Abstract
Background
Supratentorial diffuse low-grade gliomas present a slow macroscopic tumor growth that can be quantified through the measurement of their velocity of diametric expansion. We assessed whether spontaneous velocity of diametric expansion can predict long-term outcomes as a categorical variable and as a continuous predictor.
Methods
A total of 407 adult patients with newly diagnosed supratentorial diffuse low-grade gliomas in adults were studied.
Results
The mean spontaneous velocity of diametric expansion before first-line treatment was 5.8 ± 6.3 mm/year. During the follow-up (mean, 86.5 ± 59.4 months), 209 patients presented a malignant transformation, and 87 died. The malignant progression-free survival and the overall survival were significantly longer in cases of slow velocity of diametric expansion (median, 103 and 249 months, respectively) than in cases of fast velocity of diametric expansion (median, 35 and 91 months, respectively; P < .001). In multivariate analyses, spontaneous velocity of diametric expansion as a categorical variable (<4, ≥4 and <8, ≥8 and <12, ≥12 mm/year) was an independent prognostic factor for malignant progression-free survival (P < .001; hazard ratio, 3.87; 95% confidence interval [CI], 2.67–5.52) and for overall survival (P < .001; hazard ratio, 4.62; 95% CI, 2.58–7.97). Velocity of diametric expansion was also an independent prognostic factor for overall survival as a continuous predictor, showing a linear relationship between overall survival and spontaneous velocity of diametric expansion (hazard ratio, 1.09 per one unit increase; 95% CI, 1.06–1.12; P < .001).
Conclusions
Independent of the molecular status, the spontaneous velocity of diametric expansion allows the identification of rapidly growing diffuse low-grade gliomas (at higher risk of worsened evolution) during the pretherapeutic period and without delaying treatment.
Keywords: diffuse low-grade gliomas, kinetics, malignant transformation, overall survival, velocity of diametric expansion
Supratentorial hemispheric diffuse low-grade gliomas (LGGs), referred to as World Health Organization grade II gliomas,1,2 are a heterogeneous group of tumors with distinct clinical, histological, and molecular characteristics. Diffuse low-grade gliomas affect young adults3 and are characterized by a slow tumor growth,4 even in the silent period,5 and a transformation to a higher grade of malignancy, leading to neurological disability and, ultimately, to death.6,7 Early maximal safe resection while preserving eloquent brain areas with use of perioperative functional monitoring is currently considered as the first-line treatment option.3,8 Indeed, there is a growing trend that the extent of resection is a significant prognostic factor for malignant progression-free survival and overall survival among patients with LGG.7,9,10
The anatomical distribution of LGG can be visualized on conventional magnetic resonance imaging (MRI) using T2-weighted or fluid attenuated inversion recovery sequences.11 The subtle imaging changes over time reflect the macroscopic tumor growth. They can be easily misdiagnosed as stable imaging abnormalities on qualitative analyses and should be quantified. In this way, measurement of the velocity of diametric expansion, estimated from changes in mean tumor diameter over time, can reliably quantify LGG growth.12,13 It has previously been reported that LGGs demonstrate a spontaneous velocity of diametric expansion of 4 mm/year before and after surgical resection.4,14 Of interest, velocity of diametric expansion changes after oncological treatment can predict outcomes in response to chemotherapy and radiotherapy.15–17 Spontaneous velocity of diametric expansion can also reflect the biological underpinnings of LGG.16,18 Taken together, this suggests that the spontaneous velocity of diametric expansion measurement at the time of histological diagnosis may help in predicting the natural history of LGG. Indeed, outcomes of LGG vary considerably in the literature. These wide ranges of prognosis reflect the heterogeneity of LGG and could be explained, at least in part, by the limitations of the histological diagnosis.6 Molecular marker assessment, particularly 1p19q codeletion, p53 expression, and isocitrate dehydrogenase mutation, has improved the diagnosis accuracy and refined prognosis.19 In addition, spontaneous velocity of diametric expansion was shown to represent an independent prognostic parameter for overall survival with the clinical outcomes of LGG with a velocity of diametric expansion of ≥8 mm/year were similar to those of malignant gliomas.6 Thus, spontaneous velocity of diametric expansion is a promising tool to predict LGG behavior for individual patients. This pioneer study addressed only the prognostic significance on overall survival of velocity of diametric expansion as a categorical variable in a subset of 143 LGGs.6 It appears to be logical that the higher the velocity of diametric expansion is, the worse the outcomes are. In addition, the prognostic significance of the velocity of diametric expansion may not be related to those of molecular markers but may add supplemental information. However, no previous study had addressed (i) the prognostic significance of the velocity of diametric expansion as a continuous predictor on malignant transformation and overall survival and (ii) the prognostic relationships between molecular markers and velocity of diametric expansion.
The aim of the present study was to address these specific questions by determining, in a large patient population, whether the spontaneous velocity of diametric expansion was predictive of long-term outcomes of supratentorial LGG in adults, including malignant progression-free survival and overall survival, and whether the spontaneous velocity of diametric expansion was independent of the known molecular markers for LGG.
Materials and Methods
Selection Criteria
We searched the database of a French glioma study group (Réseau d'Etude des Gliomes) for adult patients with an LGG from 1992 through 2011. The minimal following criteria were required: (i) patients >18 years of age at histological diagnosis, (ii) histological diagnosis of World Health Organization grade II gliomas (gemistocytic histology excluded),1,2 (iii) supratentorial hemispheric location, and (iv) available imaging follow-up before first-line oncological treatment (at least 2 MR examinations, minimum 6-week interval) to estimate spontaneous velocity of diametric expansion.12 Central pathology review was performed based on current WHO guidelines for cases diagnosed before 2007.1,2
Estimation of the Imaging Individual Velocities of Diametric Expansion
The spontaneous imaging tumor growth (ie, velocity of diametric expansion) was measured on serial images before first-line treatment. One investigator (L.C.) performed measurements while blinded to individual prognoses and subsequent analyses. The velocity of diametric expansion was determined on T2-weighted and fluid attenuated inversion recovery sequences with use of a methodology previously described.12,13 Detailed methodology is available elsewhere.12 Tumor volumes were calculated using an ellipsoid approximation (volume = D1 × D2 × D3/2) after manual measurements of the 3 largest tumor diameters in 3 orthogonal planes (axial, coronal, and sagittal). The mean tumor diameter was deduced from the volume through the following formula: mean tumor diameter = (2 × volume)1/3. The velocity of diametric expansion (slope of the mean tumor diameter growth curve) was plotted as a function of mean tumor diameter over time.
Procedures
The recorded variables gathered from clinical reports at histological diagnosis included sex, age, seizures, neurological deficit, increased intracranial pressure, Karnofsky performance status, and Chang score.20 The recorded imaging characteristics gathered from longitudinal MRI follow-up included number of cerebral lobes involved, corpus callosum involvement, anatomical location, contrast enhancement,21 and tumor volume at radiological discovery. The recorded neuropathological characteristics included histological subtype, proliferation rates, 1p19q codeletion status, 1p deletion status, 19q deletion status, 10q deletion status, p53 expression, internexin-alpha expression, isocitrate dehydrogenase 1 expression, and epidermal growth factor receptor amplification status. The recorded therapeutic characteristics gathered from clinical reports included first-line treatment (surgical resection, chemotherapy, and radiotherapy), extent of resection,22 postoperative residual tumor,7 oncological treatment at progression and at malignant transformation (surgical resection, chemotherapy, and radiotherapy), and type and number of therapeutic modalities during the whole course of the LGG.
Overall survival was defined as the time from histological diagnosis to death. Malignant progression-free survival was defined as the time from histological diagnosis to demonstration of evidence of malignant transformation or death. Malignant transformation was considered when new contrast enhancement appeared or progressed if originally present or when histologically proven.7 These intervals were censored at the date of last follow-up for survivors. In addition to calculating the time to assessed end points on the basis of the date of histological diagnosis, we also calculated on the basis of the start of symptoms, of the radiological discovery, and of the first-line treatment. The results were not substantially different from those using outcomes measures based on the date of histological diagnosis. Therefore, only the results based on the date of histological diagnosis are presented.
Statistical Analyses
Descriptive results are presented as means ± standard deviation (median, range) for continuous data and percentages for categorical data. Analyses were tailored to address associations among clinical, imaging, neuropathological, molecular and treatment-related variables, and spontaneous velocity of diametric expansion. Univariate analyses were performed using the χ2 or Fisher's exact tests for categorical variables and the unpaired t test or Mann-Whitney rank-sum test for continuous variables, as appropriate. Kaplan-Meier analysis was used for unadjusted survival curves, using log-rank tests to assess significance. To evaluate whether spontaneous velocity of diametric expansion predicted malignant progression-free survival and overall survival, Cox proportional hazards models were constructed, adjusting for predictors previously associated with mortality or malignant transformation in univariate analyses. Specifically, spontaneous velocity of diametric expansion was initially modeled as a categorical variable (<4, ≥4 and <8, ≥8 and <12, ≥12) and then modeled as a continuous predictor to determine whether the relationship between spontaneous velocity of diametric expansion and survival was potentially linear or nonlinear using polynomial regression coefficients. The proportional-hazards assumption was tested with Schoenfeld residuals and was found to hold. A P value <.05 was considered to be statistically significant. All statistical analyses were performed using JMP, version 7.2 (SAS Institute, Cary, NC).
Results
Patients and Tumor Characteristics
Four hundred seven patients (227 males, 180 females) fulfilled eligibility criteria, and 133 (32.7%) of them were previously reported.6 The mean age at histological diagnosis was 39.0 ± 11.0 years (median, 38.0 years; range, 18–77 years). Clinical, imaging, and pathological findings are summarized in Tables 1 and 2. The mean number of MRI performed before treatment, allowing individual mean tumor diameter measurements was 3.5 ± 2 (median, 3.0; range, 2–17) per patient. The mean duration of repeated measurements before treatment was 28.3 ± 32.0 months (median, 15.0 months; range, 1.5–215.7 months). The mean spontaneous velocity of diametric expansion was 5.8 ± 6.3 mm/year (median, 3.9 mm/year; range, 0.2–42.4 mm/year). When applying the cutoff previously described,6 335 patients (82.3%) presented a velocity of diametric expansion slower than 8 mm/year, the known range for LGG (slow velocity subgroup), and 72 patients (17.7%) presented a velocity of diametric expansion at ≥8 mm/year (fast velocity subgroup) (Fig. 1).
Table 1.
Clinical and imaging parameters
| Parameter | Available cases |
Spontaneous VDE |
P value | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Median | Range | ||||
| Sex | 407 | .565 | ||||
| Male | 227 | 5.2 ± 5.1 | 3.8 | 0.2–42.4 | ||
| Female | 180 | 6.4 ± 7.5 | 4.2 | 0.2–35.8 | ||
| Age | 407 | .069 | ||||
| <40 | 257 | 5.8 ± 6.2 | 4.2 | 0.2–42.4 | ||
| ≥40 | 150 | 5.3 ± 6.1 | 3.2 | 0.3–37.2 | ||
| Increased intracranial pressure | 407 | .576 | ||||
| Yes | 33 | 6.5 ± 6.7 | 4.4 | 0.4–28.7 | ||
| No | 374 | 5.7 ± 6.3 | 3.9 | 0.2–42.4 | ||
| Neurological deficit | 407 | .825 | ||||
| Yes | 101 | 5.7 ± 5.9 | 3.8 | 0.2–33.8 | ||
| No | 306 | 5.8 ± 6.5 | 3.9 | 0.2–42.4 | ||
| Seizures | 407 | .245 | ||||
| Yes | 375 | 5.8 ± 6.4 | 3.9 | 0.2–42.4 | ||
| No | 32 | 5.1 ± 5.9 | 3.3 | 0.2–28.7 | ||
| Karnofsky Performance Status | 403 | .798 | ||||
| >70% | 396 | 5.8 ± 6.3 | 3.3 | 1.0–22.0 | ||
| ≤70% | 7 | 6.5 ± 7.2 | 3.9 | 0.2–42.4 | ||
| Cerebral lobes involved | 406 | .282 | ||||
| <2 | 211 | 5.7 ± 6.6 | 3.6 | 0.2–42.4 | ||
| ≥2 | 195 | 5.7 ± 5.7 | 4.2 | 0.2–35.8 | ||
| Corpus callosum involvement | 406 | .001 | ||||
| No | 351 | 5.3 ± 5.7 | 3.7 | 0.2–42.4 | ||
| Yes | 55 | 8.2 ± 8.2 | 5.6 | 0.2–37.2 | ||
| Tumor location | 406 | .06 | ||||
| Frontal | 181 | 6.0 ± 6.8 | 4.2 | 0.2–42.4 | ||
| Temporal | 81 | 5.4 ± 5.5 | 3.8 | 0.2–28.0 | ||
| Insular | 76 | 5.6 ± 4.4 | 4.4 | 0.4–25.2 | ||
| Parietal | 43 | 6.4 ± 8.1 | 3.5 | 0.2–35.9 | ||
| Other | 25 | 3.3 ± 3.4 | 2.3 | 0.7–16.3 | ||
| Contrast Enhancement | 402 | .003 | ||||
| No | 334 | 5.3 ± 5.8 | 3.7 | 0.2–37.1 | ||
| Faint and patchy | 54 | 7.1 ± 7.5 | 4.5 | 0.2–42.4 | ||
| Nodular-like | 14 | 11.2 ± 10.4 | 7.3 | 0.3–34.4 | ||
| Tumor volume | 405 | .003 | ||||
| <100cc | 284 | 5.5 ± 6.4 | 3.6 | 0.2–37.2 | ||
| ≥100cc | 121 | 6.4 ± 6.2 | 4.8 | 0.8–42.4 | ||
| Chang score | 400 | .117 | ||||
| Low-risk patients | 204 | 5.1 ± 5.3 | 3.8 | 0.2–34.4 | ||
| High-risk patients | 196 | 6.3 ± 6.8 | 4.0 | 0.2–42.4 | ||
| Chang number of risk factors | 400 | .193 | ||||
| None | 50 | 4.4 ± 4.4 | 3.8 | 0.2–19.8 | ||
| One | 161 | 5.3 ± 5.5 | 3.7 | 0.2–34.4 | ||
| Two or more | 189 | 6.2 ± 6.9 | 4.0 | 0.2–42.4 | ||
Table 2.
Pathological and molecular parameters
| Parameter | Available cases |
Spontaneous VDE |
P value | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Median | Range | ||||
| Histological subtype | 407 | .891 | ||||
| Astrocytoma | 76 | 5.7 ± 6.6 | 3.7 | 0.2–35.8 | ||
| Oligodendroglioma | 246 | 5.8 ± 6.5 | 4.0 | 0.2–42.4 | ||
| Mixed glioma | 85 | 5.7 ± 5.6 | 3.9 | 0.2–24.3 | ||
| 1p19q codeletion | 205 | .008 | ||||
| Yes | 67 | 4.6 ± 5.7 | 2.9 | 0.3–34.1 | ||
| No | 138 | 6.2 ± 6.1 | 4.6 | 0.2–42.4 | ||
| 1p deletion (complete) | 206 | .009 | ||||
| Yes | 64 | 4.6 ± 5.7 | 2.8 | 0.3–34.1 | ||
| No | 142 | 6.1 ± 6.1 | 4.6 | 0.2–42.4 | ||
| 1p deletion (partial) | 206 | .446 | ||||
| Yes | 17 | 6.3 ± 5.5 | 5.6 | 0.9–21.1 | ||
| No | 189 | 5.6 ± 6.1 | 3.9 | 0.2–42.4 | ||
| 19q deletion | 205 | .173 | ||||
| Yes | 94 | 5.6 ± 6.9 | 3.9 | 0.3–42.4 | ||
| No | 111 | 5.8 ± 5.3 | 4.4 | 0.2–24.3 | ||
| Proliferation rates | 195 | .620 | ||||
| <5% | 124 | 5.7 ± 5.2 | 3.9 | 0.2–31.0 | ||
| ≥5% | 71 | 5.2 ± 6.3 | 3.3 | 0.3–34.4 | ||
| EGFR amplification | 193 | .669 | ||||
| Yes | 183 | 6.7 ± 6.3 | 4.8 | 1.0–19.9 | ||
| No | 10 | 5.9 ± 6.5 | 4.2 | 0.3–42.4 | ||
| 10q deletion | 181 | .649 | ||||
| Yes | 153 | 5.8 ± 5.4 | 4.9 | 0.3–22.6 | ||
| No | 28 | 5.6 ± 6.2 | 3.8 | 0.2–42.4 | ||
| p53 overexpression | 144 | .004 | ||||
| Yes | 64 | 7.7 ± 7.8 | 5.1 | 0.3–34.1 | ||
| No | 80 | 4.5 ± 4.5 | 3.4 | 0.4–31.0 | ||
| Internexin alpha expression | 39 | .955 | ||||
| Yes | 21 | 6.2 ± 7.8 | 3.4 | 0.4–31.0 | ||
| No | 18 | 6.3 ± 9.2 | 3.4 | 0.5–33.8 | ||
| IDH1 expression | 32 | .245 | ||||
| Yes | 25 | 7.3 ± 8.8 | 3.8 | 0.4–24.7 | ||
| No | 7 | 2.9 ± 1.3 | 2.9 | 1.0–5.0 | ||
Fig. 1.
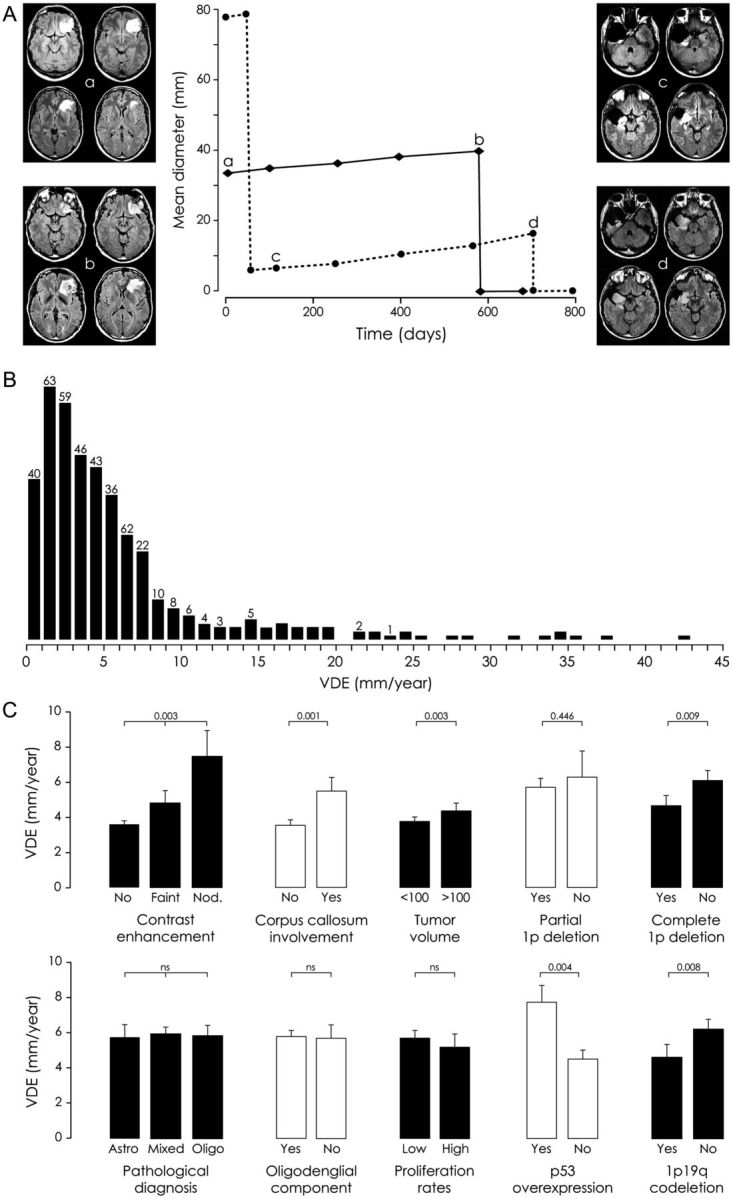
(A) Spontaneous velocity of diametric expansion of 2 diffuse low-grade gliomas (LGGs) through the evolution of their mean tumor diameter over time. Each point represents an MR examination. Before surgical resection, the tumors grew spontaneously and continuously with a spontaneous velocity of diametric expansion at 3.4 mm/year in the case of a left frontal LGG (full line) and at 3.9 mm/year in the case of a right temporo-insular LGG (dotted line). After subtotal surgical resection of the right temporo-insular LGG, the residual tumor grew at a similar range as that at presurgical evaluation (velocity of diametric expansion at 4.3 mm/year). (B) Distribution of the 407 patients by individual velocities of diametric expansion. (C) Imaging, pathological, and molecular factors influencing the spontaneous velocity of diametric expansion of LGGs. Nod. refers to nodular-like pattern of contrast enhancement.
Velocity of Diametric Expansion and Patient Outcome
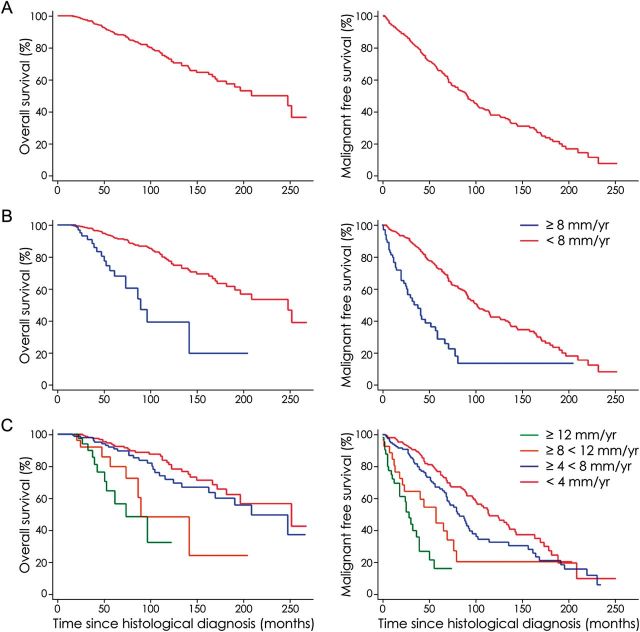
The mean follow-up was 86.5 ± 59.4 months since histological diagnosis (median, 73.0 months; range, 0–269 months). During the follow-up period, 209 patients (51.4%) presented a malignant transformation (histologically proven in 109 cases) at a mean of 108.9 ± 4.7 months (median, 92 months; range, 1–253 months) since histological diagnosis: 159 in the slow velocity subgroup (47.5%) and 50 in the fast velocity subgroup (69.5%). Patients were treated for a malignant transformation at a mean time from histological diagnosis of 74.0 ± 51.1 months (median, 65 months; range, 1–233 months) for the slow velocity subgroup and 29.8 ± 21.8 months (median, 24.5 months; range, 2–82 months) for the fast velocity subgroup. At 5 years, 73.4% of patients of the slow velocity subgroup and 27.7% of patients of the fast velocity subgroup were free of malignant transformation. The malignant progression-free survival was significantly higher in the slow velocity subgroup (median, 103 months; mean, 119.2 months; range, 1–253 months) than in the fast velocity subgroup (median, 35 months; mean, 41.4 months; range, 2–206 months; P < .001). Similarly, patients with velocity of diametric expansion <4 mm/year, with velocity of diametric expansion ≥4 and <8 mm/year, with velocity of diametric expansion ≥8 and <12 mm/year, and with velocity of diametric expansion ≥12 mm/year had a median malignant progression-free survival of 117, 83, 60, and 31 months, respectively. Patients with velocity of diametric expansion <4 mm/year had significantly longer malignant progression-free survival than did patients with velocity of diametric expansion ≥4 mm/year (P < .001). Likewise, patients with velocity of diametric expansion ≥4 and <8 mm/year had significantly longer malignant progression-free survival than did patients with velocity of diametric expansion ≥8 mm/year (P < .001). Likewise, patients with velocity of diametric expansion ≥8 and <12 mm/year had significantly longer malignant progression-free survival than did patients with velocity of diametric expansion ≥12 mm/year (P = .044).
During the follow-up period, 87 patients (21.4%) died of tumor progression at a mean of 183.5 ± 6.0 months (median, 210 months; range, 17–269 months) since histological diagnosis: 65 in the slow velocity subgroup (19.4%) and 22 in the fast velocity subgroup (30.5%). Patients died of tumor progression at a mean time from histological diagnosis of 98.0 ± 56.6 months (median, 89 months; range, 17–253 months) for the slow velocity subgroup and 58.9 ± 31.0 months (median, 50.5 months; range, 22–143 months) for the fast velocity subgroup. At 5 years, 92.8% of patients in the slow velocity subgroup and 69.2% of patients in the fast velocity subgroup were alive. The overall survival from histological diagnosis was significantly higher in the slow velocity subgroup (median, 249 months; mean, 192.6 months; range, 17–253 months) than in the fast velocity subgroup (median, 91 months; mean, 93.9 months; range, 22–206 months; P < .001). Similarly, patients with velocity of diametric expansion <4 mm/year, with velocity of diametric expansion ≥4 and <8 mm/year, with velocity of diametric expansion ≥8 and <12 mm/year, and with velocity of diametric expansion ≥12 mm/year had a median overall survival of 253, 210, 91, and 75 months, respectively. Patients with velocity of diametric expansion <4 mm/year had significantly longer overall survival than did patients with velocity of diametric expansion ≥4 mm/year (P < .001). Likewise, patients with velocity of diametric expansion ≥4 and <8 mm/year had significantly longer overall survival than did patients with velocity of diametric expansion ≥8 mm/year (P < .001). Survival curves are presented in Fig. 2.
Fig. 2.
Kaplan-Meier estimates of overall survival and malignant progression-free survival according to velocity of diametric expansion. (A) Overall survival and malignant progression-free survival in the whole series of 407 supratentorial hemispheric diffuse low-grade gliomas (LGGs) in adults. (B) Overall survival and malignant progression-free survival according to velocity of diametric expansion (cutoff at 8 mm/year). The unadjusted hazard ratio for death among patients harboring an LGG with a spontaneous velocity of diametric expansion ≥8 mm/year, as compared with those harboring an LGG with a spontaneous velocity of diametric expansion <8 mm/year was 3.96 (95% confidence interval [CI], 2.25–6.66; P < .001). The unadjusted hazard ratio for death or malignant progression among patients harboring an LGG with a spontaneous velocity of diametric expansion ≥8 mm/year, as compared with those harboring an LGG with a spontaneous velocity of diametric expansion <8 mm/year was 3.50 (95% CI, 2.44–4.94; P < .001). (C) Overall survival and malignant progression-free survival according to velocity of diametric expansion (cutoffs at 4, 8, and 12 mm/year). The unadjusted hazard ratio for death among patients harboring an LGG with a spontaneous velocity of diametric expansion ≥4 and <8 mm/year, ≥8 and <12 mm/year, and ≥ 12 mm/year, as compared with those harboring an LGG with a spontaneous velocity of diametric expansion <4 mm/year was 1.33 (95% CI, 0.59–1.50; P = .029), 3.41 (95% CI, 1.19–4.99; P < .001), and 5.91 (95% CI, 2.28–8.82; P < .001), respectively. The unadjusted hazard ratio for death or malignant progression among patients harboring an LGG with a spontaneous velocity of diametric expansion ≥4 and <8 mm/year, ≥8 and <12 mm/year, and ≥12 mm/year, as compared with those harboring an LGG with a spontaneous velocity of diametric expansion <4 mm/year was 1.43 (95% CI, 0.78–1.91; P = .07), 2.71 (95% CI, 1.15–3.16; P < .001), and 6.50 (95% CI, 3.23–7.92; P < .001), respectively.
Univariate and multivariate analyses were restricted to the population (n = 386 for overall survival, n = 380 for malignant progression-free survival) with full information on the following parameters: sex, age, seizures, neurological deficit, increased intracranial pressure, Karnofsky performance status, Chang score, number of cerebral lobes involved, corpus callosum involvement, anatomical location, contrast enhancement, tumor volume, spontaneous velocity of diametric expansion, histological subtype, first-line surgical resection, and extent of resection. In univariate analysis, spontaneous velocity of diametric expansion (P < .001), tumor volume (P = .001), corpus callosum involvement (P = .003), contrast enhancement (P = .014), first-line resection (P < .001), and extent of resection (P < .001) were predictive of malignant progression-free survival. Spontaneous velocity of diametric expansion (P < .001), tumor volume (P = .002), and first-line resection (P = .054) were predictive of overall survival. The prognostic parameters for malignant progression-free survival and overall survival in univariate analyses are detailed in Table 3. We established multivariate survival models for overall survival and malignant progression-free survival, including the following prognostic parameters: age, tumor volume, spontaneous velocity of diametric expansion, corpus callosum involvement, contrast enhancement, first-line resection, and extent of resection. Independent prognostic factors for overall survival were tumor volume and spontaneous velocity of diametric expansion, modeled as a categorical variable (<8 or ≥8mm/year)or as a continuous predictor, showing a linear relationship between overall survival and spontaneous velocity of diametric expansion (hazard ratio, 1.09 per one unit increase in velocity of diametric expansion; 95% confidence interval [CI], 1.06–1.12; P < .001). Independent prognostic factors for malignant progression-free survival were tumor volume, contrast enhancement, first-line resection, and spontaneous velocity of diametric expansion. The latter predictor was modeled as a categorical variable (<8 or ≥8 mm/year) or as a continuous variable, showing a second-order polynomial relationship between malignant progression-free survival and velocity of diametric expansion (hazard ratio for spontaneous velocity of diametric expansion: 1.20 [95% CI, 1.13–1.27; P < .001]; hazard ratio for spontaneous velocity of diametric expansion2: 0.997 [95% CI, 0.995–0.999; P = .001]), indicating that the decrease in malignant progression-free survival observed with increasing velocity of diametric expansion progressively reached a plateau for the highest velocity of diametric expansion. Relative risk estimates and CIs are summarized in Table 4. We performed the same analyses in the subgroup of patients (n = 170 for overall survival, n = 197 for malignant progression-free survival) with full information on the 1p19q codeletion status in addition to the selected parameters. Independent prognostic factors for overall survival were spontaneous velocity of diametric expansion and 1p19q codeletion. Independent prognostic factors for malignant progression-free survival were spontaneous velocity of diametric expansion, 1p19q codeletion, tumor volume, contrast enhancement, and first-line resection.
Table 3.
Univariate analyses
| Parameter | n = 386 | Overall survival since histological diagnosis |
|||
|---|---|---|---|---|---|
| Median (months) | P value | Hazard ratio | 95% CI | ||
| Tumor volume | .002 | ||||
| <100cc | 335 | 249 | 1 (ref) | ||
| ≥100cc | 51 | 142 | 2.31 | 1.27–3.96 | |
| Spontaneous VDE | <.001 | ||||
| <8 mm/year | 320 | 249 | 1 (ref) | ||
| ≥8 mm/year | 66 | 91 | 3.96 | 2.25–6.66 | |
| Spontaneous VDE | <.001 | ||||
| <4 mm/year | 196 | 253 | 1 (ref) | ||
| 4 and <8 mm/year | 126 | 210 | 1.33 | 0.59–1.50 | |
| 8 and <12 mm/year | 26 | 91 | 3.41 | 1.19–4.99 | |
| 12 and >mm/year | 40 | 75 | 5.91 | 2.28–8.82 | |
| First-line surgical resection | .054 | ||||
| No | 196 | 210 | 1 (ref) | ||
| Yes | 190 | 249 | 0.74 | 0.47–1.15 | |
| Parameter | n = 380 | Malignant free survival since histological diagnosis | |||
| Corpus callosum involvement | .003 | ||||
| No | 330 | 95 | 1 (ref) | ||
| Yes | 50 | 59 | 1.73 | 1.16–2.50 | |
| Contrast enhancement | .014 | ||||
| No | 317 | 93 | 1 (ref) | ||
| Faint and patchy | 51 | 89 | 1.46 | 1.11–1.88 | |
| Nodular-like | 12 | 39 | 2.19 | 1.22–3.72 | |
| Tumor volume | .001 | ||||
| <100cc | 330 | 93 | 1 (ref) | ||
| ≥100cc | 50 | 48 | 1.90 | 1.26–2.76 | |
| Spontaneous VDE | <.001 | ||||
| <8 mm/year | 316 | 103 | 1 (ref) | ||
| ≥8 mm/year | 64 | 39 | 3.50 | 2.44–4.94 | |
| Spontaneous VDE | <.001 | ||||
| <4 mm/year | 195 | 117 | 1 (ref) | ||
| 4 and <8 mm/year | 123 | 83 | 1.43 | 0.78–1.91 | |
| 8 and <12 mm/year | 25 | 60 | 2.71 | 1.15–3.16 | |
| 12 and >mm/year | 39 | 31 | 6.50 | 3.23–7.92 | |
| First-line surgical resection | <.001 | ||||
| No | 192 | 68 | 1 (ref) | ||
| Yes | 188 | 127 | 0.42 | 0.31–0.57 | |
| Extent of surgical resection | <.001 | ||||
| Biopsy | 192 | 68 | 1 (ref) | ||
| Partial removal | 86 | 103 | 0.59 | 0.22–0.96 | |
| Subtotal removal | 64 | 133 | 0.39 | 0.14–0.78 | |
| Total removal | 38 | . | 0.09 | 0.003–0.17 | |
Table 4.
Multivariate analyses
| Parameter, n = 386 | Overall survival since histological diagnosis |
||
|---|---|---|---|
| P value | Hazard ratio | 95% CI | |
| Tumor volume ≥100 cc | .001 | 2.92 | 1.54–5.16 |
| Spontaneous VDE ≥ 8 mm/year | <.001 | 4.62 | 2.58–7.97 |
| Parameter, n = 380 | Malignant free survival since histological diagnosis | ||
| Tumor volume ≥100 cc | .008 | 1.76 | 1.16–2.60 |
| Spontaneous VDE ≥ 8 mm/year | <.001 | 3.87 | 2.67–5.52 |
| Contrast enhancement | .011 | 1.44 | 1.09–1.87 |
| First line surgical resection | <.001 | 0.44 | 0.32–0.59 |
| Parameter, n = 170 | Overall survival since histological diagnosis | ||
| 1p19q codeletion | .040 | 0.45 | 0.21–0.91 |
| Spontaneous VDE ≥ 8 mm/year | <.001 | 6.25 | 2.80–13.62 |
| Parameter, n = 197 | Malignant free survival since histological diagnosis | ||
| 1p19q codeletion | .021 | 0.59 | 0.37–0.92 |
| Tumor volume ≥100 cc | .031 | 1.87 | 1.06–3.29 |
| Spontaneous VDE ≥ 8 mm/year | <.001 | 3.27 | 1.92–5.53 |
| Contrast enhancement | .049 | 1.58 | 1.01–2.51 |
| First line surgical resection | <.001 | 0.33 | 0.22–0.51 |
Multivariate analyses in the whole population (n = 386 for overall survival, n = 380 for malignant progression-free survival) and in the subgroup with available 1p19q codeletion status (n = 170 for overall survival, n = 197 for malignant progression-free survival).
Velocities of Diametric Expansion and Correlations with Clinical, Imaging, Pathological, and Molecular Findings
Correlations between velocity of diametric expansion and clinical, imaging, pathological, and molecular characteristics are summarized in Tables 1 and 2 and Fig. 1. The spontaneous velocity of diametric expansion was significantly higher in tumors with a corpus callosum involvement (P = .001), with a large volume (P = .003), and with contrast enhancements (P = .003) than were those without such abnormalities. Spontaneous velocity of diametric expansion increased with Chang scoring system and with regard to the number of risk factors of this scoring system20 without reaching significance. The spontaneous velocity of diametric expansion was significantly slower in tumors with 1p19q codeletion (P = .008) and in tumors with complete 1p deletion (P = .042) and was significantly faster in tumors with p53 overexpression (P = .003), compared with those without such abnormalities. The spontaneous velocity of diametric expansion did not significantly differ with regard to types of oncological treatments (first-line, progression, malignancy). In accordance with the observed worsened evolution of LGG with fast velocity of diametric expansion, the spontaneous velocity of diametric expansion measured before oncological treatment was significantly faster in patients who received ≥2 therapeutic modalities (P < .001), who received radiotherapy (P < .001), and who received chemotherapy (P < .001) during the course of the disease.
Discussion
Velocity of Diametric Expansion Prognostic Significance
We show that spontaneous velocity of diametric expansion, reflecting the imaging tumor growth, represents an independent prognostic factor for LGG clinical outcome among adult patients as a continuous predictor and as a categorical variable. Specifically, we find that slow-growing LGGs are less clinically aggressive than rapidly growing LGGs and are associated with better malignant progression-free survival and overall survival, regardless of the histopathological findings and the molecular status. This information was not previously quantified for LGGs. This underlies the heterogeneity of LGGs that are only partially detailed by pathological and molecular analyses. Thus, the early identification of rapidly growing LGGs by measuring the spontaneous velocity of diametric expansion, during the pretherapeutic evaluation period and without delaying oncological treatment, may be useful to overcome biological heterogeneity of LGGs. Here, we did not consider low-grade progression as an end point, because imaging follow-up was not uniform among all the cases in this series and because LGGs in adults are known to show a spontaneous growth in absence of oncological treatment4–6,11,15,16,23,24 and after surgery.14
With regard to malignant progression-free survival, Hlaihel et al. previously demonstrated that an elevated velocity of diametric expansion was associated with a greater risk of malignant transformation.24 Brasil Caseiras et al. previously demonstrated that spontaneous tumor volume increase was an independent predictor of malignant transformation.23 We have previously suggested the confounding prognostic significance of the evolution of the tumor volume (and not mean tumor diameter) over time that combines 2 independent parameters: initial tumor volume and imaging tumor growth rate.25 We suggest that velocity of diametric expansion measurements could be a more reliable parameter than changes in tumor volume to assess LGG imaging tumor growth.12,25 Altogether, these data reinforce spontaneous velocity of diametric expansion as a reliable predictor of early malignant transformation for LGG.
For patient overall survival, we find that histologically proven LGGs with a fast spontaneous velocity of diametric present outcomes closer than those of malignant gliomas. If the 8 mm/year cutoff is easier in clinical practice to identify patients with an LGG at higher risk of worsened evolution, we identified a continuous gradation of velocity of diametric expansion contributing to survival differences (including malignant progression-free survival and overall survival) in this large series in accordance with modeling predictions.26 The measurement of the spontaneous velocity of diametric expansion without delaying oncological treatment will help inform patients about the outcomes and to tailor treatments and imaging follow-up on an individual basis.
Velocity of Diametric Expansion Reflects Tumor-Intrinsic Properties
We show that known imaging risk factors for LGG are associated with faster spontaneous velocity of diametric expansion6,27 and that proliferation rates do not exhibit significant differences in spontaneous velocity of diametric expansion. This underlies that both proliferation rates and tumor cell migration account for LGG imaging growth. Indeed, within the framework of proliferation-diffusion models, the velocity of diametric expansion is proportional to the square root of the product of proliferation rates and cell diffusion coefficient.13 Therefore, tumors with fast spontaneous velocity of diametric expansion, despite mild proliferation rates, should have a high cell diffusion coefficient. Accordingly, cell density in such tumors may not be high enough to generate hypoxic foci triggering neoangiogenesis and may lead to a LGG appearance on pathological examination.28 Indeed, pathological findings do not exhibit significant differences in the spontaneous velocity of diametric expansion. This underlines the importance of an imaging kinetics grading, based on velocity of diametric expansion, independently and in addition to the pathological grading and molecular status.
Limitation of the study is the various sizes of available data for molecular markers and, particularly, isocitrate dehydrogenase 1 expression, which precluded their entrance in survival analyses modeling. However, our additional analysis restricted to the population with available 1p19q codeletion status showed that spontaneous velocity of diametric expansion and 1p19q codeletion status are both independent prognostic factors for LGG clinical outcome among adult patients. Thus, the determination of spontaneous velocity of diametric expansion adds to the 1p19q codeletion status in determining outcome. We confirm that LGGs with 1p19q codeletion grow significantly slower and those with p53 overexpression grow significantly faster than LGGs without such abnormalities, whereas isocitrate dehydrogenase 1 expression has no significant impact on spontaneous velocity of diametric expansion.16,18 In addition, we revealed that the presence of a complete 1p deletion is associated with a significant slower spontaneous velocity of diametric expansion in accordance with the known differences in prognosis for complete and partial 1p deletions for LGG.29
Taken together, these data emphasize that spontaneous velocity of diametric expansion identify LGGs with tumor-intrinsic properties leading to a higher risk of tumor progression and malignant transformation. However, additional extrinsic factors might affect LGG growth, as suggested by the negative impact of pregnancy.30
Practical Implications
We propose that the velocity of diametric expansion could be integrated together with the other static known prognostic parameters in a multidimensional approach to better predict the outcomes of an LGG at the individual level. Because the spontaneous velocity of diametric expansion is a strong prognostic parameter, it should be measured systematically at the beginning of the management of a LGG without delaying treatment. Patients should get a second MRI before oncological treatment, thus allowing the measurement of the velocity of diametric expansion through the evolution of the mean tumor diameter over time. This implies use of the first discovery MRI and a second one performed just before surgery for surgical planning, thus allowing velocity of diametric expansion measurement through the mean tumor diameter evolution over time.12 In rare patients presenting with increased intracranial pressure and/or neurological deficit, treatment should be prompted, precluding performing a second MRI after a short observational period. In the other cases, patients presenting with a spontaneous velocity of diametric expansion >8 mm/year (ie, an increase in the mean tumor diameter >1 mm over a 6-week interval and >2 mm over a 3-month period) should be considered as high-risk patients together with those without IDH mutation and those with known risk factors according to Pignatti and Chang scoring systems.20,27 Because these LGGs share outcomes similar to those of malignant gliomas, treatment modalities should be selected accordingly, and a 3-month follow-up should be preferred.
Funding
None declared.
Acknowledgments
Johan Pallud: literature search, study design, data collection, data analysis, data interpretation, figures and table, writing; Marie Blonski: data analysis, data interpretation, writing; Emmanuel Mandonnet: data analysis, data interpretation, writing; Etienne Audureau: statistical analyses, data interpretation, tables, writing; Denys Fontaine: data collection, writing; Nader Sanai: data analysis, data inter pretation, writing; Luc Bauchet: data collection, writing; Philippe Peruzzi: data collection, writing; Marc Frénay: data collection, writing; Philippe Colin: data collection, writing; Rémy Guillevin: data collection, writing; Valérie Bernier: data collection, writing; Marie-Héléne Baron: data collection, writing; Jacques Guyotat: data collection, writing; Hugues Duffau: study design, data collection, writing; Luc Taillandier: study design, data collection, writing; Laurent Capelle: study design, data collection, writing.
Conflict of interest statement. None declared.
References
- 1.Reifenberger G, Kros JM, Louis DN, Collins VP. Oligodendroglioma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer (IARC); 2007. pp. 54–59. [Google Scholar]
- 2.von Deimling A, Burger PC, Nakazato Y, et al. Diffuse astrocytomas. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer (IARC); 2007. pp. 25–29. [Google Scholar]
- 3.Soffietti R, Baumert BG, Bello L, et al. Guidelines on management of low-grade gliomas: report of an EFNS-EANO* Task Force. Eur J Neurol. 2010;17:1124–1133. doi: 10.1111/j.1468-1331.2010.03151.x. [DOI] [PubMed] [Google Scholar]
- 4.Mandonnet E, Delattre JY, Tanguy ML, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Annals of Neurology. 2003;53:524–528. doi: 10.1002/ana.10528. [DOI] [PubMed] [Google Scholar]
- 5.Pallud J, Fontaine D, Duffau H, et al. Natural history of incidental World Health Organization grade II gliomas. Annals of Neurology. 2010;68:727–733. doi: 10.1002/ana.22106. [DOI] [PubMed] [Google Scholar]
- 6.Pallud J, Mandonnet E, Duffau H, et al. Prognostic value of initial magnetic resonance imaging growth rates for World Health Organization grade II gliomas. Annals of Neurology. 2006;60:380–383. doi: 10.1002/ana.20946. [DOI] [PubMed] [Google Scholar]
- 7.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 8.Duffau H. Surgery of low-grade gliomas: towards a ‘functional neurooncology. Current Opinion in Oncology. 2009;21:543–549. doi: 10.1097/CCO.0b013e3283305996. [DOI] [PubMed] [Google Scholar]
- 9.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–764. doi: 10.1227/01.neu.0000318159.21731.cf. discussion 264–756. [DOI] [PubMed] [Google Scholar]
- 10.Pallud J. Diffuse low-grade gliomas. What does complete resection mean? In: Hayat MA, editor. Tumors of the Central Nervous System. 1st ed. Vol. 2. Springer; 2011. pp. 153–162. [Google Scholar]
- 11.Pallud J, Varlet P, Devaux B, et al. Diffuse low-grade oligodendrogliomas extend beyond MRI-defined abnormalities. Neurology. 2010;74:1724–1731. doi: 10.1212/WNL.0b013e3181e04264. [DOI] [PubMed] [Google Scholar]
- 12.Pallud J, Taillandier L, Capelle L, et al. Quantitative morphological MRI follow-up of low-grade gliomas: a plead for systematic measurement of growth rates. Neurosurgery. 2012;71:729–739. doi: 10.1227/NEU.0b013e31826213de. [DOI] [PubMed] [Google Scholar]
- 13.Mandonnet E, Pallud J, Clatz O, et al. Computational modeling of the WHO grade II glioma dynamics: principles and applications to management paradigm. Neurosurgical Review. 2008;31:263–269. doi: 10.1007/s10143-008-0128-6. [DOI] [PubMed] [Google Scholar]
- 14.Mandonnet E, Pallud J, Fontaine D, et al. Inter- and intrapatients comparison of WHO grade II glioma kinetics before and after surgical resection. Neurosurgical Review. 2010;33:91–96. doi: 10.1007/s10143-009-0229-x. [DOI] [PubMed] [Google Scholar]
- 15.Peyre M, Cartalat-Carel S, Meyronet D, et al. Prolonged response without prolonged chemotherapy: a lesson from PCV chemotherapy in low-grade gliomas. Neuro Oncol. 2010;12:1078–1082. doi: 10.1093/neuonc/noq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricard D, Kaloshi G, Amiel-Benouaich A, et al. Dynamic history of low-grade gliomas before and after temozolomide treatment. Annals of Neurology. 2007;61:484–490. doi: 10.1002/ana.21125. [DOI] [PubMed] [Google Scholar]
- 17.Pallud J, Llitjos JF, Dhermain F, et al. Dynamic imaging response following radiation therapy predicts long-term outcomes for diffuse low-grade gliomas. Neuro-Oncology. 2012;14:496–505. doi: 10.1093/neuonc/nos069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goze C, Bezzina C, Goze E, et al. 1P19Q loss but not IDH1 mutations influences WHO grade II gliomas spontaneous growth. Journal of Neuro-Oncology. 2012;108:69–75. doi: 10.1007/s11060-012-0831-6. [DOI] [PubMed] [Google Scholar]
- 19.Bourne TD, Schiff D. Update on molecular findings, management and outcome in low-grade gliomas. Nat Rev Neurol. 2010;6:695–701. doi: 10.1038/nrneurol.2010.159. [DOI] [PubMed] [Google Scholar]
- 20.Chang EF, Clark A, Jensen RL, et al. Multiinstitutional validation of the University of California at San Francisco Low-Grade Glioma Prognostic Scoring System. Clinical article. Journal of Neurosurgery. 2009;111:203–210. doi: 10.3171/2009.2.JNS081101. [DOI] [PubMed] [Google Scholar]
- 21.Pallud J, Capelle L, Taillandier L, et al. Prognostic Significance of Imaging Contrast Enhancement for WHO grade II Gliomas. Neuro-Oncology. 2008;11:176–182. doi: 10.1215/15228517-2008-066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger MS, Deliganis AV, Dobbins J, Keles GE. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74:1784–1791. doi: 10.1002/1097-0142(19940915)74:6<1784::aid-cncr2820740622>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Brasil Caseiras G, Ciccarelli O, Altmann DR, et al. Low-grade gliomas: six-month tumor growth predicts patient outcome better than admission tumor volume, relative cerebral blood volume, and apparent diffusion coefficient. Radiology. 2009;253:505–512. doi: 10.1148/radiol.2532081623. [DOI] [PubMed] [Google Scholar]
- 24.Hlaihel C, Guilloton L, Guyotat J, et al. Predictive value of multimodality MRI using conventional, perfusion, and spectroscopy MR in anaplastic transformation of low-grade oligodendrogliomas. Journal of Neuro-Oncology. 2010;97:73–80. doi: 10.1007/s11060-009-9991-4. [DOI] [PubMed] [Google Scholar]
- 25.Pallud J, Capelle L, Mandonnet E. Comment on parameters of low-grade glioma as predictors. Radiology. 2010;256:1014. doi: 10.1148/radiol.100299. [DOI] [PubMed] [Google Scholar]
- 26.Alvord EC, Jr., Swanson KR. Using mathematical modeling to predict survival of low-grade gliomas. Annals of Neurology. 2007;61:496. doi: 10.1002/ana.21042. author reply 496–497. [DOI] [PubMed] [Google Scholar]
- 27.Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076–2084. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 28.Swanson KR, Rockne RC, Claridge J, et al. Quantifying the role of angiogenesis in malignant progression of gliomas: in silico modeling integrates imaging and histology. Cancer Research. 2011;71:7366–7375. doi: 10.1158/0008-5472.CAN-11-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Idbaih A, Marie Y, Pierron G, et al. Two types of chromosome 1p losses with opposite significance in gliomas. Annals of Neurology. 2005;58:483–487. doi: 10.1002/ana.20607. [DOI] [PubMed] [Google Scholar]
- 30.Pallud J, Mandonnet E, Deroulers C, et al. Pregnancy increases the growth rates of World Health Organization grade II gliomas. Annals of Neurology. 2010;67:398–404. doi: 10.1002/ana.21888. [DOI] [PubMed] [Google Scholar]