |
|
| ||
| Renal biomarkers: | ||
| eGFR (mL/min/1.73m2) | − 0.37 | <0.001 |
| Cystatin C (mg/L) | 0.31 | 0.001 |
| Blood urea nitrogen (mg/dL) | 0.46 | <0.001 |
|
| ||
| Inflammatory biomakers | ||
| Myeloperoxidase (pM) | 0.01 | 0.966 |
| hsCRP (pg/L) | − 0.16 | 0.102 |
Abbreviations: as in Table 1.
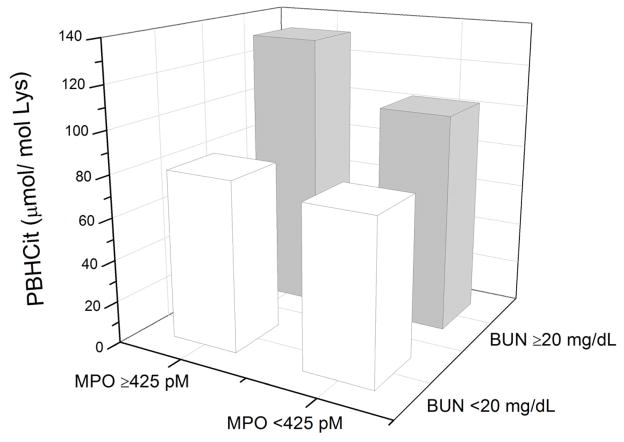
When stratified according to high versus low plasma MPO and serum blood urea nitrogen (BUN) levels, there was a noted increase in PBHcit levels in those with both elevated MPO and BUN levels compared to other subsets (Figure 1).
Figure 1.
Relationship between Plasma Myeloperoxidase and Blood Urea Nitrogen (BUN) in Median Levels of Protein-Bound Homocitrulline (PBHcit)
Protein-Bound Homocitrulline and Adverse Outcomes
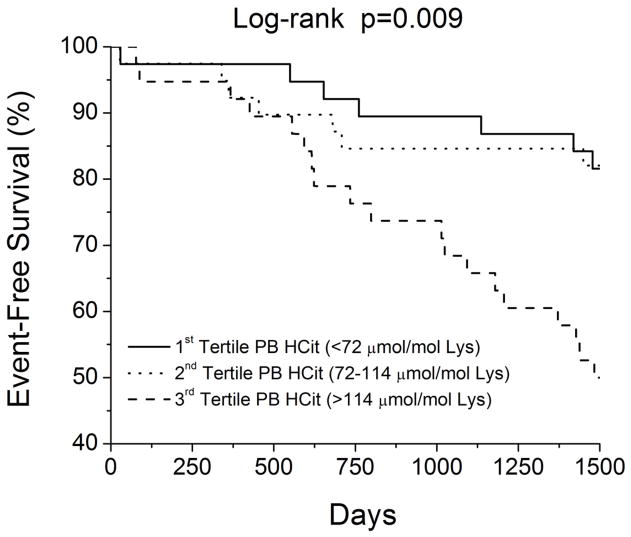
In our study cohort, a total of 40 subjects reached the composite endpoint of all-cause mortality or cardiac transplantations. Increasing PBHCit predicted increased risk of death or cardiac transplantation (unadjusted Hazard ratio [HR] per standard deviation increment of Logn PBHcit = 1.79, 95% confidence interval [CI] 1.26 – 2.57, p<0.001). Elevated PBHCit levels predicted adverse clinical events when analyzed as in tertiles, with the upper tertile portending the greatest mortality risk (Figure 2). Upon adjustment for age, eGFR, MPO, and NT-proBNP, elevated PBHCit remained an independent risk factor for adverse clinical events (adjusted HR = 1.85, 95%CI 1.18–3.09, p=0.047). Substituting cystatin C for eGFR in the multivariate model, PBHCit still remained significant even though the Hazard ratio was attenuated (adjusted HR = 1.47, 95%CI 1.01 – 2.22, p=0.043). This also holds true when adjusted for BUN (adjusted HR = 1.63, 95%CI 1.05–2.64, p=0.028). Furthermore, subjects with both elevated PBHcit and MPO (stratified by median values) demonstrated the poorest long-term outcomes, while those with lower PBHcit despite elevated MPO had more favorable outcomes (Figure 3).
Figure 2.
Prognostic value of Protein-Bound Homocitrulline (PBHcit) in Chronic Systolic Heart Failure stratified by Tertiles
Figure 3.
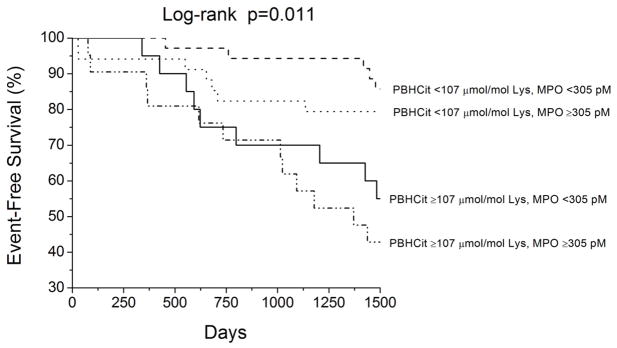
Relative Prognostic value of Protein-Bound Homocitrulline (PBHcit) and Plasma Myeloperoxidase (MPO) in Chronic Systolic Heart Failure
DISCUSSION
There has been recognition that carbamylated proteins formed in the setting of renal insufficiency from the spontaneous decomposition of urea or at sites of inflammation exert a variety of adverse metabolic effects3, 5, 12. There are several key findings from our study, and to our knowledge the first study to examine the association between indices of systemic protein carbamylation and adverse prognosis in chronic systolic heart failure. First, we observed elevated plasma levels of PBHCit levels, an amino acid formed by the carbamylation of the ε-amino groups of lysine residues, were not substantially increased in patients with stable but symptomatic heart failure. Second, increases in PBHcit appear to relate more with renal than cardiac impairment, as there were no significant associations with both systolic and diastolic function assessed by transthoracic echocardiography. Third, the presence of elevated PBHcit levels portends poorer long-term survival in patients with heart failure, even when adjusting for standard cardio-renal covariates, although it did not remain significant when adjusted for BUN as it depends on the process of protein carbamylation linked with urea. Although there was a lack of association between PBHcit and systemic MPO mass measurement, patients with elevation of both levels portend the highest risk of adverse outcomes, and interestingly higher levels of PBHcit was observed in subjects with both elevated MPO and BUN. These findings are suggestive that process of protein carbamylation may be linked to progressive metabolic derangements, particularly with progressive uremia in the setting of systolic heart failure.
Metabolic defects invariably occur in the setting of end-organ dysfunction such as heart failure or renal failure. Catabolic processes and post-translational modifications of proteins and lipids directly or indirectly affect cellular processes and may prompt further progression of organ dysfunction12, 13. In particular, the presence of elevated BUN has been considered an important biomarker of neurohormonal activation in heart failure14. Our study confirms the close association between BUN and PBHcit with the significant correlation between the two (r=0.46, p<0.001). Nevertheless, it is important to emphasize that the overall degree of “uremia” (as assessed by BUN levels) still largely falls within the normal reference range. That may also explain why PBHcit levels were also not substantially elevated in our heart failure cohort, even though subjects with low levels of PBHcit still demonstrates the most favorable long-term outcomes even in the setting of elevated MPO levels (Figure 3).
Reversing cardio-renal impairment has been a long-standing focus of heart failure management, and has been increasingly targeted for treatments15. However, much emphasis has been put into improving hemodynamic flow to the kidneys without considering the metabolic defects and contributions from renal impairment16. In our study, the prognostic value of PBHcit still holds true when adjusting for renal predictors of adverse long-term outcomes. This observation, coupled with the fact that significant PBHCit levels are observed within subjects with relatively preserved renal function, suggests that inflammation-driven PBHCit formation may in part contributes to the extent of protein carbamylation monitored that is not adequately quantified by indices of systemic inflammation in the present cohort. Interestingly, it was recently reported that protein carbamylation may play a role in the decreased plasma protein binding of some acidic drugs (e.g. loop diuretics or thiazide diuretics)7, which has important implications in heart failure.
The lack of association between circulating MPO mass measurements and PBHCit deserves some discussion. Our group and others have previously identified increased MPO in the setting of heart failure, both in the form of circulating MPO mass and in leukocyte activities and expression. It is therefore conceivable that oxidative targets of MPO such as thiocyanate may lead to more protein carbamylation and thus detectable PBHCit. First, it should be noted that circulating MPO levels are a measure of systemic MPO release from activated leukocytes, not a measure of MPO catalytic activity. Our group has recently demonstrated that PBHCit is a product formed by MPO catalysis at sites of inflammation such as within the chronically inflamed atherosclerotic artery wall, with carbamylation conferring multiple pro-atherosclerotic biological activities5. Plasma MPO levels are generally higher in patients with heart failure8, 10, but our data did not observe any significant association between circulating MPO and systemic levels of PBHcit. Thus, the prognostic values of circulating MPO and PBHcit may reflect independent disease processes beyond their mechanistic link. Nevertheless, in the setting where both measures are elevated, it appears that the propensity to experience future adverse events is significantly higher.
There are several limitations in our study that warrant discussion. First, this is a single-center study with relatively small sample size and event rates, and therefore our multivariate adjustments were limited. Also, this is a relatively young cohort of predominantly male patients with systolic heart failure. It therefore remains to be determined if these findings are readily generalized to a general heart failure population, or to the elderly, females, or HF subjects with preserved systolic function. Further, only a single time point assessment of circulating PBHcit was made, and it is also conceivable that levels can be affected by processes not measured in our study procedures. Despite comprehensive, research-grade echocardiographic evaluation, we do not have any invasive hemodynamic measures, detailed metabolic profiling, or direct measurements of glomerular filtration rate (e.g. iothalamate) or urea levels to better characterize the cardio-renal phenotype. Nevertheless, our findings point to a potentially intriguing concept of a heightened uremic and inflammation driven toxicity in the setting of heart failure that is predominantly renally-related. It is intriguing that Holzer et al recently reported that MPO may also facilitate protein carbamylation indirectly via accelerated decomposition of urea locally at sites of inflammation17. Further investigations into the clinical significance of PBHcit and strategies to modulate mechanisms leading to uremia- and MPO-mediated protein carbamylation are therefore warranted, as levels tend to be higher in the setting of heart failure especially with concomitant renal impairment.
CONCLUSION
In the setting of chronic systolic heart failure, protein carbamylation, as quantified by elevated circulating protein-bound homocitrulline levels, is associated with poorer renal but not left ventricular dysfunction, and portends poorer long-term adverse clinical outcomes even when adjusted for cardio-renal indices.
Acknowledgments
FUNDING
This research was supported by National Institutes of Health grants P01HL076491 and P01HL103453. The GeneBank study has been supported by NIH grants P01HL098055, R01HL103866, R01HL103931, P20HL113452 and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439-06). SLH is also partially supported by a gift from the Leonard Krieger endowment. The main ADEPT study was supported in part by grant funding from American Society of Echocardiography, GlaxoSmithKline Pharmaceuticals, and Roche Diagnostics Inc. Mass spectrometry instrumentation used was housed within the Cleveland Clinic Mass Spectrometry Facility with partial support through a Center of Innovation by AB SCIEX.
Footnotes
DISCLOSURE
Dr. Tang has previously received research grant support from Abbott Laboratories. Mr. Shrestha and Borowski, Drs. Wang, Troughton, and Klein all reported no relationships to disclose. Drs. Hazen and Wang report being listed as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Hazen reports having been paid as a consultant for the following companies: Abbott Diagnostics, Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck & Co., Inc., and Pfizer Inc. Dr. Hazen reports receiving research funds from Abbott, Cleveland Heart Lab, Liposcience Inc., and Pfizer Inc. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Abbott Laboratories, Inc., Cleveland Heart Lab., Esperion, Frantz Biomarkers, LLC, Liposcience Inc., and Siemens. Dr. Wang reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics from Liposcience Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stark GR. Reactions of Cyanate with Functional Groups of Proteins. II. Formation, Decomposition, and Properties of N-Carbamylimidazole. Biochemistry. 1965;4:588–95. doi: 10.1021/bi00879a032. [DOI] [PubMed] [Google Scholar]
- 2.Stark GR, Stein WH, Moore S. Reactions of the Cyanate Present in Aqueous Urea with Amino Acids and Proteins. J Biol Chem. 1960;235:3177–81. [Google Scholar]
- 3.Apostolov EO, Ray D, Savenka AV, Shah SV, Basnakian AG. Chronic uremia stimulates LDL carbamylation and atherosclerosis. J Am Soc Nephrol. 2010;21:1852–7. doi: 10.1681/ASN.2010040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirpal S. Myeloperoxidase-mediated lipoprotein carbamylation as a mechanistic pathway for atherosclerotic vascular disease. Clin Sci (Lond) 2009;116:681–95. doi: 10.1042/CS20080322. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–84. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 6.Horkko S, Savolainen MJ, Kervinen K, Kesaniemi YA. Carbamylation-induced alterations in low-density lipoprotein metabolism. Kidney Int. 1992;41:1175–81. doi: 10.1038/ki.1992.179. [DOI] [PubMed] [Google Scholar]
- 7.Erill S, Calvo R, Carlos R. Plasma protein carbamylation and decreased acidic drug protein binding in uremia. Clin Pharmacol Ther. 1980;27:612–8. doi: 10.1038/clpt.1980.87. [DOI] [PubMed] [Google Scholar]
- 8.Tang WH, Tong W, Troughton RW, Martin MG, Shrestha K, Borowski A, et al. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007;49:2364–70. doi: 10.1016/j.jacc.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 9.Meuwese MC, Stroes ES, Hazen SL, van Miert JN, Kuivenhoven JA, Schaub RG, et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;50:159–65. doi: 10.1016/j.jacc.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Tang WH, Brennan ML, Philip K, Tong W, Mann S, Van Lente F, et al. Plasma myeloperoxidase levels in patients with chronic heart failure. Am J Cardiol. 2006;98:796–9. doi: 10.1016/j.amjcard.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Tang WH, Katz R, Brennan ML, Aviles RJ, Tracy RP, Psaty BM, et al. Usefulness of myeloperoxidase levels in healthy elderly subjects to predict risk of developing heart failure. Am J Cardiol. 2009;103:1269–74. doi: 10.1016/j.amjcard.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaisson S, Pietrement C, Gillery P. Carbamylation-derived products: bioactive compounds and potential biomarkers in chronic renal failure and atherosclerosis. Clin Chem. 2011;57:1499–505. doi: 10.1373/clinchem.2011.163188. [DOI] [PubMed] [Google Scholar]
- 13.Kraus LM, Kraus AP., Jr Carbamoylation of amino acids and proteins in uremia. Kidney Int Suppl. 2001;78:S102–7. doi: 10.1046/j.1523-1755.2001.59780102.x. [DOI] [PubMed] [Google Scholar]
- 14.Kazory A. Emergence of blood urea nitrogen as a biomarker of neurohormonal activation in heart failure. Am J Cardiol. 2010;106:694–700. doi: 10.1016/j.amjcard.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Shrestha K, Tang WH. Cardiorenal syndrome: diagnosis, treatment, and clinical outcomes. Curr Heart Fail Rep. 2010;7:167–74. doi: 10.1007/s11897-010-0025-5. [DOI] [PubMed] [Google Scholar]
- 16.Dupont M, Shrestha K, Tang WH. Revisiting the cardio-renal hypothesis: the pivotal role of the kidney in congestive heart failure. Eur J Heart Fail. 2011;13:820–2. doi: 10.1093/eurjhf/hfr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holzer M, Gauster M, Pfeifer T, Wadsack C, Fauler G, Stiegler P, et al. Protein carbamylation renders high-density lipoprotein dysfunctional. Antioxid Redox Signal. 2011;14:2337–46. doi: 10.1089/ars.2010.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]