Abstract
Background
Glutamine synthetase (GS) is an astrocytic enzyme catalyzing the conversion of glutamate and ammonia to glutamine. Its up-regulation has been related to higher tumor proliferation and poor prognosis in extra-cerebral tumors. We have previously reported a GS deficiency in patients with glioblastoma multiforme (GBM) who also developed epilepsy, which is a favorable prognostic factor in glioma. Here, we investigated the prognostic value of GS expression in patients with GBM with or without epilepsy and its correlation with survival.
Methods
We conducted a clinical and histopathological study on 83 (52 males) consecutive patients with newly diagnosed GBM. Immunohistochemical expression of GS was scored semi-quantitatively on the basis of cell number, staining intensity, and distribution of immunoreactive cells. Several clinical and neuropathological variables were analyzed in relation to survival and GS expression.
Results
Median age at diagnosis was 62 years. At the last evaluation, with a median follow-up of 11.5 months (range, 1.5–58 months), 5 patients (6%) were still alive and 78 (94%) were dead. GS expression patterns in neoplastic cells were inversely correlated to the presence of epilepsy (P < .0001 for intensity and P < .009 for homogeneity of GS distribution, respectively). Univariate analysis showed that RPA score, epilepsy, O6-methylguanine-DNA methyltransferase (MGM)T status, application of Stupp protocol, and GS intensity pattern had a significant impact on survival. Absent/low intensity of GS expression was significantly associated with a longer survival in both uni- (19 vs 8 months; P < .0005) and multivariate (P = .003) analyses.
Conclusions
Absent/low-intensity GS expression pattern represents a valuable biomarker of both epilepsy and overall survival in GBM.
Keywords: epilepsy, glutamine synthetase, glioblastoma multiforme, immunohistochemistry, survival
Glioblastoma multiforme (GBM) represents the most common and most malignant type of primary brain tumor in humans, with a median survival of 14 months.1 Several clinical, therapeutic, and histopathological features have been reported as significant to influence survival in GBM, but the exact value of each prognostic factor remains controversial.2–6 Only young age, good initial performance score, and MGMT (O6-methylguanine-DNA methyltransferase) promoter hypermethylation have been strongly associated with a significantly longer survival among patients who were treated with concomitant radiotherapy and chemotherapy with temozolomide (Stupp protocol).4,6
A few studies have also shown that history of seizures before GBM diagnosis may be a reliable positive prognostic factor, regardless of age, extent of surgery, radiotherapy field, and dosage.2,7–10 We have previously demonstrated a strong correlation between level of glutamine synthetase (GS) and epilepsy in GBM, suggesting that glutamate increase by GS deficiency in neoplastic cells is a key molecular mechanism associated with seizure generation.11 Of interest, GS catalyzes the conversion of glutamate and ammonia to glutamine, which is the crucial energy substrate for optimal growth of both normal and neoplastic cells in culture, and its up-regulation is related to higher tumor growth and proliferation.12–14
Here, we investigated the prognostic value of GS expression in a group of patients with newly diagnosed GBM with or without epilepsy. Our hypothesis was that low GS expression might have a favorable impact on survival.
Materials and Methods
Patient Selection
We included 83 patients with a histologically confirmed new diagnosis of GBM who were consecutively admitted at the Department of Neurological Sciences and Vision of Spedali Civili of Brescia from February 1, 2006 through February 1, 2010. The 2 principal inclusion criteria were surgery with availability of pathological specimen and complete follow-up data. Data were collected with regard to the age at diagnosis, sex, symptoms at onset, neurological examination, Karnofsky performance status (KPS) score, tumor localization, surgical treatment, and adjuvant treatment. All patients underwent an extensive interview to determine the stereotypic symptoms suggestive of focal seizures. Seizures were categorized on the basis of the Classification and Terminology of the International League Against Epilepsy.15 Individuals only with a diagnosis of symptomatic epilepsy were treated with antiepileptic drug. In detail, a diagnosis of symptomatic epilepsy was made in the following circumstances: (1) recurrent seizures with or without interictal epileptiform abnormalities (IEAs) on EEG recordings, (2) single convulsive seizure and IEAs, (3) single convulsive seizure and history of spells suggestive of focal seizures with or without IEAs, and (4) seizures occurring for the first time during the follow-up.
Imaging analysis was based on tumor properties obtained from conventional magnetic resonance (MR) T1- and T2-weighted before and after gadolinium injection and from MR spectroscopy. All patients underwent a contrast CT examination before surgery and within 3–6 h after surgery; on the basis of the operative report and postoperative CT, tumor removal was defined as partial (>20% of residual tumor), subtotal (<20% of residual tumor), or total (absence of residual tumor), respectively. CTs were evaluated by a single neuro-radiologist in the neuro-oncology group who was unaware of clinical data. Then, patients received adjuvant radiotherapy (RT) with concurrent and adjuvant chemotherapy, according to the Stupp protocol.1 The Recursive Partitioning Analysis (RPA) class score, incorporating the most significant prognostic clinical variables, was used to guide clinical decisions.16 Postoperative RT was delivered to the tumor bed with adequate margins up to 60 Gy (2 Gy/fraction). Patients then received followed-up with MR at regular intervals of 3 months.
Histopathological Analysis
Tumor specimens were formalin fixed and paraffin embedded, and representative sections were selected and submitted to hematoxylin and eosin and routine immunohistochemical (IHC) staining for evaluation of histological diagnosis according to the World Health Organization (WHO) classification.17 All the samples were obtained from the archives of the Pathology Service of Spedali Civili of Brescia according to the protocols of the local ethical board. For histopathological characterization of GBM, the following parameters were evaluated: brain anatomical sublocalization, cellularity, pleomorphism, presence gemistocytic, oligodendroglial and small cell component, amount of tumor infiltrating inflammatory cells, necrosis, and vascular proliferation. The different parameters were semi-quantitatively scored as follows: 0, absence; 1, scarce; 2, moderate; 3, high. For statistical purposes, 0–1 and 2–3 combined scores were considered.
Immunohistochemistry
For IHC staining, 2 µm thick representative sections from paraffin-embedded blocks were deparaffinized and rehydrated. Endogenous peroxidase activity was blocked with 0.3% H2O2 in methanol for 20 min. Antigen retrieval (if necessary) was performed using a 0.05% protease type XIV (Sigma-Aldrich, St Louis, MO) solution at 37°C for 20 min or a microwave oven in 1.0 mM EDTA buffer (pH, 8.00). Sections were then washed in TBS (pH, 7.4) and incubated for 1 h with the following primary antibodies diluted in TBS/1% bovine serum albumin: GS (Sigma-Aldrich, St Louis; 1:2000), epidermal growth factor receptor (Clone E-30, Dako Cytomation, Glostrup, Denmark; 1:20), p53 (Clone D07, Neomarkers; 1:100), Ki67 (clone MIB-1, Dako Cytomation; 1:100), and glial fibrillary acidic protein (Dako Cytomation; 1:200). Immunostain for GS was tested and optimized on normal brain tissue specimens. Isocitrate dehydrogenase 1 (IDH1) status was evaluated using the anti-IDH1-R132H antibody (clone H09, Dianova GmbH; 1:100) that specifically recognizes the R132H mutation, expressed in >80% of low-grade gliomas and secondary GBMs but rarely present in primary GBMs. Signal was revealed using the Dako EnVision+Dual Link System Peroxidase (Dako Cytomation), followed by diaminobenzydine as chromogen and hematoxylin as counterstain. Images were acquired using an Olympus DP70 camera mounted on Olympus B×60 microscope using CellF imaging software (Soft Imaging System GmbH).
Evaluation of Immunohistochemical Staining
Analyses of the immunostain results were performed blindly, without knowledge of the clinical findings, by 3 independent observers (P.L.P., M.C., A.T.). Expression of GS was scored semi-quantitatively on the basis of cell number, staining intensity, and distribution of immunoreactive (IR) cells. Only gliomas exhibiting a positive cytoplasmic reaction in at least 10% of the tumor cells were considered to be positive. The intensity of the staining in IR cells has been evaluated as follows: 0, no staining; 1, low staining intensity; 2, low to moderate staining intensity; 3, moderate to strong staining intensity in the large majority of IR cells. For statistical purposes, 0–1 and 2–3 combined scores were considered. Furthermore, distribution of GS-positive cells was scored as homogeneous, with the large majority of IR cells being uniformly distributed in the tumor, and not homogeneous, with IR cells being positive only in selected areas. IDH1-R132H expression was classified as either positive or negative. Cases with different inter-observer evaluations have been plenary discussed to reach a final consensus, although most of the cases have reached a common judgment, because the immunostaining is highly specific and highly sensitive. Moreover, most of the inter-observer variations fit in the differences between score 0 or 1 and score 2 or 3, for which the statistical analysis were combined.
DNA Extraction and Methylation Assay
Chloroform/phenol DNA extraction was performed on representative formalin-fixed, paraffin-embedded sections and used for methylation-specific polymerase chain reaction (PCR) to investigate MGMT methylation status of tumor samples from patients who received concomitant radio-chemoherapy. Two micrograms of DNA was denatured by NaOH and modified using DNA Bisulphite Modification Kit (Human Genetic Signatures Pty Ltd, NSW2113, Australia), and methylation-specific PCR was performed using the following primers: 5′-TTTGTGTTTTGATGTTTGTAGGTTTTTGT-3′ (forward primer) and 5′-AACTCCACACTCTTCCAAAAACAAAACA-3′ (reverse primer) for unmethylated reaction and 5′-TTTCGACGTTCGTAGGTTTTCGC-3′ (forward primer) and 5′-GCACTCTTCCGAAAACGAAACG-3′ (reverse primer) for methylated reaction. PCR products were analyzed with 3130 Genetic Analyzer (Applied Biosystem) using Fragment Analysis Software Gene Mapper, version 4.0, and the peak height ratio of PCR products deriving from methylated and unmethylated DNA of the same tumor was calculated as previously described.18
Statistical Analysis
We first investigated semiquantitative IHC expression levels of the different immunostains in patients with GBM and their relationship with clinico-pathological variables. For statistical purposes, clinical and pathological features were considered to be categorical variables and were dichotomized. The following variables and relative cutoff levels were used: sex (male vs female), age (<50 years vs ≥50 years), KPS (<70 vs ≥70), RPA class (III + IV + V vs VI), epilepsy (present vs absent), MGMT status (methylated vs unmethylated), GS expression (absent vs present), GS intensity (absent/low vs moderate/strong), and GS distribution (absent/not homogeneous vs homogeneous). The χ2 test was used to test associations in univariate analysis. We then verified the hypothesis that GS expression may have a prognostic value in terms of overall survival. Overall survival was defined as the time elapsed from radiological diagnosis to death or last follow-up. Analysis of prognostic factors was conducted in univariate analysis on the whole cohort. Variables with known prognostic impact, including RPA, MGMT status, and treatment, were combined with experimental variables (GS and epilepsy). Kaplan-Meier survival curves were stratified by single covariate, and the difference between curves was analyzed using the Log-rank test. All tests were 2-sided, and P ≤ .05 was considered to be statistically significant. A Bonferroni correction was applied to correct for multiple testing. Values remaining significant after Bonferroni's correction (for p .05/5 = .01) were considered to be robust prognostic factors.
Prognostic value of statistically significant covariates in univariate analysis was evaluated using a backward stepwise Cox proportional hazards model in multivariate analysis. However, because MGMT data were available only for a restricted series of 44 patients (53%), we conducted 2 separate multivariate analyses, one on the whole cohort and another on the restricted MGMT-determined population. Results are presented as hazard ratio (HR) with their relative 95% confidence intervals (CIs). All statistical analyses were conducted using the SPSS software, version 17.0 (SPSS Inc., Chicago, IL).
Results
Patient Demographic Characteristics
From February 1, 2006 through February 1, 2010, 114 patients had a histologically confirmed new diagnosis of GBM. Thirty-one individuals (27%) were excluded because of a too limited diagnostic biopsy with insufficient pathological specimen. Of these, 19 were treated with palliative RT and/or temozolomide (TMZ), 9 did not receive any treatment because of rapid clinical deterioration, and 3 were lost at follow-up. The frequency of epilepsy at GBM diagnosis in the 31 excluded patients was 26%.
Demographic data of the remaining 83 patients are reported in Table 1. Age at diagnosis ranged from 25 through 80 years (median, 62 years). The symptom at onset was epilepsy in 24 individuals (28.9%), focal neurological deficit in 24 (28.9%), headache in 16 (19.3%), psychiatric manifestation in 11 (13.3%), and a single convulsive seizure in 8 (9.6%).
Table 1.
Demographic and clinical characteristics
| Characteristic | No. (%) of Patients |
|---|---|
| Sex | |
| Male | 52 (63) |
| Female | 31 (37) |
| Age, years | |
| Median 62 y (25–80 y) | |
| <50 | 13 (16) |
| ≥50 | 70 (84) |
| NE | |
| Pathological | 38 (46) |
| Normal | 45 (54) |
| KPS | |
| <70% | 12 (14) |
| ≥70% | 71 (86) |
| Epilepsy | |
| Yes | 34 (41) |
| Not | 49 (59) |
| GBM sitea | |
| Frontal lobe | 18 (22) |
| Fronto-temporal lobe | 7 (8) |
| Temporal-occipital lobe | 3 (4) |
| Fronto-parietal lobe | 6 (7) |
| Temporal lobe | 24 (29) |
| Temporo-parietal lobe | 7 (8) |
| Parietal lobe | 7 (8) |
| Parieto-occipital lobe | 4 (5) |
| Corpo callosum, talamic | 3 (4) |
| Multifocal | 4 (5) |
| Extent of surgery | |
| Residual >20% | 29 (35) |
| Residual <20% | 5 (6) |
| Total | 49 (59) |
| RPA Class | |
| III | 3 (4) |
| IV | 47 (57) |
| V | 18 (22) |
| VI | 15 (17) |
| Stupp Protocol | |
| Yes | 47 (57) |
| No/Palliative care | 36 (43) |
| MGMT | |
| Methylated | 14 (36) |
| Unmethylated | 30 (17) |
| Not available | 39 (47) |
Abbreviations: GBM, glioblastoma multiforme; KPS, Karnofsky performance status; MGMT, O6-methylguanine- methyltransferase enzyme; NE, neurological examination; RPA, Recursive Partitioning Analysis.
aCorrelation between GBM localization and epilepsy at the χ2 test, P .021.
As a part of established protocol, all patients with a diagnosis of epilepsy were treated with levetiracetam because of its absence of pharmacokinetic interactions.19 Tumor localization was in the left cerebral hemisphere in 42 patients (50.6%) and in the right cerebral hemisphere in 37 patients (44.6%); bilateral disease crossing the corpus callosum was observed in 4 patients (4.8%). Thirty-four GBMs (40.9%) involved >1 lobe. The temporal lobe was involved in 41 patients, followed by frontal (31), parietal (24), and occipital (4). Two cases were classified as glioblastoma with sarcoma-like component (gliosarcoma, WHO grade IV), and 2 cases exhibited a significant oligodendroglial component in addition to the typical areas of glioblastoma with astrocytic differentiation (glioblastoma with oligodendroglial component, WHO grade IV). Adjuvant treatment according to the Stupp protocol was administered to 47 patients (56.6%). Four weeks after completing concomitant RT, TMZ was administered for an additional 6 cycles. Thirty-four patients (72%) received the full 6-cycle regimen of TMZ, and the remaining 13 individuals (28%) received treatment with a reduced dose of drug. Causes of early discontinuation or dose reduction were GBM progression or unacceptable toxicity. Among 36 individuals (43.4%) not treated according to Stupp protocol, 29 patients received palliative hypofractionated RT and 7 were addressed to palliative care.
Thirty-four patients (41%) finally received a diagnosis of epilepsy. Seizures occurred at onset in 24 (70.6%) of 34 individuals, at GBM progression in 6 (17.6%), at GBM recurrence in 2 (5.9%), and during RT in 2 (5.9%). Ictal semiology of focal seizures was motor (5 patients), sensitive (1), simple focal (10), and complex focal (6), with (8) and without (14) secondary generalization. Twelve patients had convulsive seizures without clear clinical evidence of focal symptoms.
Eight patients (9.6%) did not receive a diagnosis of epilepsy, because they had an isolated single seizure at onset and normal interictal EEG recording. They were not treated with antiepileptic drug and did not develop epilepsy until death.
Patterns of GS Expression
Patterns of GS expression are reported in Table 2 and Fig. 1. GS was expressed in the vast majority (87%) of patients and was absent in 11 patients (13%). GS was widely expressed in reactive astrocytes in both normal-appearing brain tissue sections adjacent to the lesions and peritumoral and intratumoral regions (Fig. 1A–C). However, the presence of reactive astrocytes did not show any significant correlation with the presence of epilepsy (data not shown). Patterns of GS expression in neoplastic cells were inversely correlated to the presence of epilepsy, particularly regarding intensity (P < .0001) and distribution (P < .009) (Fig. 1D–F). GS patterns in the 8 patients with a single seizure at onset paralleled those in patients without epilepsy. Of interest, in positive GBM cases, GS staining has been found to be either particularly intense around blood vessels or weakly expressed in nearby necrotic areas.
Table 2.
Immunohistochemical staining patterns of GS in patients with and without epilepsy
| Immunostaining | No. (%) of Patients |
||
|---|---|---|---|
| Pts with epilepsy (n = 34) | Pts without epilepsy (n = 49) | P | |
| GS expression | |||
| Absent | 7 (20.6) | 4 (8.2) | |
| Present | 27 (79.4) | 45 (91.8) | .180 |
| Intensity of staining | |||
| Absent/low | 17 (50) | 7 (14) | <.0001 |
| Moderate/strong | 17 (50) | 42 (86) | |
| Homogeneity of staining | |||
| Absent/not homogeneous | 27 (79.4) | 25 (51) | .009 |
| Homogeneous | 7 (20.6) | 24 (49) | |
Abbreviation: GS glutamine synthetase.
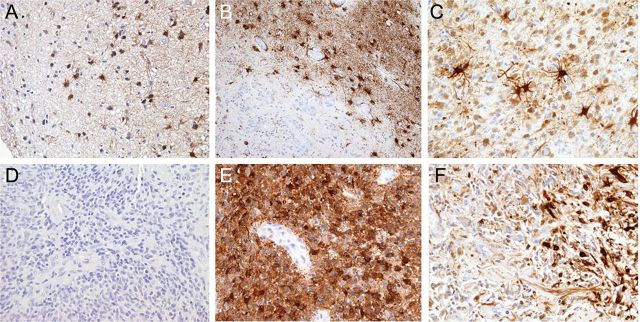
Fig. 1.
Expression of glutamine synthetase (GS) in reactive astrocytes and neoplastic cells. Reactive astrocytes, showing typical hypertrophic ramified morphology, have been found to be strongly and widely immunoreactive for GS in normal-appearing tissue sections adjacent to the tumor (A) and in the peritumoral (B) and intratumoral (C) regions. In neoplastic cells, GS expression has been found to be inversely correlated to the presence of epilepsy. Tumor samples from patients presenting with epilepsy showed to be heavily depleted or completely negative for GS-expressing cells (D), compared with tumors from patients without a clinical history of epilepsy that showed to be consistently and homogenously positive for GS (E). In terms of distribution, tumor samples from patients with epilepsy, when GS positive, showed a biphasic pattern of expression with areas severely depleted of GS-positive cells and focal areas with GS-labeled cells (F). Original magnification: 10× (A and B) and 20× (C–F).
Analysis of Survival and Prognostic Factors
At the last evaluation (December 31, 2011), after a median follow-up of 11.5 months (range, 1.5–58 months), 5 patients (6%) were still alive and 78 patients (94%) were dead. Univariate analysis of the whole cohort showed that RPA score, GS intensity pattern, MGMT status, and application of Stupp protocol had a significant impact on survival (Table 3). At a significance level of P =.05, epilepsy was significantly associated with better survival (P =.016), but the effect was lost after Bonferroni's correction. Absolute GS expression (present vs absent) did not show any relationship with survival. This was probably attributable to the very low frequency of patients with completely negative GS expression. However, the pattern of intensity of GS staining had a marked impact on overall survival, because absent or low intensity of GS expression was significantly associated with a better prognosis in uni- and multivariate analyses (Table 3), both in the whole cohort (19 vs 8 months; P < .0005 and P =.003, respectively) and in the restricted MGMT-determined population (19.5 vs 12.5 months; P < .003 and P =.006, respectively). The presence of epilepsy also had a significant favorable effect on overall survival in the whole cohort (16.5 vs 7.5 months; P =.016).
Table 3.
Uni- and multivariate analyses of prognostic factors in the whole cohort (a) and in the MGMT restricted cohort (b)
| Variables | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|
| χ | P | HR | 95% CI | P | |
| (A) whole cohort, 83 patients | |||||
| Epilepsy | |||||
| yes vs no | 5.817 | .016 | NS | NS | NS |
| RPA | |||||
| III-IV-V vs VI | 21.615 | <.0005 | .406 | .213–.774 | .006 |
| Stupp protocol | |||||
| yes vs no | 36.886 | <.0005 | .334 | .194–.577 | <.0005 |
| MGMT | |||||
| methylated/ unmethylated | 19.855 | <.0005 | ND | ND | ND |
| GS intensity | |||||
| absent/low vs moderate/strong | 15.472 | <.0005 | .436 | .252–.754 | .003 |
| (B) MGMT restricted cohort, 47 patients | |||||
| MGMT | |||||
| methylated/ unmethylated | 12.402 | <.0005 | .265 | .117–.601 | .001 |
| GS intensity | |||||
| absent/low vs moderate/strong | 9.040 | <.003 | .401 | .208–.771 | .006 |
Abbreviations: GS, glutamine synthetase; MGMT, O6-methylguanine-methyltransferase enzyme; ND, not determined; NS, not significant; RPA, Recursive Partitioning Analysis.
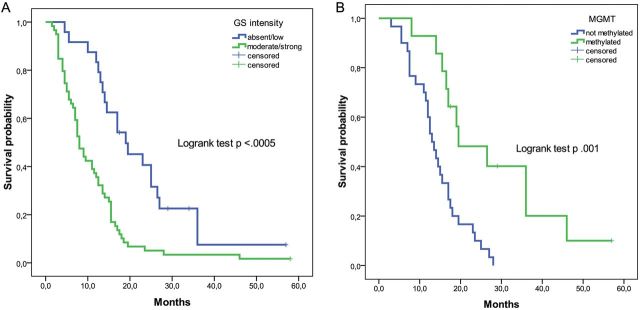
MGMT methylation status was available for 44 (53%) of 83 patients. All of them received chemo-radiotherapy according to the Stupp protocol. Most (68%) of these patients had an unmethylated MGMT (Table 1). MGMT status was not associated to either GS intensity (P =.604) or epilepsy (P =.281). As expected, MGMT status (methylated vs unmethylated) identified patients with good and poor prognosis (19.5 vs 13.0 months; P =.001) in univariate and multivariate analyses (Table 3). In the unmethylated group, low vs high GS intensity showed a trend toward good vs poor prognosis without reaching statistical significance (14.5 vs 11 months; P =.157) (Fig. 2). In the methylated group, low vs high GS intensity was significantly associated with a better prognosis (36 vs 16.5 months; P =.029) (Fig. 2).
Fig. 2.
Kaplan–Meier survival curves, stratified by glutamine synthetase (GS) staining intensity in the whole cohort (n = 83) (A) and by methylation status of MGMT in the MGMT-determined subcohort (n = 44) (B).
No additional neurological symptoms at onset other than epilepsy had any significant impact on survival. Clinical variables, such as age, extent of surgery, performance status, and neurological function, did not have impact on survival when singularly analyzed outside the RPA score (data not shown).
Discussion
Using a semiquantitative IHC analysis, we revealed that GS expression pattern strongly correlates with survival among patients with newly diagnosed GBM, and thus, this pattern represents a valuable prognostic biomarker in GBM. In our analysis, statistical significance was demonstrated for the intensity of GS expression, rather than for its absolute expression (absent vs present), because only 11 patients (13%) showed absence of GS, according to the pathological criteria used in this study to assess GS staining. An absent or low pattern of expression strongly correlated with better survival. Epilepsy had a favorable impact on survival in univariate analysis, although this impact was not significant (P =.016) after Bonferroni correction. However, GS expression was predictive of survival independently of epilepsy.
We believe that there is a plausible biochemical explanation of the favorable prognostic value of GS deficiency in GBM. Indeed, GS catalyzes the synthesis of glutamine, which is the crucial energy substrate for optimal growth of both normal and neoplastic cells in culture, and its up-regulation is related to higher tumor growth and proliferation.12–14 Several lines of evidence indicate that the metabolic pathway, including glutamine, glutamine synthetase, glutamate, and the oncogene MYC, has a growing role in tumor growth and metabolism.20 Previous studies have shown that GS expression is a reliable prognostic indicator of extra-cerebral tumors, because up-regulated GS protein and GS enzyme activity in human hepatocellular carcinoma were strongly associated with recurrence and poor prognosis.21–24
The observation of a lower and different GS expression pattern in individuals with GBM and epilepsy, compared with those without epilepsy, is in agreement with our previous findings obtained through a Western blot analysis in a different population with GBM.11 GS deficiency and gliosis were already reported in experimental models of epilepsy,25–27 and in patients with mesial temporal sclerosis and epilepsy,28 glutamate increase from GS deficiency may play a role in epileptogenesis. On the basis of this finding, GS deficiency in neoplastic astrocytes may represent a possible molecular mechanism for seizure generation and a plausible biochemical explanation of the favorable prognostic value of epilepsy in patients with GBM.
Certainly, down-regulation of GS expression could help to strengthen our hypothesis, and additional studies should be performed in murine GBM models to answer this crucial issue.
Of interest, a link between IDH1 mutation and epilepsy has been recently postulated.29 Analysis of the IDH1 mutational status confirmed that most of the patients with GBM who were included in our study (none of them with a clinical history of low-grade glioma) belong to the primary de novo GBM group, which did not show the IDH1 mutation (2 [2.4%] of 83 cases), indicating that, at least for primary GBMs, IDH1 mutation does not associate with seizures.
Although temporal and frontal lobes were more frequently involved in patients with epilepsy, extents of surgical resection were comparable in those without epilepsy. Therefore, the possibility that a longer survival among patients with epilepsy was related to a more extensive surgery appears to be unlikely,5,10 and our results further support the notion that differences in survival may reflect the different biological behavior of the tumor rather than its localization.30 The GBM location is probably an important determinant of tumor epileptogenesis, which is a multifactorial phenomenon in which several factors play a role.30 Our assessment of the extent of resection by only CT and standard MRI may be a possible limitation of our study, because advanced imaging methods, such as volumetric MRI, seem to be useful to better define the level of GBM resection.31
Other clinical variables, such as age, extent of surgery, performance status, and neurological function, did not have a great impact on survival. However, they are part of the RPA score, which was also a highly significant prognostic factor. Although in our series, not all patients were evaluated for MGMT, most of whom had unmethylated cases and could be further stratified (although not significantly) by better or worse prognosis according to GS. As hypothesis generating, this observation is interesting, because it introduces a possible predictive substratification in patients with unmethylated MGMT. However, this evidence should be considered with caution and needs to be confirmed in a prospective, larger study.
In conclusion, GS expression appears to be a novel potential prognostic marker in patients with GBM, and it may become a useful tool for the clinical management of GBM. Moreover, manipulation of GS activity might indicate new therapeutic opportunities in patients with malignant glioma, as already proposed for other tumors.32
Conflict of interest statement. None declared.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Mineo JF, Bordron A, Baroncini M, et al. Prognosis factors of survival time in patients with glioblastoma multiforme: a multivariate analysis of 340 patients. Acta Neurochir. 2007;149(3):245–253. doi: 10.1007/s00701-006-1092-y. [DOI] [PubMed] [Google Scholar]
- 3.Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981–22981/CE.3. Lancet Oncol. 2008;9(1):29–38. doi: 10.1016/S1470-2045(07)70384-4. [DOI] [PubMed] [Google Scholar]
- 4.Vecht CJ. Neuro-oncology: clinical and molecular predictive factors. Lancet Neurol. 2009;8(1):17–19. doi: 10.1016/S1474-4422(08)70278-3. [DOI] [PubMed] [Google Scholar]
- 5.McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 6.Weller M, Stupp R, Reifenberger G, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010;6(1):39–51. doi: 10.1038/nrneurol.2009.197. [DOI] [PubMed] [Google Scholar]
- 7.Miller PJ, Hassanein RS, Giri PG, Kimler BF, O'Boynick P, Evans RG. Univariate and multivariate statistical analysis of high-grade gliomas: the relationship of radiation dose and other prognostic factors. Int J Radiat Oncol Biol Phys. 1990;19(2):275–280. doi: 10.1016/0360-3016(90)90534-q. [DOI] [PubMed] [Google Scholar]
- 8.Kilpatrick C, Kaye A, Dohrmann P, Gonzales M, Hopper J. Epilepsy and primary cerebral tumours. J Clin Neurosci. 1994;1(3):178–181. doi: 10.1016/0967-5868(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 9.Lote K, Stenwig AE, Skullerud K, Hirschberg H. Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer. 1998;34(1):98–102. doi: 10.1016/s0959-8049(97)00374-2. [DOI] [PubMed] [Google Scholar]
- 10.Ozbek N, Cakir S, Gursel B, Meydan D. Prognostic significance of seizure in patients with glioblastoma multiforme. Neurol India. 2004;52(1):76–78. [PubMed] [Google Scholar]
- 11.Rosati A, Marconi S, Pollo B, et al. Epilepsy in glioblastoma multiforme: correlation with glutamine synthetase levels. J Neurooncol. 2009;93:319–324. doi: 10.1007/s11060-008-9794-z. [DOI] [PubMed] [Google Scholar]
- 12.Zielke HR, Zielke CL, Ozand PT. Glutamine a major energy source for cultured mammalian cells. Fed Proc. 1984;43:121–125. [PubMed] [Google Scholar]
- 13.Lu W, Pelicano H, Huang P. Cancer metabolism: is glutamine sweeter than glucose? Cancer Cell. 2010;18:199–200. doi: 10.1016/j.ccr.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang JB, Erickson JW, Fuji R, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 16.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three RTOG malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 17.Kleihues P, Burger PC, Aldape KD, et al. Glioblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2007. pp. 33–49. Chapter 1. [Google Scholar]
- 18.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 19.Rosati A, Buttolo L, Stefini R, Todeschini A, Cenzato M, Padovani A. Efficacy and safety of levetiracetam in patients with glioma. Arch Neurol. 2010;67:343–346. doi: 10.1001/archneurol.2009.335. [DOI] [PubMed] [Google Scholar]
- 20.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 21.Osada T, Sakamoto M, Nagawa H, et al. Acquisition of glutamine synthetase expression in human hepatocarcinogenesis: relation to disease recurrence and possible regulation by ubiquitin-dependent proteolysis. Cancer. 1999;85:819–831. doi: 10.1002/(sici)1097-0142(19990215)85:4<819::aid-cncr9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Osada T, Nagashima I, Tsuno NH, Kitayama J, Nagawa H. Prognostic significance of glutamine synthetase expression in unifocal advanced hepatocellular carcinoma. J Hepatol. 2000;33:247–253. doi: 10.1016/s0168-8278(00)80365-7. [DOI] [PubMed] [Google Scholar]
- 23.Long J, Lang ZW, Wang HG, Wang TL, Wang BE, Liu SQ. Glutamine synthetase as a n early marker for hepatocellular carcinoma based on proteomic analysis of resected small hepatocellular carcinomas. Hepatobiliary Pancreat Dis Int. 2010;9:296–305. [PubMed] [Google Scholar]
- 24.Dal Bello B, Rosa L, Campanini N, et al. Glutamine synthetase immunostaining correlates with pathologic features of hepatocellular carcinoma and better survival after radiofrequency thermal ablation. Clin Cancer Res. 2010;16:2157–2166. doi: 10.1158/1078-0432.CCR-09-1978. [DOI] [PubMed] [Google Scholar]
- 25.Laming PR, Cosby SL, O'Neill JK. Seizures in the Mongolian gerbil are related to a deficiency in cerebral glutamine synthetase. Comp Biochem Physiol C. 1989;94:399–404. doi: 10.1016/0742-8413(89)90088-1. [DOI] [PubMed] [Google Scholar]
- 26.Dutuit M, Didier-Bazès M, Vergnes M, et al. Specific alteration in the expression of glial fibrillary acid protein, glutamate deydrogenase, and glutamine synthetase in rats with genetic absence epilepsy. Glia. 2000;32:15–24. doi: 10.1002/1098-1136(200010)32:1<15::aid-glia20>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Ortinski PI, Dong J, Mungenast A, et al. Selective induction of astrocytic gliosis generates in neuronal inhibition. Nat Neurosci. 2010;13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stockhammer F, Misch M, Helms HJ, et al. IDH1/2 mutations in WHO grade II astrocytomas associated with localization and seizure as the initial symptom. Seizure. 2012;21:194–7. doi: 10.1016/j.seizure.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Eid T, Thomas MJ, Spencer DD, et al. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363:28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- 30.Berntsson SG, Malmer B, Bondy ML, Qu M, Smits A. Tumor-associated epilepsy and gliomas: Are there common genetic pathways? Acta Oncol. 2009;48:955–963. doi: 10.1080/02841860903104145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iliadis G, Kotoula V, Chatzisotiriou A, et al. Volumetric and MGMT parameters in glioblastoma patients: survival analysis. BMC Cancer. 2012;12:3. doi: 10.1186/1471-2407-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]