Abstract
Background
Premature arterial stiffening and atherosclerosis are increased in patients with inflammatory arthropathies such as rheumatoid arthritis (RA), ankylosing spondylitis (AS) and psoriatic arthritis (PsA). The proinflammatory protein calprotectin is associated with inflammatory arthropathies, vascular pathology, and acute coronary events. We examined the long-term effects of treatment with tumor necrosis factor (TNF)-α antagonists on aortic stiffness and carotid intima media thickness (CIMT) in patients with inflammatory arthropathies, and the relationships to the levels of calprotectin.
Methods
Fifty-five patients with RA, AS, or PsA and a clinical indication for anti-TNF-α therapy were included and followed with regular examinations for 1 year. Thirty-six patients starting with anti-TNF-α therapy were compared with a nontreatment group of 19 patients. Examinations included assessments of aortic stiffness (aortic pulse wave velocity, aPWV), CIMT, and plasma calprotectin.
Results
After 1 year, aPWV (mean (s.d.)) was improved in the treatment group, but not in the control group (−0.54 [0.79] m/s vs. 0.06 [0.61] m/s, respectively; P = 0.004), and CIMT progression (median (quartile cut-points, 25th and 75th percentiles)) was reduced in the treatment group compared to the control group (−0.002 [–0.038, 0.030] mm vs. 0.030 [0.011, 0.043] mm, respectively; P = 0.01). In multivariable analyses, anti-TNF-α therapy over time was associated with improved aPWV (P = 0.02) and reduced CIMT progression (P = 0.04), and calprotectin was longitudinally associated with aPWV (P = 0.02).
Conclusions
Long-term anti-TNF-α therapy improved aortic stiffness and CIMT progression in patients with inflammatory arthropathies. Calprotectin may be a soluble biomarker reflecting aortic stiffening in these patients.
Keywords: anti-TNF-α, arterial stiffness, blood pressure, carotid intima media thickness, hypertension, rheumatoid arthritis
Cardiovascular morbidity and mortality are increased in patients with inflammatory arthropathies such as rheumatoid arthritis (RA), ankylosing spondylitis (AS), and psoriatic arthritis (PsA).1 The systemic inflammation associated with these diseases seems to contribute importantly to the observed accelerated atherosclerosis. Previous results suggest that treatment with tumor necrosis factor (TNF)-α antagonists may reduce the risk of cardiovascular events in patients with RA.2 Aortic pulse wave velocity (aPWV), a measure of aortic stiffness, and carotid intima media thickness (CIMT) are both predictors of cardiovascular events3,4 and are increased in patients with inflammatory arthropathies.5,6 We have recently reported that 3 months of anti-TNF-α therapy improved aortic stiffness in such patients.7 However, results regarding the long-term effects of this treatment on aortic stiffness are limited, and data concerning CIMT are conflicting.6,8–11
There is also a lack of knowledge about associations between soluble inflammatory biomarkers and aPWV and CIMT in patients with inflammatory arthropathies. Calprotectin is a protein complex compounded by the S100A8 and S100A9 proteins, also referred to as myeloid-related proteins (MRP)-8 and MRP-14, which is secreted by activated neutrophils and monocytes.12 In inflammatory arthropathies, calprotectin is associated with the disease activity and synovial inflammation,13,14 and anti-TNF-α therapy has been demonstrated to reduce both the number of infiltrating MRP-8 and MRP-14 expressing macrophages in synovial tissue and serum calprotectin levels.14 Plasma calprotectin has also been reported to predict cardiovascular events15 and to be a marker of acute coronary syndromes.16 Recent results have indicated that the protein complex promote vascular inflammation by recruitment of neutrophils and macrophages, stimulates vascular smooth muscle cell proliferation, and enhances atherosclerotic lesion formation in a MRP-14 knockout mice model.17 Thus, calprotectin might have a direct role in vascular pathology.
The objective of this study was to evaluate the effects of 1-year anti-TNF-α therapy on aPWV and CIMT in patients with RA, AS, and PsA compared to a nontreatment group of patients with similar inflammatory activity and to explore possible associations with calprotectin.
Methods
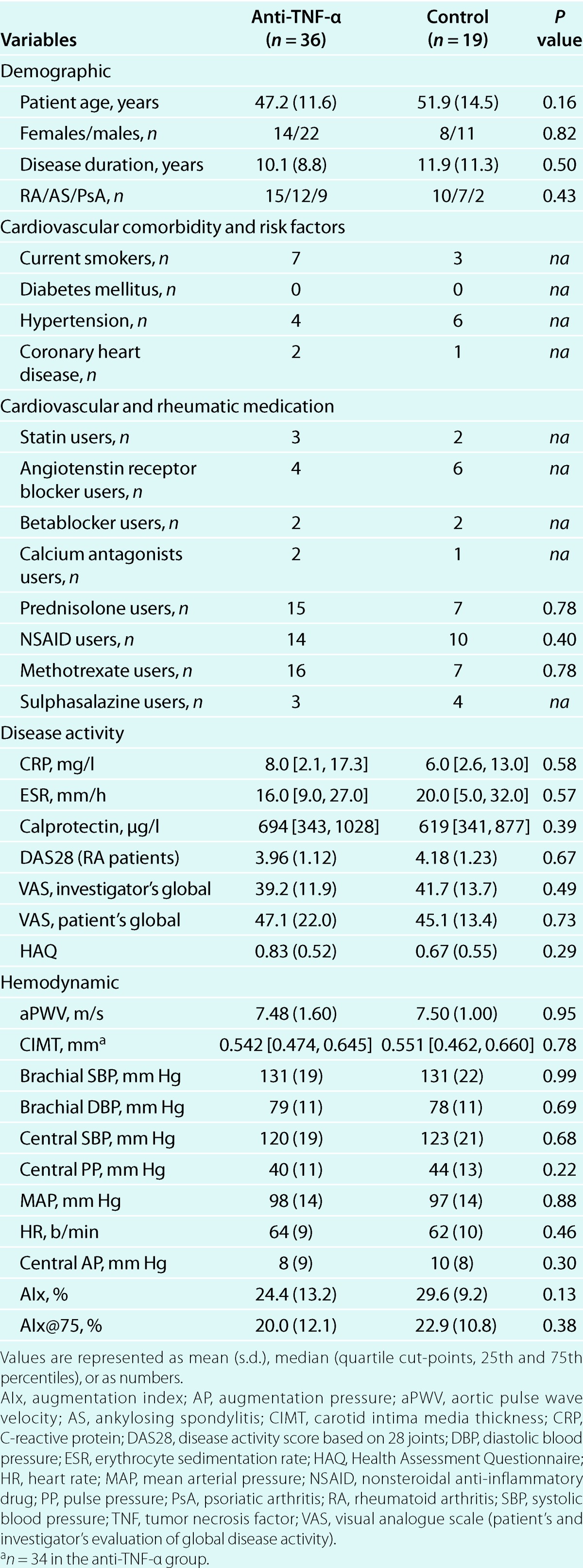
Design and patients. This study was prospective and not randomized. Patients with RA, AS, or PsA and age > 18 years were recruited between June 2006 and January 2008 from two rheumatology outpatient clinics in the Oslo area. Inclusion criteria were an active inflammatory disease and a clinical indication for anti-TNF-α therapy. Exclusion criteria were uncontrolled arterial hypertension (systolic pressure ≥ 140 mm Hg and/or diastolic pressure ≥ 90 mm Hg), change in antihypertensive or lipid-lowering treatment the last 6 months prior to inclusion or permanent cardiac arrhythmia. Additionally, patients with changes in antihypertensive or lipid-lowering medication, or patients who initiated treatment with other biological disease-modifying antirheumatic therapy such as treatment targeting B cells (rituximab), T cells (abatacept), interleukin-1 (anakinra), or interleukin-6 (tocilizumab) during the study period would be excluded. Sixty patients were included. The short-term (3 months) effects of anti-TNF-α therapy on aPWV in this population have been reported before7 and the present analyses focus on the long-term (1 year) effects on aPWV and the effects on CIMT. After the short-term follow-up period, additionally six patients initiated anti-TNF-α therapy, giving a total of 41 patients in the treatment group and 19 patients in the control group in the present long-term follow-up study. The 19 patients in the control group had to postpone therapy initiation because of a positive Mantoux' test, their working situation or planned operations. Five patients ended therapy during the follow-up period because of treatment failure, allergic reactions, or suspected cancer. The 36 patients continuing anti-TNF-α treatment for 1 year (etanercept: 17, adalimumab: 10, infliximab: 9) were compared to the 19 patients who remained without anti-TNF-α therapy during the total follow-up period. The patients were examined with measurements of aPWV, central hemodynamic measures and the augmentation index (AIx), evaluation of disease activity and collection of fasting blood samples at baseline, and thereafter every third month until a total follow-up period of 1 year. CIMT was obtained at the baseline, 6 month, and 1-year visits.
Approval was obtained from the regional research ethics committee, and written informed consent was obtained from each participant. The study was performed according to the Helsinki Declaration.
Vascular measurements. Patients were examined in a quiet, temperature-controlled room after an overnight fast. All examinations were performed with the patients in a supine position after 10 min of relaxation. Systolic and diastolic brachial blood pressures were measured with an appropriately sized cuff using a validated automated device (Omron HEM-757, Kyoto, Japan).18
aPWV, central hemodynamic measures, and the AIx were assessed with the Sphygmocor device version 7.1 (AtCor Medical, Sydney, Australia) and a validated tonometer (SPC-301; Millar instruments, Houston,TX) as previously reported.7 In short, central pressures, the unadjusted AIx, and the heart rate adjusted AIx (AIx@75) were derived by applying a validated transfer system to recordings of the arterial pressure waveforms at the radial artery. aPWV was assessed as carotid-femoral pulse wave velocity. The carotid-femoral travel distance was obtained by subtracting the distance from the carotid measurement location to the sternal notch from the distance between the sternal notch and the femoral site of recording.3 The distances were measured on the body surface with a tape. Mean arterial pressure (MAP) was determined from the pressure waveforms obtained at the radial artery calibrated with brachial blood pressures.
CIMT was measured using the multiarray echotracking system Art.Lab (Esaote, Maastricht, the Netherlands) equipped with a 10–5 MHz linear array transducer.19 The system utilizes rough radio frequency data to register CIMT automatically for each subsequent cardiac cycle over a period of 6 s. CIMT was measured in the far wall of ~2-cm-long segments in the distal common carotid artery close to the carotid bulb on both sides.4 During examination, the patient's neck was slightly hyperextended and the head rotated away from the probe. We used a protractor frame to standardize the position of measurements at the consecutive visits. The rough radio frequency data were analyzed from stored cineloops. CIMT measurements in two patients were excluded from analyses because of insufficient quality.
All vascular measurements were made in triplicate by the same examiner (K.A.), and their mean was used in the analyses. The repeatability of the vascular measurements are given in the Supplementary Figure S1 online. The examiner did not participate in the treatment of the rheumatic disorder, and information of the patients' treatment was disclosed after completion of data collection and analyses.
Disease activity and laboratory measurements. Disease activity was evaluated as previously reported7 and included the Health Assessment Questionnaire (HAQ), visual analog scales for pain and global disease activity and the disease activity score based on 28 joints (DAS28). Erythrocyte sedimentation rate, C-reactive protein, total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol and triglycerides were determined by routine methodology at the time of examinations (Sysmex, Kobe, Japan and Roche Diagnostics GmbH, Mannheim, Germany).
Calprotectin was analyzed from EDTA plasma samples taken at the baseline, 3, and 12 months visits and stored at −80 °C. Samples were run in triplicate on calprotectin ELISA kits from Calpro AS, Lysaker, Norway. The mean of the closest two of triplicates was used for the calculations.
Statistical analyses. Continuous data are presented as mean with standard deviation or as median with quartile cut-points (25th and 75th percentiles) in the case of skewed variables and were compared using Student's independent sample, paired t tests, Mann–Whitney U test or related samples Wilcoxon test as appropriate. Categorical variables are given as numbers and were compared using Pearson's χ2 test. Bivariate relations were analyzed using Pearson's, or for skewed variables, Spearman's correlation coefficient. Associations with aPWV or CIMT were examined with multivariable mixed model repeated measures analyses. Mixed model repeated measures analysis is a linear regression analysis that controls for multiple testing of the same patient by modeling the covariance between the repeated measurements of each individual as a clustered random effect. An unstructured covariance matrix was used in our analyses. The longitudinal effects of anti-TNF-α therapy on aPWV and CIMT during the follow-up period were examined as the interaction between the variables anti-TNF-α therapy and time. Variables that were associated (P value < 0.25) with aPWV or CIMT in bivariate analyses, or variables known to influence aPWV or CIMT, were entered into the multivariable models. Variables were then removed in a step-down manner according to levels of significance. The models were examined for relevant interactions and confounding in a standard manner. Three patients failed to attend either visit 3 or visit 4. These visits are only included in the mixed model analyses. The mixed model procedure deals with missing values by assuming them to be missing at random without removing the individual from the dataset. The mixed model analyses were repeated after matching the 19 patients in the control group with 19 patients in the treatment group by sex, age, and MAP, and including the matched pairs as a random factor. P values ≤ 0.05 were considered significant. Statistical analyses were performed with SPSS, version 17.0 (SPSS, Chicago, IL) and R (R Development Core Team, 2008. R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients' demographic, comorbidity and baseline medication, disease activity, and hemodynamic parameters did not differ between the two patient groups (Table 1). All patients were Caucasians. None of the patients changed dosage or type of antihypertensive or lipid-lowering medication, or initiated treatment with other biological biological disease-modifying antirheumatic drugs during the follow-up period. Changes in methotrexate, prednisolone, nonsteroidal anti-inflammatory drugs, and sulphasalazine were not different between groups (data not shown).
Table 1.
Patients' demographic and baseline characteristics
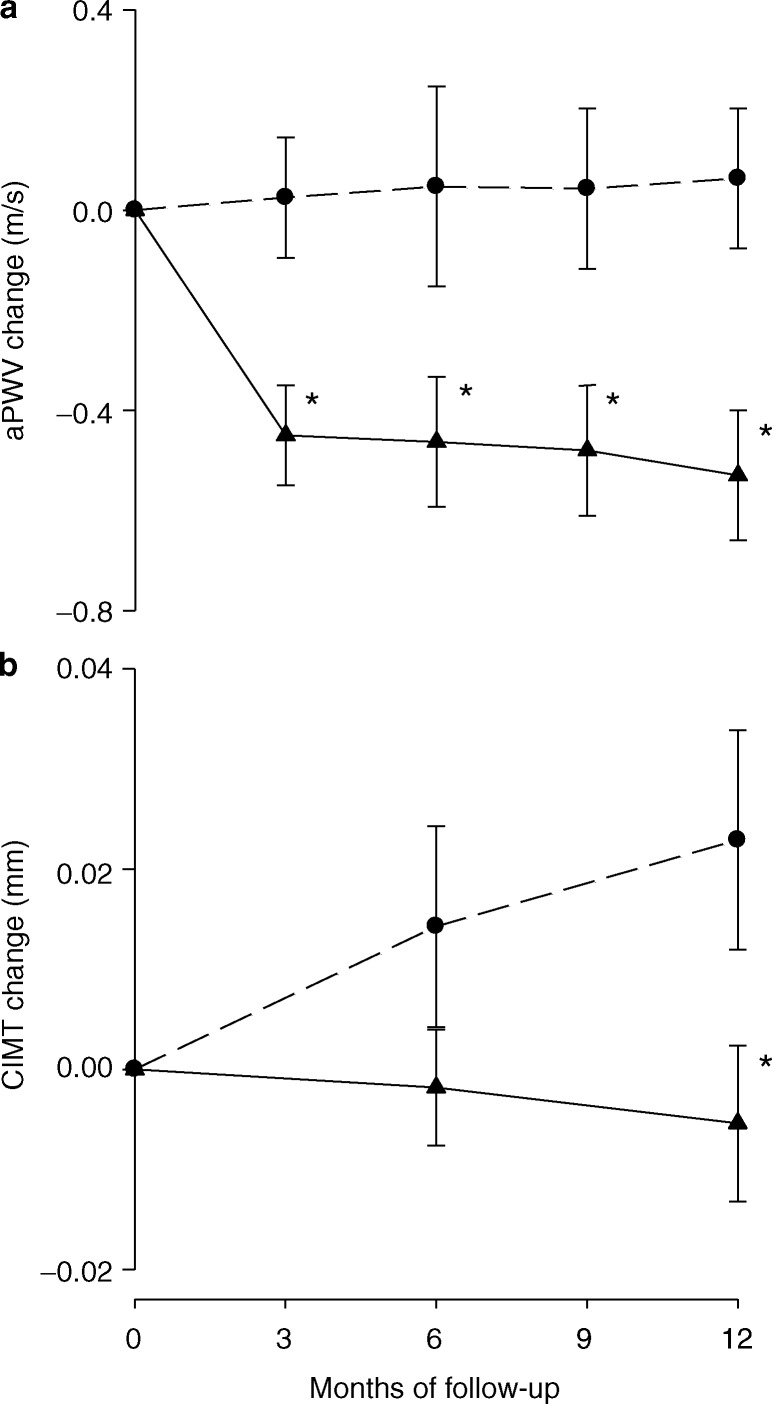
After 12 months, both aPWV and CIMT progression were significantly reduced in the treatment group compared to the control group (Table 2). Within-group analyses demonstrated a significant reduction in aPWV in the treatment group (baseline: 7.48 [1.60] m/s, 12 months: 6.94 [1.26] m/s, P < 0.001) but not in the control group (baseline: 7.50 [1.00] m/s, 12 months: 7.56 [1.48] m/s, P = 0.66), whereas CIMT increased only in the control group (baseline: 0.551 [0.462, 0.660] mm, 12 months: 0.569 [0.499, 0.668] mm, P = 0.02) but not in the treatment group (baseline: 0.542 [0.474, 0.645] mm, 12 months: 0.542 [0.437, 0.651] mm, P = 0.60). Changes in central pressures, AIx, and AIx@75 were not different between groups (Table 2). Within-group analyses showed that neither AIx (Treatment group; baseline: 24.4 [13.2] %, 12 months: 26.2 [10.8] %, P = 0.13. Control group; baseline: 29.6 [9.2] %, 12 months: 28.8 [8.6] %, P = 0.52) nor AIx@75 (treatment group, baseline: 20.0 [12.1] %, 12 months: 19.5 [10.5] %, P = 0.62; control group, baseline: 22.9 [10.8] %, 12 months: 22.6 [11.0] %, P = 0.72) changed in any of the groups. The greatest reduction in aPWV in the treatment group during the follow-up period was observed at the first 3 months after initiation of anti-TNF-α therapy (−0.46 [0.60] m/s, P < 0.001), whereas CIMT progression was constant during the 1-year follow-up (Figure 1 a) and b)). Changes in the level of disease activity, including changes in visual analog scales for pain (data not shown), were also favorable in the treatment group, whereas changes in lipids were similar between groups (Table 2).
Table 2.
Changes in hemodynamic, disease activity, and biochemical variables after 1 year
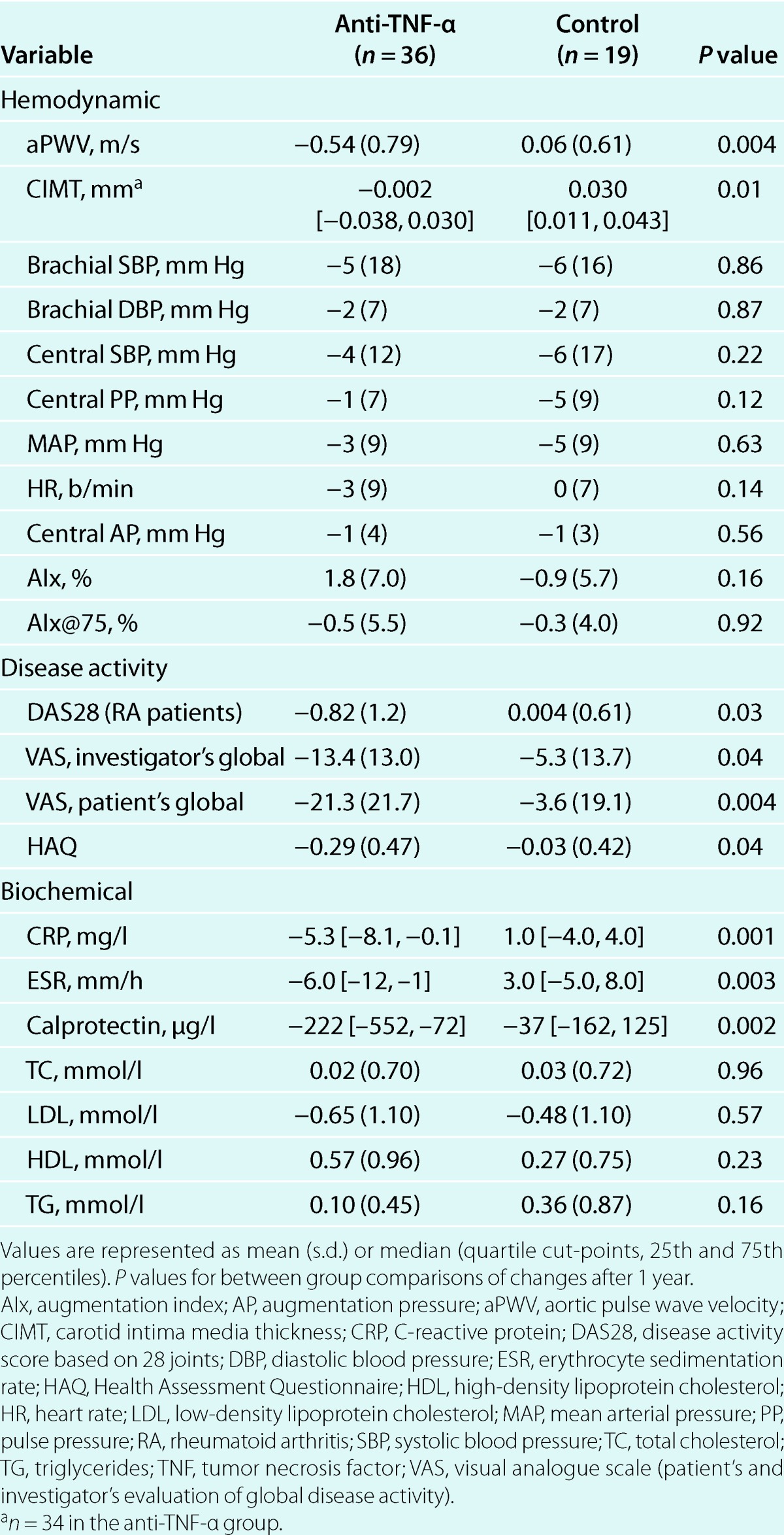
Figure 1. Changes in (a) aPWV and (b) CIMT from baseline to 1 year in the anti-TNF-α and the control group. Dark triangle, anti-TNF-α group, (a) n = 36, (b) n = 34. Open circle, control group, n = 19. Values are represented as mean (standard error). * Indicates significant changes from baseline in the anti-TNF-α group compared to the control group. aPWV, aortic pulse wave velocity; CIMT, carotid intima media thickness. TNF, tumor necrosis factor.
In the treatment group, significant reductions in aPWV were shown in all three diseases (RA: −0.47 [1.0] m/s, P = 0.04, AS: −0.36 [0.47] m/s, P = 0.05, PsA: −0.69 [0.61] m/s, P = 0.01). For changes in aPWV and CIMT by RA, AS, and PsA subgroups in the anti-TNF-α group see Supplementary Figure S2 online. The three different TNF-α antagonists used in the study had comparable effects on aPWV (adalimumab; −0.54 [1.1] m/s, P = 0.06, etanercept; −0.55 [0.65] m/s, P = 0.01, infliximab; −0.49 [0.65] m/s, P = 0.02).
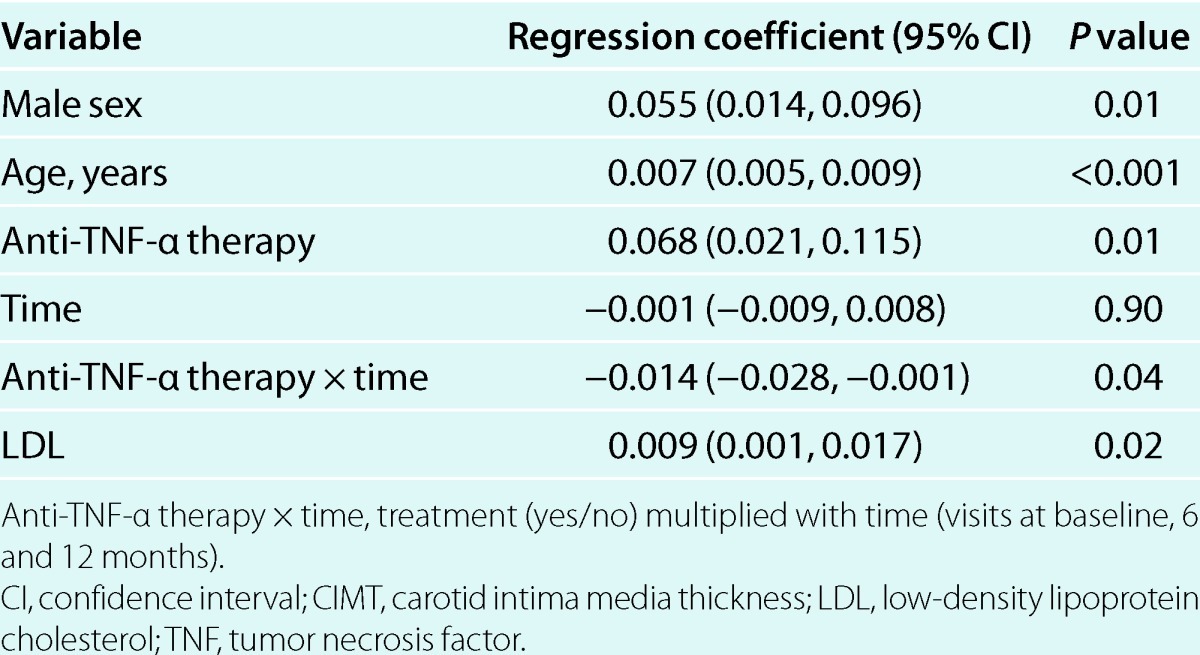
The multivariable mixed model repeated measures analyses showed that anti-TNF-α therapy over time was significantly associated with improvement in both aPWV and CIMT (dependent variables) (Tables 3 and 4). The final multivariable models were adjusted for age, sex, and MAP. MAP was neither significantly associated with CIMT in the final model (P = 0.07) nor a confounder, and are therefore not included in Table 4. Adjustment for duration of the inflammatory disease, rheumatic diagnose, use of other medication than TNF-α antagonists, or comorbidites did not change the results from the multivariable analyses. In bivariate analyses, changes in calprotectin after 1 year correlated significantly with changes in aPWV (ρ = 0.36, P = 0.04) but not CIMT (ρ = 0.12, P = 0.39). These findings were consistent with the results from the multivariate mixed model analyses, which showed that calprotectin was longitudinally associated with aPWV but not CIMT (Tables 3 and 4). Associations between C-reactive protein, erythrocyte sedimentation rate, or other disease activity measures and the vascular parameters were not observed, and adjustment for C-reactive protein (see Supplementary Table S1 online) or erythrocyte sedimentation rate in the multivariable models did not change the results. LDL was not associated with aPWV. Repeating the mixed model analyses after matching by sex, age and MAP did not change the results (data not shown). Furthermore, removal of premenopausal women did not influence the results (data not shown).
Table 3.
Mixed model with aPWV as dependent variable (n = 55, 5 visits)
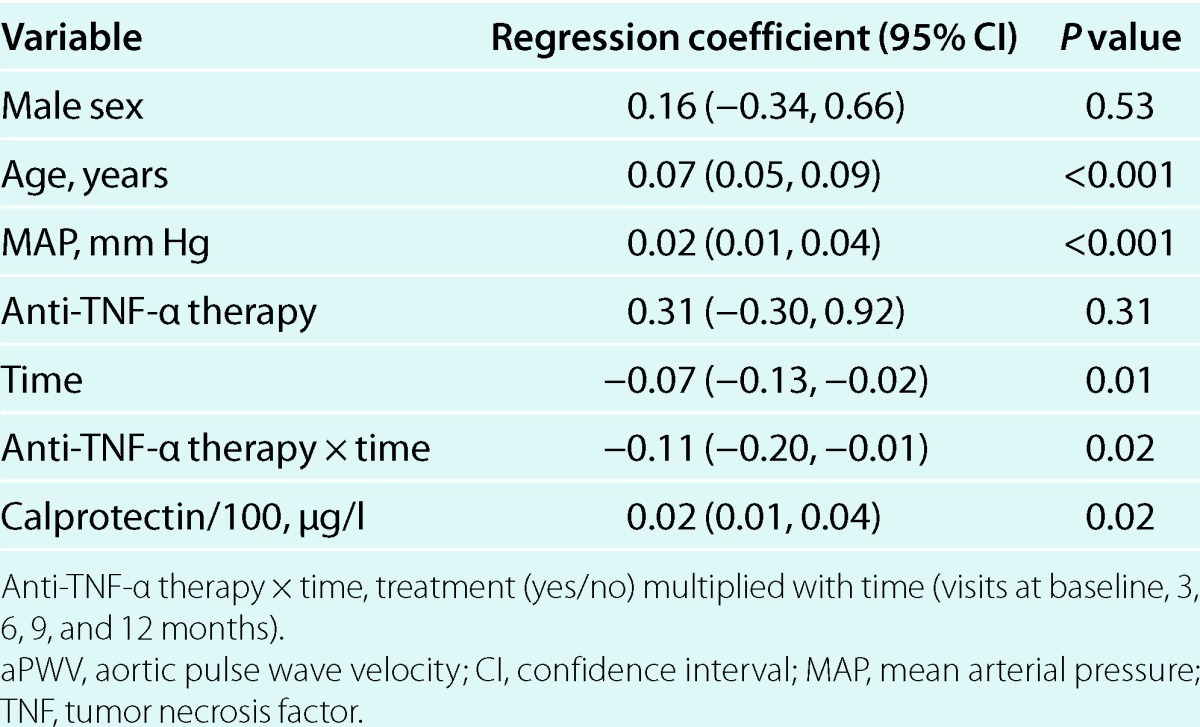
Table 4.
Mixed model with CIMT as dependent variable (n = 53, 3 visits)
Discussion
This study evaluated the effects of 1 year with anti-TNF-α therapy on aortic stiffness and CIMT in patients with inflammatory arthropathies. We showed that the patients who received treatment with a TNF-α antagonist maintained a beneficial effect on aPWV and had a reduced CIMT progression in comparison to an untreated control group (Table 2). Furthermore, we found for the first time that aPWV was longitudinally associated with calprotectin plasma levels (Table 3).
aPWV has previously been demonstrated to improve shortly after initiation of anti-TNF-α therapy in patients with inflammatory arthropathies.5,7 However, results regarding the long-term effects in AS and PsA have been missing, and the only previous report in RA is by Wong et al. who have shown in an uncontrolled study that aPWV improved after 56 weeks of therapy.8 Our data are in line with the results by Wong et al., and we have further expanded the results by including patients with AS and PsA. We observed that the main improvement in aPWV occurred during the first 3 months of anti-TNF-α therapy, while the treatment effect was maintained throughout the 1-year follow-up period (Figure 1a). According to the recently published pulse wave velocity reference values from The Reference Values for Arterial Stiffness Collaboration,20 the mean aPWV values in the treatment group at the visits after initiation of anti-TNF-α therapy were in the normal range. Thus, one possible interpretation of our results could be that anti-TNF-α therapy reduced aPWV to the expected level for an individual without systemic inflammation. In agreement with this explanation, Mäki-Petäjä et al. have previously reported that 12 weeks of anti-TNF-α therapy in patients with RA reduced aPWV to the level of healthy controls.5 Considering AIx and central pressures, the majority of previous studies have shown no change during treatment with TNF-α antagonists, supporting our finding.5,7,8,21,22 This may be explained by a normalization of peripheral vasculature tonus and an increase in wave reflection after initiation of anti-TNF-α therapy.23
CIMT is a validated surrogate endpoint for cardiovascular disease in statin and antihypertensive trials, and its progression is reduced in a linear fashion by most lipid- and blood pressure-lowering agents.24,25 Previous data regarding the effect of anti-TNF-α therapy on CIMT are inconsistent and only include patients with RA. Two studies, which both included RA patients receiving conventional therapy as controls, have shown a reduction in CIMT after anti-TNF-α therapy.6,9 In contrast, Wong et al., Sidiropoulos et al., and Gonzalez-Juanatey et al. did not find any effect of such treatment on CIMT.8,10,11 In the present study, anti-TNF-α therapy had a beneficial effect on the CIMT progression (Tables 2 and 4). The absolute difference in CIMT progression between the patient groups was small and could be attributed to normal flucations in repeated CIMT measurements (Supplementary Figure S1 online). However, the CIMT progression rates in the present study were significantly different both in univariate and multivariable analyses, and the effect of anti-TNF-α therapy was comparable with that reported in studies of statin efficacy also using far wall common carotid artery intima media thickness as an endpoint.26 The negative findings reported by Wong et al., Sidiropoulos et al., and Gonzalez-Juanatey et al. might be because of small patient numbers and lack of control groups, which make significant effects on CIMT progression difficult to detect.
Increased aortic stiffness and CIMT have previously been shown in cross-sectional studies to be related to inflammatory biomarkers and disease activity in RA.5,9 The patients receiving treatment in the present study displayed significant improvement in both biochemical and clinical measures of inflammatory activity (Table 2). We specifically addressed whether calprotectin could be associated with changes in aPWV and CIMT because of its association both to the inflammatory activity in inflammatory arthropathies13,14 and to atherogenesis and vascular pathology.15–17 The systemic inflammation in inflammatory arthropathies may promote arterial stiffening and CIMT progression by inducing endothelial dysfunction and inflammatory cell influx to the artery wall. Calprotectin has been demonstrated to interact with the receptor for advanced glycation end products and toll-like receptor 4, which both augment vascular inflammatory processes through increased cytokine expression.27,28 In the present study, calprotectin was significantly associated with aPWV in both bivariate and multivariable longitudinal analyses, but not to CIMT. To our knowledge, previous data regarding the association between calprotectin and aPWV have been missing, and only one study by Larsson et al. has examined correlations between circulating calprotectin and CIMT.29 Larsson et al. found a positive correlation between carotid plaque occurrence but not CIMT and calprotectin, which is in line with our results. Previous results suggest that CIMT and arterial stiffness may be differently related to diverse cardiovascular risk factors such as LDL cholesterol and C-reactive protein,30,31 with LDL cholesterol being more strongly associated to CIMT than to arterial stiffness. Therefore, LDL cholesterol together with age, sex, and treatment over time may dominate as the explaining factors for CIMT in the present study.
There are some study limitations to consider. This study had a nonrandomized design. As the inclusion criterion was a clinical indication for anti-TNF-α therapy, we considered it unethical to randomize to treatment or placebo. The choice of control group could introduce potential bias, but the treatment and control groups were recruited from the same clinical setting and did not differ with regard to demographic characteristics or baseline disease activity. Furthermore, the results favoring the effect of anti-TNF-α therapy were consistent in multivariable analyses even after matching the patients by sex, age, and MAP. Although we consider the association between calprotectin and aPWV in the present study robust, it does not prove any cause-effect relationship. Calprotectin's influence on aortic stiffening obviously needs further exploration in both clinical and translational research. Finally, the missing association between calprotectin and CIMT, and the lack of change in central hemodynamics and AIx, may be because of the limited patient number in our study, and future studies in larger populations are needed.
In conclusion, the present study shows that long-term anti-TNF-α therapy has a maintained beneficial effect on aortic stiffness and reduces CIMT progression in patients with inflammatory arthropathies. The proinflammatory protein complex calprotectin was longitudinally associated with aortic stiffness and might be a candidate soluble biomarker of vascular dysfunction in these patients.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ajh
Supplementary Figure S1
Supplementary Figure S2
Supplementary Table S1
Acknowledgments
K.A. was supported by the Norwegian Foundation for Health and Rehabilitation (grant number 2004/2/0255) and the Norwegian Rheumatism Association.
Disclosure
T.K.K. has received research grants and/or consultancy fees from Abbott, BMS, MSD/Schering-Plough, Pfizer/Wyeth, Roche and UBS. M.K.F. shares patent rights on calprotectin. The other authors declared no conflict of interest.
References
- 1.Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, McInnes IB, Haentzschel H, Gonzalez-Gay MA, Provan S, Semb A, Sidiropoulos P, Kitas G, Smulders YM, Soubrier M, Szekanecz Z, Sattar N, Nurmohamed MT. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 2010;69:325–331 [DOI] [PubMed] [Google Scholar]
- 2.Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor a therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011;63:522–529 [DOI] [PubMed] [Google Scholar]
- 3.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588–2605 [DOI] [PubMed] [Google Scholar]
- 4.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Fatar M, Hernandez Hernandez R, Jaff M, Kownator S, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS, Zannad F, Zureik M. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis 2007;23:75–80 [DOI] [PubMed] [Google Scholar]
- 5.Mäki-Petäjä KM, Hall FC, Booth AD, Wallace SM, Yasmin, Bearcroft PW, Harish S, Furlong A, McEniery CM, Brown J, Wilkinson IB. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation 2006;114:1185–1192 [DOI] [PubMed] [Google Scholar]
- 6.Ferrante A, Giardina AR, Ciccia F, Parrinello G, Licata G, Avellone G, Giardina E, Impastato R, Triolo G. Long-term anti-tumour necrosis factor therapy reverses the progression of carotid intima-media thickness in female patients with active rheumatoid arthritis. Rheumatol Int 2009;30:193–198 [DOI] [PubMed] [Google Scholar]
- 7.Angel K, Provan SA, Gulseth HL, Mowinckel P, Kvien TK, Atar D. Tumor necrosis factor-alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: a controlled study. Hypertension 2010;55:333–338 [DOI] [PubMed] [Google Scholar]
- 8.Wong M, Oakley SP, Young L, Jiang BY, Wierzbicki A, Panayi G, Chowienczyk P, Kirkham B. Infliximab improves vascular stiffness in patients with rheumatoid arthritis. Ann Rheum Dis 2009;68:1277–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Porto F, Laganà B, Lai S, Nofroni I, Tinti F, Vitale M, Podestà E, Mitterhofer AP, D'Amelio R. Response to anti-tumour necrosis factor alpha blockade is associated with reduction of carotid intima-media thickness in patients with active rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1111–1115 [DOI] [PubMed] [Google Scholar]
- 10.Sidiropoulos PI, Siakka P, Pagonidis K, Raptopoulou A, Kritikos H, Tsetis D, Boumpas DT. Sustained improvement of vascular endothelial function during anti-TNFalpha treatment in rheumatoid arthritis patients. Scand J Rheumatol 2009;38:6–10 [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Juanatey C, Llorca J, Garcia-Porrua C, Martin J, Gonzalez-Gay MA. Effect of anti-tumor necrosis factor alpha therapy on the progression of subclinical atherosclerosis in severe rheumatoid arthritis. Arthritis Rheum 2006;55:150–153 [DOI] [PubMed] [Google Scholar]
- 12.Steinbakk M, Naess-Andresen CF, Lingaas E, Dale I, Brandtzaeg P, Fagerhol MK. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet 1990;336:763–765 [DOI] [PubMed] [Google Scholar]
- 13.Brun JG, Jonsson R, Haga HJ. Measurement of plasma calprotectin as an indicator of arthritis and disease activity in patients with inflammatory rheumatic diseases. J Rheumatol 1994;21:733–738 [PubMed] [Google Scholar]
- 14.De Rycke L, Baeten D, Foell D, Kruithof E, Veys EM, Roth J, De Keyser F. Differential expression and response to anti-TNFalpha treatment of infiltrating versus resident tissue macrophage subsets in autoimmune arthritis. J Pathol 2005;206:17–27 [DOI] [PubMed] [Google Scholar]
- 15.Healy AM, Pickard MD, Pradhan AD, Wang Y, Chen Z, Croce K, Sakuma M, Shi C, Zago AC, Garasic J, Damokosh AI, Dowie TL, Poisson L, Lillie J, Libby P, Ridker PM, Simon DI. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation 2006;113:2278–2284 [DOI] [PubMed] [Google Scholar]
- 16.Altwegg LA, Neidhart M, Hersberger M, Müller S, Eberli FR, Corti R, Roffi M, Sütsch G, Gay S, von Eckardstein A, Wischnewsky MB, Lüscher TF, Maier W. Myeloid-related protein 8/14 complex is released by monocytes and granulocytes at the site of coronary occlusion: a novel, early, and sensitive marker of acute coronary syndromes. Eur Heart J 2007;28:941–948 [DOI] [PubMed] [Google Scholar]
- 17.Croce K, Gao H, Wang Y, Mooroka T, Sakuma M, Shi C, Sukhova GK, Packard RR, Hogg N, Libby P, Simon DI. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation 2009;120:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Assaad MA, Topouchian JA, Asmar RG. Evaluation of two devices for self-measurement of blood pressure according to the international protocol: the Omron M5-I and the Omron 705IT. Blood Press Monit 2003;8:127–133 [DOI] [PubMed] [Google Scholar]
- 19.Brands PJ, Hoeks AP, Willigers J, Willekes C, Reneman RS. An integrated system for the non-invasive assessment of vessel wall and hemodynamic properties of large arteries by means of ultrasound. Eur J Ultrasound 1999;9:257–266 [DOI] [PubMed] [Google Scholar]
- 20.The Reference Values for Arterial Stiffness' Collaboration Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values'. Eur Heart J 2010;31:2338–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathieu S, Joly H, Baron G, Tournadre A, Dubost JJ, Ristori JM, Lusson JR, Soubrier M. Trend towards increased arterial stiffness or intima-media thickness in ankylosing spondylitis patients without clinically evident cardiovascular disease. Rheumatology (Oxford) 2008;47:1203–1207 [DOI] [PubMed] [Google Scholar]
- 22.Van Doornum S, McColl G, Wicks IP. Tumour necrosis factor antagonists improve disease activity but not arterial stiffness in rheumatoid arthritis. Rheumatology (Oxford) 2005;44:1428–1432 [DOI] [PubMed] [Google Scholar]
- 23.Irace C, Mancuso G, Fiaschi E, Madia A, Sesti G, Gnasso A. Effect of anti TNFalpha therapy on arterial diameter and wall shear stress and HDL cholesterol. Atherosclerosis 2004;177:113–118 [DOI] [PubMed] [Google Scholar]
- 24.Espeland MA, O'leary DH, Terry JG, Morgan T, Evans G, Mudra H. Carotid intimal-media thickness as a surrogate for cardiovascular disease events in trials of HMG-CoA reductase inhibitors. Curr Control Trials Cardiovasc Med 2005;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JG, Staessen JA, Li Y, Van Bortel LM, Nawrot T, Fagard R, Messerli FH, Safar M. Carotid intima-media thickness and antihypertensive treatment: a meta-analysis of randomized controlled trials. Stroke 2006;37:1933–1940 [DOI] [PubMed] [Google Scholar]
- 26.Bots ML. Carotid intima-media thickness as a surrogate marker for cardiovascular disease in intervention studies. Curr Med Res Opin 2006;22:2181–2190 [DOI] [PubMed] [Google Scholar]
- 27.Boyd JH, Kan B, Roberts H, Wang Y, Walley K. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation and products. Circ res 2008;102:1239–1246 [DOI] [PubMed] [Google Scholar]
- 28.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 2007;13:1042–1049 [DOI] [PubMed] [Google Scholar]
- 29.Larsson PT, Hallerstam S, Rosfors S, Wallén NH. Circulating markers of inflammation are related to carotid artery atherosclerosis. Int Angiol 2005;24:43–51 [PubMed] [Google Scholar]
- 30.Kampus P, Kals J, Ristimäe T, Muda P, Ulst K, Zilmer K, Salonen RM, Tuomainen TP, Teesalu R, Zilmer M. Augmentation index and carotid intima-media thickness are differently related to age, C-reactive protein and oxidized low-density lipoprotein. J Hypertens 2007;25:819–825 [DOI] [PubMed] [Google Scholar]
- 31.Oren A, Vos LE, Uiterwaal CS, Grobbee DE, Bots ML. Aortic stiffness and carotid intima-media thickness: two independent markers of subclinical vascular damage in young adults? Eur J Clin Invest 2003;33:949–954 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Table S1