Abstract
Background. Pregnancy-associated malaria (PAM) produces poor birth outcomes, but its prevalence is commonly estimated in convenience samples.
Methods. We assessed the prevalence of malaria using real-time polymerase chain reaction (PCR) and estimated the consequences of infection on birth outcomes, using specimens from a nationally representative sample of 4570 women of childbearing age (WOCBA) responding to the 2007 Demographic and Health Survey in Democratic Republic of the Congo (DRC).
Results. Overall, 31.2% (95% confidence interval [CI], 29.2–33.1) of WOCBA were parasitemic, which was significantly more common in pregnant (37.2% [31.0–43.5]) than nonpregnant women (30.4% [CI, 28.4–32.5], prevalence ratio [PR] 1.22 [1.02–1.47]). Plasmodium falciparum was highest among pregnant women (36.6% vs 28.8%, PR 1.27 [1.05–1.53]). By contrast, P malariae was less common in pregnant (0.6%) compared with nonpregnant women (2.7%, PR 0.23 [0.09–0.56]). Extrapolation of the prevalence estimate to the population at risk of malaria in DRC suggests 1.015 million births are affected by P falciparum infection annually, and that adherence to preventive measures could prevent up to 549 000 episodes of pregnancy-associated malaria and 47 000 low-birth-weight births.
Conclusions. Pregnancy-associated malaria and its consequences are highly prevalent in the DRC. Increasing the uptake of malaria preventive measures represents a significant opportunity to improve birth outcomes and neonatal health.
Pregnancy-associated malaria (PAM) is the most important preventable cause of poor birth outcomes in sub–Saharan Africa [1]. Most malaria-endemic countries have adopted PAM-prevention measures such as insecticide-treated bednets (ITNs) use and intermittent preventive therapy during pregnancy with sulfadoxine-pyrimethamine (IPTp-SP) [2, 3]. Both ITNs and IPTp-SP are clinically and cost-effective interventions to prevent PAM-associated low birth-weight (LBW) [4–7]. Ambitious targets are endorsed for near-universal deployment of these interventions [8], though uptake in sub–Saharan Africa is inadequate [3].
Nationally representative estimates of PAM prevalence are lacking owing to a dearth of surveys that utilize scientific probability sampling of a random sample of the entire population. Thus, current estimates of burden are typically derived from convenience samples that do not reflect national populations. In malaria-endemic sub–Saharan Africa, a recent spatial demographic study estimated that 31 million pregnancies occur annually that result in approximately 23 million live births [9]. The prevalence of malaria during pregnancy is reported as high as 25%–28% [10, 11], but more sensitive parasite detection methods using polymerase chain reaction (PCR) suggest that the true prevalence may be considerably higher [12–14].
The Democratic Republic of the Congo (DRC) has the second-highest population at risk of malaria in Africa [15] and over 3.5 million annual pregnancies at risk [3, 9], but there are no data on the prevalence of malaria parasitemia among pregnant and nonpregnant women of childbearing age (WOCBA). Herein, we describe the first nationally representative, cross-sectional molecular survey of pregnancy-associated malaria. Using specimens collected during the 2007 Demographic and Health Survey (DHS) in DRC, we employed real-time PCR to estimate both the burden of infection and the birth consequences of PAM in this highly endemic region.
MATERIALS AND METHODS
Ethics Statement
Study procedures were approved by the review boards of Macro International, the University of Kinshasa School of Public Health, and the University of North Carolina. All survey respondents provided informed consent verbally owing to the need for immediate deidentification of all data.
Survey Design and Sample Collection
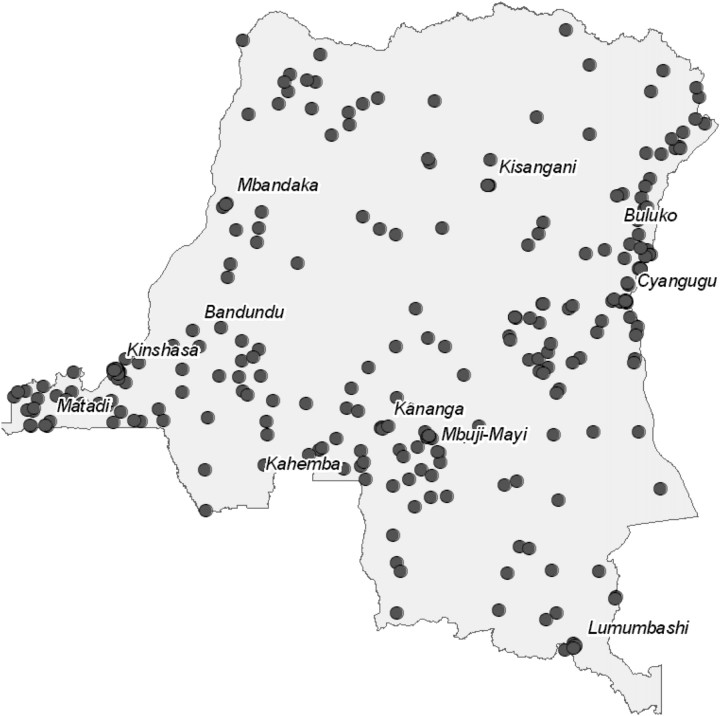
The DHS surveyed women aged 15 to 49 years within 300 geographic clusters randomly selected from demographic population density data obtained before the 2006 national elections (Figure 1) [16]. From these clusters, 9000 households were randomly selected for inclusion, in which all women were surveyed. The survey was designed to collect blood samples in order to determine human immunodeficiency virus (HIV) serostatus and measure hemoglobin (Hgb) levels of women from every other household. Pregnancy status and estimated trimester were determined by self-report. Clusters near Kinshasa were surveyed during the rainy season in February and March; the difficulty of transportation necessitated surveying outlying clusters during the dry season between May and August.
Figure 1.
Geographic clusters in Democratic Republic of the Congo in which women of childbearing age were sampled for survey.
After consent was obtained, a fingerprick was taken for hemoglobin assessment and dried blood spots (DBSs). HIV was determined by enzyme-linked immunosorbent assay and Western blot testing. Hemoglobin point-of-care testing was performed using a HemoCue photometer. Hemoglobin results were immediately communicated to participants, and those with moderate to severe anemia (Hgb < 9 g/dL or < 7 g/dL for pregnant and nonpregnant women, respectively) were referred to local health care services. DBSs were stored at room temperature in individual bags prior to PCR testing.
Molecular Testing
Real-time PCR testing has been described in detail elsewhere [17]. Briefly, testing employed 2 assays that target the 18S ribosomal DNA sequence of Plasmodia in order to distinguish between Plasmodium falciparum, P. malariae, and P. ovale. To minimize the risk of sample contamination, filtered pipet tips were exclusively employed in all steps, and separate work areas were maintained for punching discs from DBSs, extracting genomic DNA, preparing reaction mixtures, and assembling reaction plates.
Statistical Analyses
The overall and species-specific prevalence of parasitemia were calculated for the overall sample and in pregnant and nonpregnant women. The species distributions of parasitemias were compared with Fisher exact test between pregnant and nonpregnant WOCBA. To determine the effect of pregnancy and other risk factors on the risk of malaria parasitemia, adjusted prevalence ratios (aPRs) for parasitemias and anemia were calculated using robust Poisson regression models with pregnancy as the exposure variable of interest using the overall sample. Separate models of risk factors were then developed for WOCBA and pregnant women. Independent variables with prevalence ratios (PRs) for parasitemia significant at a level of 0.2 were included in the multivariate models. In addition, known determinants of parasitemia risk were included in the full model irrespective of bivariate statistical significance.
The contribution of malaria parasitemia to maternal anemia was assessed in separate Poisson models using a similar approach, but with anemia (defined as Hgb < 11 g/dL, adjusted for altitude) as the outcome variable and with malaria and common risk factors as the exposure variable and covariates. Because parasitemia was the main exposure of interest, it was always included in the final multivariate model. For this analysis, severe anemia was defined as Hgb < 7 g/dL.
All statistical analyses were performed with Stata/IC (v10, Stata Corp, College Station, TX). All statistical analyses incorporated survey sampling weights to account for survey design in generating nationally representative estimates.
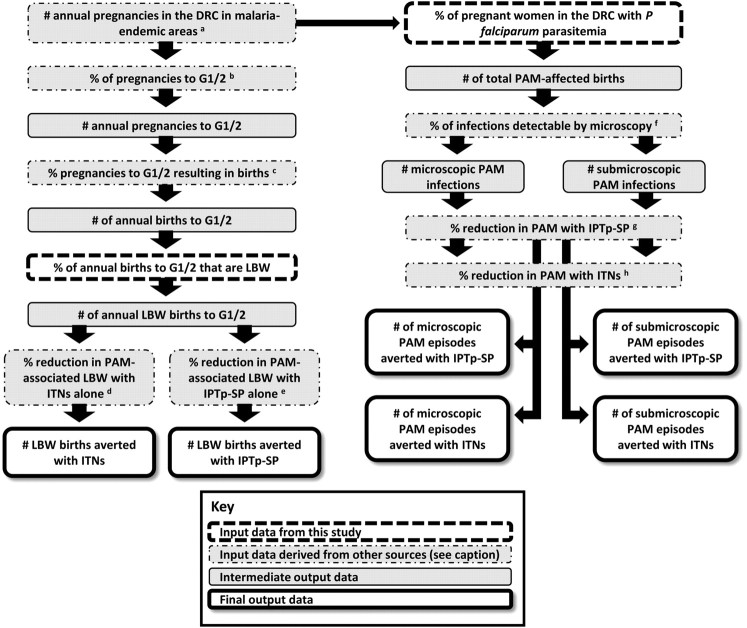
Extrapolation to Annual Number of Pregnancies Affected and Impact of Malaria Control
The nationally representative sample provided the opportunity to estimate (1) the total annual number of pregnancies affected by malaria in DRC, and (2) the potential impact of successful malaria control by ITNs and IPTp-SP (Figure 2). The lack of reliable estimates of the risk of mortality attributable to LBW in sub–Saharan Africa precluded an extrapolated estimate of PAM-associated infant mortality.
Figure 2.
Schematic approach to the extrapolation of survey results to PAM burden and control estimates.
Abbreviations: PAM, pregnancy-associated malaria; DRC, Democratic Republic of the Congo; G1/2, primi- and secundigravidae; ITN, insecticide-treated bednet; IPTp-SP, intermittent preventive therapy with sulfadoxine-pyrimethamine; LBW: low birth weight.
b Reference [16].
c Reference [9].
d Reference [7].
e Reference [4].
f Reference [17].
h Reference [20].
To obtain estimates of the number of pregnancies per year and the number of births per year, we used the national estimates of the annual number of live births for 2005–2010 from the 2008 revision of the population database of the United Nations Population Division [18] and added estimates of stillbirths and induced abortions projected for 2007 [9]. The reported national fertility rate for DRC from 2005 to 2010 was used to estimate the fraction and numbers of births in each gravidity category. We deducted 6.2% of the national estimates of pregnancies and births to obtain the number at risk of malaria because of the reported absence of malaria risk in this proportion of DRC’s population [[15], protocol S1].
The potential impact of successful malaria control in pregnancy on placental malaria and LBW was estimated by using the summary protective efficacy of ITNs and IPTp-SP obtained from previous meta-analyses of randomized controlled trials: These suggest that ITNs and IPTp-SP can reduce the prevalence of malaria at delivery among all gravidae by 24% and 57%, respectively [4, 19, 20]. Additionally, ITNs or IPTp-SP have been shown to reduce the prevalence of LBW among primi- and secundigravidae (G1/2) by 23% [7] and 29% [4], respectively. We estimated that microscopy would detect 68% of PCR-detectable parasitemias, based on a previous study of Congolese pregnant women using identical PCR protocols [21].
The prevalence estimates of LBW, combined with the absolute number of pregnancies at risk per gravidity group, were then used to compute the number of births resulting in LBW, which in turn was used to estimate the number that could be prevented by successful malaria control in pregnancy. Because IPTp-SP is provided in the second and third trimester only, the number of births was used for these impact estimates. We estimated the impact of IPTp-SP and ITNs on LBW in the first 2 pregnancies only. In order to indirectly obtain the number of LBW births to G1/2 only, we combined national estimates of the prevalence of LBW in all gravidae in DRC in 2007 (reported as 12.5% by the World Health Organization Department of Making Pregnancy Safer [22]) with data on the relative distribution of LBW by these gravidae groups obtained from other studies in malaria-endemic areas and with the number of annual births in these strata obtained from demographic data [3, 9]. The summary relative risk of LBW among G1/2 versus G3+ (2.06) was obtained from a random effect meta-analysis model using data from 6 observational studies in malaria-endemic areas in Africa since 1997 [23–28]. These studies were identified using the Malaria in Pregnancy Library [29], and meta-analysis was performed using Comprehensive Meta-Analysis (version 2.0, Biostat, Englewood, NJ).
RESULTS
Overall, 9995 WOCBA were surveyed, of which 1100 (11.0%) reported to be pregnant. Because the survey was designed to collect blood samples from only half of the women, biometric data were available from 4570 WOCBA, of whom 520 (11.3%; 95% confidence interval [CI], 10.0–12.6) reported being currently pregnant. These 4570 women constituted the sample of analysis, and there were no significant differences in age, residence, bednet use, pregnancy, or wealth between women with and without blood samples collected (data not shown).
Among the subset of 2669 WOCBA queried about bednets, bednet use the night prior to the interview was infrequent (23.4%; 95% CI, 21.1–25.7) either with an ITN (6.6%; 95% CI, 5.3–7.9) or an untreated net (16.8%; 95% CI, 14.8–18.8); use was highly variable between clusters [17]. Similar use was reported when the analysis was restricted to pregnant women (data available for 390): only 7.0% (95% CI, 2.5–11.4) reported using an ITN and 14.6% (95% CI, 10.0–19.2) reported the use of an untreated net. There were no data regarding bednet source, antenatal care, or the use of antenatal antimalarials.
Prevalence of Parasitemia
The overall prevalence of malaria parasitemia among WOCBA was 31.2% (95% CI, 29.2–33.1), and this was 37.2% among the 520 pregnant women (95% CI, 31.0–43.5) compared with 30.4% (95% CI, 28.4–32.5) among the nonpregnant women (PR, 1.22; 95% CI, 1.02–1.47) (Table 1).
Table 1.
Parasitemias Among Pregnant and Nonpregnant Women
| All WOCBA, % (n = 4570) | Pregnant, % (n = 520) | Nonpregnant, % (n = 4050) | PRa | p valuea | |
| P. falciparum b | 29.7 (27.8–31.6) | 36.6 (30.3–42.9) | 28.8 (26.8–30.8) | 1.27 (1.05–1.53) | .0114 |
| P. malariae b | 0.6 (0.1–1.2) | 0.6 (0.08–1.2) | 2.7 (2–3.5) | 0.23 (0.09–0.56) | .0015 |
| P. ovale b | 0.5 (0.2–0.8) | 0.7 (0–1.6) | 0.5 (0.1–0.8) | 1.49 (0.36–6.09) | .5781 |
| Any species | 31.2 (29.2–33.1) | 37.2 (31–43.5) | 30.4 (28.4–32.5) | 1.22 (1.02–1.47) | .0284 |
Abbreviations: WOCBA, women of childbearing age; PR, prevalence ratio. Proportions and prevalence ratios were calculated using sampling weights. Values are percentages; those in parentheses are 95% confidence intervals.
For comparison of pregnant versus nonpregnant women; calculated using Poisson regression.
Includes mixed-species infections.
P. falciparum accounted for most infections (95.3%) and P. malariae and P. ovale monoinfections for most of the remainder (Figure 3). While the prevalence of P. falciparum was higher among pregnant women (36.6%) than their nonpregnant (28.8%) peers (PR, 1.27; 95% CI, 1.05–1.53), P. malariae was less common in pregnant (0.6%) compared with nonpregnant (2.7%) women (PR, 0.23; 95% CI, 0.09–0.56). Nevertheless, among parasitemic women, the overall distribution of infecting species did not differ significantly between pregnant and nonpregnant women (Fisher exact P value .222). P. ovale was rare within parasitemias in both groups (overall prevalence 1.7%; 95% CI, 0.6–2.7).
Figure 3.
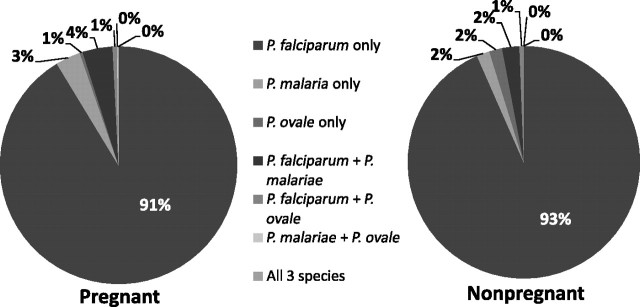
Infecting Plasmodium species among parasitemic pregnant and nonpregnant women.
Predictors of Parasitemia
Nonpregnant WOCBA
Among 4050 women who were not currently pregnant, bivariate analyses demonstrated that malaria parasitemia was significantly associated only with urban (compared with rural) residence and all wealth quintiles below the highest (Table 2). In a multivariate model, only increasing age and increasing wealth quintiles were significantly associated with lower prevalences of parasitemia.
Table 2.
Bivariate and Multivariate Models of the Risk of Malaria Parasitemia (any Species) Among All WOCBA, Pregnant, and Nonpregnant Women
| No. of women | Parasitemic, % (95% CI) | PR (95% CI) | PR P value | aPRa (95% CI) | aPR P valuea | |
| All WOCBA (n = 4570) | ||||||
| Age | ||||||
| 15–24 | 1932 | 34.1 (31.1–37.1) | REF | .0357 | REF | .008 |
| 25–34 | 1407 | 28.4 (24.9–31.8) | 0.83 (0.72–0.97) | 0.82 (0.70–0.95) | ||
| 35–49 | 1231 | 29.7 (25.9–33.6) | 0.87 (0.75–1.02) | 0.83 (0.71–0.97) | ||
| HIV | ||||||
| Negative | 449 | 31.4 (29.4–33.4) | REF | .0889 | REF | .1683 |
| Positive | 179 | 15.9 (3.4–28.3) | 0.51 (0.23–1.11) | 0.58 (0.27–1.26) | ||
| Residence | ||||||
| Urban | 2195 | 24.9 (22.4–27.3) | REF | <.0001 | REF | .2484 |
| Rural | 2375 | 36.3 (33.4–39.3) | 1.46 (1.29–1.66) | 0.91 (0.76–1.07) | ||
| Wealth index | ||||||
| Highest | 1090 | 13.9 (11.6–16.2) | REF | <.0001 | REF | <.0001 |
| High | 946 | 30.4 (26.3–34.5) | 2.18 (1.76–2.7) | 2.23 (1.79–2.77) | ||
| Moderate | 870 | 35.1 (30.1–40.1) | 2.52 (2.02–3.14) | 2.70 (2.11–3.45) | ||
| Low | 812 | 41.0 (36.1–46.0) | 2.94 (2.4–3.62) | 3.23 (2.51–4.14) | ||
| Lowest | 852 | 37.7 (32.8–42.6) | 2.71 (2.19–3.34) | 2.96 (2.27–3.86) | ||
| Currently pregnant | ||||||
| No | 4050 | 30.4 (28.4–32.5) | REF | .0284 | REF | .1886 |
| Yes | 520 | 37.2 (31.0–43.5) | 1.22 (1.02–1.47) | 1.13 (0.94–1.35) | ||
| Pregnant women (n = 520) | ||||||
| Age | ||||||
| 15–24 | 237 | 42.6 (33.3–52.0) | REF | .2993 | REF | .5814 |
| 25–34 | 207 | 32.8 (22.6–42.9) | 0.77 (0.53–1.12) | 0.81 (0.54–1.21) | ||
| 35–49 | 76 | 32.1 (16.6–47.3) | 0.75 (0.44–1.28) | 0.87 (0.51–1.50) | ||
| HIV | ||||||
| Negative | 514 | 37.4 (31.1–43.7) | REF | .1557 | REF | .4072 |
| Positive | 6 | 8.3 (0–25.6) | 0.22 (0.03–1.77) | 0.43 (0.06–3.16) | ||
| Residence | ||||||
| Urban | 207 | 30.1 (20.8–39.3) | REF | .821 | REF | .7615 |
| Rural | 313 | 41.6 (33.3–50.0) | 1.38 (0.96–2) | 0.94 (0.63–1.41) | ||
| Wealth index | ||||||
| Highest | 78 | 12.0 (4.0–20.1) | REF | .0002 | REF | .0009 |
| High | 107 | 31.5 (18.4–44.5) | 2.61 (1.19–5.73) | 2.58 (1.16–5.75) | ||
| Moderate | 116 | 52.5 (39.0–65.9) | 4.36 (2.13–8.9) | 4.24 (1.94–9.24) | ||
| Low | 99 | 49.3 (34.1–64.5) | 4.1 (1.96–8.53) | 4.16 (1.87–9.24) | ||
| Lowest | 120 | 30.7 (19.2–42.1) | 2.55 (1.19–5.47) | 2.47 (1.06–5.75) | ||
| Gravidity | ||||||
| Primigravidae | 110 | 43.7 (30.4–57.0) | REF | .5501 | REF | .5101 |
| Secundigravidae | 81 | 34.2 (18.9–49.5) | 0.78 (0.46–1.34) | 0.74 (0.44–1.24) | ||
| Multigravidae | 329 | 36.3 (28.2–44.3) | 0.83 (0.57–1.21) | 0.93 (0.63–1.40) | ||
| Trimester | ||||||
| First | 136 | 40.8 (28.1–53.4) | REF | .6259 | REF | .6701 |
| Second | 223 | 37.8 (28.1–47.5) | 0.93 (0.62–1.39) | 0.92 (0.64–1.33) | ||
| Third | 161 | 32.9 (22.5–43.3) | 0.81 (0.52–1.25) | 0.83 (0.55–1.25) | ||
| Bednet use | ||||||
| ITN | 23 | 48.5 (14.4–82.7) | REF | .3614 | Not included | … |
| Untreated | 73 | 26.4 (12.3–40.5) | 0.72 (0.40–1.28) | |||
| None | 294 | 36.8 (28.6–45.1) | 1.32 (0.63–2.75) | |||
| Nonpregnant WOCBA (n = 4050) | ||||||
| Age | ||||||
| 15–24 | 1695 | 32.9 (29.7–36.1) | REF | .0917 | REF | .0166 |
| 25–34 | 1200 | 27.6 (24.0–31.2) | 0.84 (0.71–0.99) | 0.82 (0.70–0.96) | ||
| 35–49 | 1155 | 29.6 (25.6–33.6) | 0.90 (0.76–1.06) | 0.83 (0.71–0.98) | ||
| HIV | ||||||
| Negative | 3977 | 30.7 (28.6–32.7) | REF | .1165 | REF | .1768 |
| Positive | 73 | 16.2 (3.3–29.0) | 0.53 (0.24–1.17) | 0.59 (0.27–1.27) | ||
| Residence | ||||||
| Urban | 1988 | 24.3 (21.8–26.9) | REF | <.0001 | 0.89 (0.74–1.07) | .2213 |
| Rural | 2062 | 35.6 (32.5–38.7) | 1.46 (1.28–1.68) | 0.89 (0.74–1.07) | ||
| Wealth index | ||||||
| Highest | 1012 | 14.1 (11.7–16.5) | REF | <.0001 | REF | <.0001 |
| High | 839 | 30.2 (25.9–34.5) | 2.14 (1.72–2.68) | 2.22 (1.78–2.79) | ||
| Moderate | 754 | 32.6 (27.3–37.9) | 2.31 (1.83–2.93) | 2.55 (1.96–3.32) | ||
| Low | 713 | 40.0 (34.8–45.2) | 2.84 (2.29–3.52) | 3.19 (2.44–4.16) | ||
| Lowest | 732 | 38.8 (33.5–44.1) | 2.76 (2.21–3.43) | 3.11 (2.35–4.11) | ||
Abbreviations: WOCBA, women of childbearing age; PR, prevalence ratio; aPR, adjusted prevalence ratio; REF, reference group; HIV, human immunodeficiency virus. The wealth index was a household score of goods owned and lodging characteristics that was subsequently partitioned into quintiles. All proportions, PR, and aPR were calculated using sampling weights.
Results of multivariate Poisson regression model, including all covariates for which aPR values are listed.
Pregnant Women
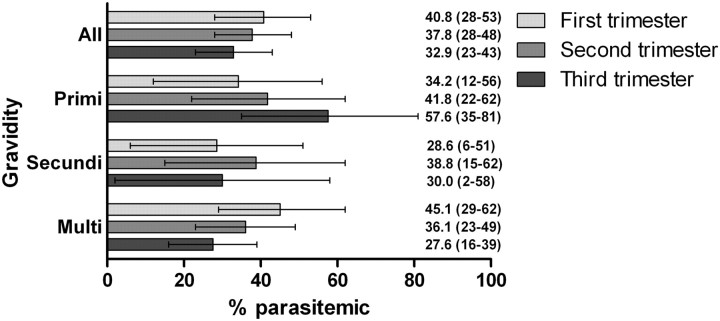
Among 520 pregnant women, bivariate analyses demonstrated that gravidity, trimester of pregnancy, and increasing age were inversely associated with the prevalence of parasitemia (Table 2). In primigravidae, parasite prevalence increased from the first to the third trimester, while in multigravidae, the parasite prevalence decreased with increasing gestation (interaction term P value = .3298) (Figure 4). Other predictors of malaria included rural residence (compared with urban) and all categories of wealth below the highest quintile.
Figure 4.
Parasite prevalence by gravidity and trimester among 520 pregnant women. Parasitemias include any malaria species. Values are percentages; those in parentheses are 95% confidence intervals. Gravidity and trimester determined by self-report. Overall differences in proportions were nonsignificant in a Poisson regression model. All analyses calculated using sampling weights.
In a multivariate model incorporating the significant covariates as well as known risk factors for parasitemia in pregnancy, only lower wealth was significantly associated with higher prevalence of parasitemia (Table 2).
Prevalence of Anemia
The prevalence of anemia was 32.3% among WOCBA (95% CI, 30.3–34.3), and was more common in pregnant women (56.4%; 95% CI, 50.2–62.7) than in nonpregnant (29.1%; 95% CI, 27.1–31.2) women (PR, 1.94; 95% CI, 1.7–2.2). Mean Hgb among WOCBA was 11.6 g/dL, and was significantly lower in pregnant (10.6 g/dL) than nonpregnant (11.8 g/dL) women (P < .001). The prevalence of severe anemia (Hgb < 7 g/dL) was 1.1% (95% CI, 0.7–1.5) among WOCBA, and was more prevalent in pregnant women (2.7%; 95% CI, 0.9–4.5) than nonpregnant (0.6%; 95% CI, 0.3–0.9) women (PR, 4.5; 95% CI, 1.9–10.7).
Predictors of Anemia in Pregnant Women
Among 516 pregnant women who had Hgb measurements, malaria parasitemia was associated with a small increased prevalence of anemia (PR, 1.09; 95% CI, 0.87–1.37) and severe anemia (PR, 1.2; 95% CI, 0.41–3.51) (Table 3). In a multivariate model including place of residence, parasitemia was associated with a small increased prevalence of anemia (adjusted PR, 1.07; 95% CI, 0.85–1.35).
Table 3.
Bivariate and Multivariate Models of the Risk of Moderate Anemia (Hgb < 11 g/dL) Among 516 Pregnant Women
| No. of women | Anemic, % (95% CI) | PR (95% CI) | PR P value | aPR a (95% CI) | aPR P valuea | |
| Age | ||||||
| 15–24 | 236 | 56.0 (46.5–65.4) | REF | .226 | Not included | … |
| 25–34 | 204 | 61.2 (51.4–71.0) | 1.09 (0.87–1.38) | |||
| 35–49 | 76 | 44.0 (28.8–59.2) | 0.79 (0.54–1.15) | |||
| HIV | ||||||
| Negative | 514 | 56.4 (50.1–62.7) | REF | .3413 | Not included | … |
| Positive | 6 | 74.3 (32.9–100) | 1.32 (0.75–2.32) | |||
| Residence | ||||||
| Urban | 207 | 50.2 (40.7–59.8) | REF | .1233 | REF | .1449 |
| Rural | 313 | 60.3 (52.2–68.4) | 1.20 (0.95–1.51) | 1.19 (0.94–1.51) | ||
| Wealth index | ||||||
| Highest | 78 | 62.0 (49.2–74.8) | REF | .3899 | Not included | … |
| High | 107 | 43.8 (30.6–57.0) | 0.71 (0.49–1.02) | |||
| Moderate | 116 | 61.4 (48.7–74.1) | 0.99 (0.74–1.33) | |||
| Low | 99 | 59.8 (45.0–74.7) | 0.96 (0.70–1.33) | |||
| Lowest | 120 | 57.6 (44.3–70.9) | 0.93 (0.68–1.27) | |||
| Gravidity | ||||||
| Primigravidae | 110 | 58.9 (45.4–72.4) | REF | .6077 | Not included | … |
| Secundigravidae | 81 | 62.1 (46.8–77.3) | 1.05 (0.75–1.47) | |||
| Multigravidae | 329 | 54.2 (46.3–62.1) | 0.92 (0.70–1.21) | |||
| Trimester | ||||||
| First | 136 | 46.0 (33.3–58.8) | REF | .2002 | Not included | … |
| Second | 223 | 61.4 (51.7–71.1) | 1.33 (0.97–1.83) | |||
| Third | 161 | 58.9 (48.1–69.6) | 1.28 (0.92–1.78) | |||
| Malaria parasitemia | ||||||
| No | 354 | 54.6 (47.0–62.3) | REF | .4481 | REF | .5571 |
| Yes | 166 | 59.7 (48.9–70.4) | 1.09 (0.87–1.37) | 1.07 (0.85–1.35) |
Abbreviations: Hgb, hemoglobin, PR, prevalence ratio; aPR, adjusted prevalence ratio; REF, reference group; HIV, human immunodeficiency virus. The wealth index was a household score of good owned and lodging characteristics that was subsequently partitioned into quintiles. All proportions, PR, and aPR calculated using sampling weights.
Results of multivariate Poisson regression model.
Extrapolation of Parasite Prevalence to National Estimates
In 2007, an estimated 3.807 million pregnancies occurred in DRC, resulting in 2.958 million births, 93.8% of which occurred in malaria-endemic areas (3.571 million pregnancies and 2.775 million births). Given the nationally representative parasite prevalence in pregnant women of 37.2% (95% CI, 31.0%–43.5%) in this study, 1.328 million (95% CI, 1.107–1.553) pregnancies and 1.032 million (95% CI, 0.860–1.207) births may be affected by malaria infection every year in DRC. The corresponding figures for P. falciparum based on the prevalence of 36.6% (95% CI, 30.3–42.9) were 1.307 million (95% CI, 1.082–1.532) pregnancies and 1.015 million (95% CI, 0.841–1.190) births.
Approximately 68% of P. falciparum infections detected by this real-time PCR assay are detectable by microscopy, suggesting that 690 539 pregnancies resulting in births (0.68 × 1.015 million) have a P. falciparum infection detectable by microscopy. Of the 1.015 million births affected by P. falciparum, ITNs alone could reduce the number of infections by 24% or 243 720 (PCR) and 165 729 (microscopy) births. Two-dose IPTp-SP could reduce this by 57% or 578 834 (PCR) and 393 607 (microscopy) births. Given the low uptake of ITNs (7.1%) and IPTp-SP (5.1%), optimal adherence to ITNs or IPTp-SP could prevent 226 415 or 549 313 episodes of PAM, respectively.
Among 880 821 first- and second-time pregnancies, 169 704 were estimated to deliver LBW newborns. ITNs have the potential to reduce LBW by 23% in primi- and secundigravidae, or 39 032 births, and IPTp-SP by 29%, or 49 214 births. ITN coverage among pregnant women during the survey was only 7% (see above), potentially preventing only 2771 LBW births; increasing this to 100% would thus prevent an additional 36 261 LBW births. Increasing the coverage of IPTp-SP from the reported 5.1% [3, 16] to the Roll Back Malaria initiative target of 100% would result in an additional 46 704 LBW births prevented among women in their first and second pregnancies.
DISCUSSION
In this population-based cross-sectional survey, the prevalence of malaria parasitemia in pregnant women was over 37%, and was significantly more common than in nonpregnant women of childbearing age. P. falciparum accounted for most parasitemias in both groups; though, interestingly, P. malariae was more prevalent in nonpregnant WOCBA than pregnant women. The overall prevalence of parasitemia translates into 1.3 million pregnancies affected per year, or approximately 1 million births. Uptake of PAM-preventive measures was very low, and optimal adherence could prevent between 36 000 to 47 000 LBW births annually. Because these estimates are based on a point-prevalence of parasitemia, the rate of PAM and the benefit of preventive measures in DRC are likely to be greater.
Quantifying the burden of PAM and the number of infected pregnant women is critical to evaluate the implementation of proven preventive measures. Recent developments in the methodology of spatial epidemiology of malaria endemicity [30] have enabled improved estimation of the number of pregnancies at potential risk of malaria [9], though no studies have yet supplied the number of pregnancies affected by malaria using a nationally representative sample to generate better estimates of PAM burden. As such, our study adds to the understanding of both the epidemiology and consequences of PAM in DRC, as well as the potential benefits of preventing it.
The low coverage of proven measures such as IPTp-SP and ITNs to prevent PAM represent one of the largest missed opportunities of programs to prevent maternal and newborn death in sub–Saharan Africa [31], particularly in DRC [3]. DRC PAM prevention policy endorsed IPTp-SP in 2004 and ITNs in 2006. The uptake is anticipated to improve considerably over the coming years with incipient financial support by the Global Fund to Fight AIDS, Tuberculosis and Malaria, and the U.S. President’s Malaria Initiative. We have used the extant gap in coverage to estimate the morbidity reductions possible with enhanced uptake of ITNs and IPTp-SP, using published estimates of the protective efficacy of such measures. The estimated 36 000 to 47 000 LBW births that could be prevented every year in the DRC by full adherence to ITNs or IPTp-SP, respectively, represents a substantial opportunity to improve childhood health in a country that suffers the fifth-largest global burden of neonatal deaths [32].
On an individual level, we observed differences in the prevalences of parasitemia and anemia consonant with traditional risk factors, though these differences did not generally achieve statistical significance. Regarding parasitemia, we note a differential effect of gravidity and gestational age on parasitemia, whereby, as gestational age increased, parasitemia was less prevalent in multigravidae and more prevalent in primigravidae. Nevertheless, these differences did not achieve statistical significance, and given the cross-sectional nature of the study and the lack of sufficient antenatal care information, we are unable to study this relationship rigorously. Although we found that pregnancy was associated with increased risk of parasitemia in the univariate analysis, the effect was smaller and nonsignificant in our multivariate models. Possible explanations include the miscategorization of women as nonpregnant when in the early stage of pregnancy, or the use of IPTp and ITNs by a small but significant number of women.
Two other findings merit further attention. First, HIV infection was inversely associated with the prevalence of parasitemia, in contrast with most other studies in sub–Saharan Africa in which the risk of parasitemia is greater in HIV-infected patients [33, 34]. This relationship was consistent in subanalyses of pregnant and nonpregnant women, and in multivariate models incorporating available potential confounders of the relationship. The lack of additional clinical or laboratory data prevents further exploration, though more frequent use of antimalarials or adherence to daily cotrimoxazole prophylaxis could account for the reduced risk, as recently reported in Malawi [35]. Women were not specifically queried regarding the use of cotrimoxazole. Second, among all WOCBA, P. malariae was significantly less prevalent among pregnant women compared with nonpregnant women. To our knowledge, this relationship has not previously been demonstrated, though because of the focal spatial distribution of P. malariae [17], we cannot rule out confounding by the parasite’s limited geographic transmission.
As expected, anemia was substantial among pregnant women, though was not associated with parasitemia, perhaps owing to the use of PCR for parasite detection (which detects low-level parasitemias). Very few women suffered from severe anemia, in contrast to women in other malarious African settings [36]. Given the multiple etiologies of anemia in similar populations [37], the lack of additional nutritional and biometric data points precluded a more detailed causal analysis of the effect of parasitemia on anemia.
Our cross-sectional molecular survey of women of childbearing age has several limitations. Part of the survey was conducted during the drier season, which may underestimate year-round prevalence. Without longitudinal data, we cannot directly assess the effect of individual parasitemias on birth outcomes in our survey respondents. Thus, we have used estimates of the individual consequences of PAM derived from prior studies to estimate the burden of the population-level consequences of PAM. Additionally, we employed a sensitive PCR assay that detects substantially more parasitemias than microscopy [21], though the clinical significance of such “submicroscopic” parasitemias is uncertain [38, 39]. Nevertheless, the increased prevalence in pregnant women and the association with gravidity suggest that such parasitemias represent a genuine biological consequence of pregnancy. We relied upon self-report for determination of pregnancy status. Though suboptimal, familiarity with pregnancy was high in the surveyed women, suggesting reliability. Finally, no data were available regarding antenatal care or the use of IPTp, preventing assessment of their effect on parasitemia.
To our knowledge, these are the first data to estimate the infection risk, potential consequences, and potential benefits of the control of PAM on a national scale using representative data. Though our data highlight an untoward gap between PAM policies and practices in one of the most intensely malarious regions on earth, they also suggest a means by which DRC and similar countries may achieve substantial improvements in maternal and child health.
Notes
Acknowledgments.
We thank Dr Augustin Okenge (Programme National de Lutte Contre le SIDA, Kinshasa, DRC) for his help in obtaining access to the dried blood spots. We are also grateful to Ann Way, Mohamed Ayad, and Martin Vaessen (MeasureDHS, Calverton, MD) for guidance and helpful discussions. We also thank Stephanie Carrier and Hannah Hoffman (University of North Carolina, Chapel Hill) for their contributions to the molecular testing. This work would not have been possible without the work of the scores of survey administrators and, ultimately, we are indebted to the DHS respondents for their participation.
Financial support.
This work was supported by a Gillings Innovation Laboratory award from the UNC Gillings School of Global Public Health (to S. R. M.) and by the Malaria in Pregnancy Consortium (MiP), which is funded through a grant from the Bill & Melinda Gates Foundation to the Liverpool School of Tropical Medicine (to F. O. tK.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Umbers AJ, Aitken EH, Rogerson SJ. Malaria in pregnancy: small babies, big problem. Trends Parasitol. 2011;27:168–75. doi: 10.1016/j.pt.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Menendez C, D'Alessandro U, ter Kuile FO. Reducing the burden of malaria in pregnancy by preventive strategies. Lancet Infect Dis. 2007;7:126–35. doi: 10.1016/S1473-3099(07)70024-5. [DOI] [PubMed] [Google Scholar]
- 3.van Eijk AM, Hill J, Alegana VA, et al. Coverage of malaria protection in pregnant women in sub–Saharan Africa: a synthesis and analysis of national survey data. Lancet Infect Dis. 2011;11:190–207. doi: 10.1016/S1473-3099(10)70295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297:2603–16. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe EB, Parise ME, Haddix AC, et al. Cost-effectiveness of sulfadoxine-pyrimethamine for the prevention of malaria-associated low birth weight. Am J Trop Med Hyg. 2001;64:178–86. doi: 10.4269/ajtmh.2001.64.178. [DOI] [PubMed] [Google Scholar]
- 6.Becker-Dreps SI, Biddle AK, Pettifor A, et al. Cost-effectiveness of adding bed net distribution for malaria prevention to antenatal services in Kinshasa, Democratic Republic of the Congo. Am J Trop Med Hyg. 2009;81:496–502. [PubMed] [Google Scholar]
- 7.Gamble C, Ekwaru PJ, Garner P, ter Kuile FO. Insecticide-treated nets for the prevention of malaria in pregnancy: a systematic review of randomised controlled trials. PLoS Med. 2007;4:e107. doi: 10.1371/journal.pmed.0040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roll Back Malaria Partnership. Global malaria action plan. http://www.rollbackmalaria.org/gmap/gmap.pdf. Accessed 26 September 2011. 2008. [Google Scholar]
- 9.Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med. 2010;7:e1000221. doi: 10.1371/journal.pmed.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64(1–2 Suppl):28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 11.Guyatt H, Snow R. Impact of malaria during pregnancy on low birth weight in sub–Saharan Africa. Clin Microbiol Rev. 2004;17:760–9. doi: 10.1128/CMR.17.4.760-769.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker-Abbey A, Djokam RR, Eno A, et al. Malaria in pregnant Cameroonian women: the effect of age and gravidity on submicroscopic and mixed-species infections and multiple parasite genotypes. Am J Trop Med Hyg. 2005;72:229–35. [PubMed] [Google Scholar]
- 13.Mockenhaupt FP, Rong B, Till H, et al. Submicroscopic Plasmodium falciparum infections in pregnancy in Ghana. Trop Med Int Health. 2000;5:167–73. doi: 10.1046/j.1365-3156.2000.00532.x. [DOI] [PubMed] [Google Scholar]
- 14.Rantala AM, Taylor SM, Trottman PA, et al. Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women. Malar J. 2010;9:269. doi: 10.1186/1475-2875-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gething PW, Patil AP, Hay SI. Quantifying aggregated uncertainty in Plasmodium falciparum malaria prevalence and populations at risk via efficient space-time geostatistical joint simulation. PLoS Comput Biol. 2010;6:e1000724. doi: 10.1371/journal.pcbi.1000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministere du Plan et Macro International. Enquete Demographique et de Sante, Republique Democratique du Congo 2007. Calverton, MD: Measure DHS; 2008. [Google Scholar]
- 17.Taylor S, Messina JP, Hand CC, et al. Molecular malaria epidemiology: mapping and burden estimates for the Democratic Republic of the Congo, 2007. PLoS One. 2011;6:e16420. doi: 10.1371/journal.pone.0016420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.United Nations Population Division. World population prospects: the 2008 revision population database. http://esa.un.org/unpp/. Accessed 25 July 2010. [Google Scholar]
- 19.Mbaye A, Richardson K, Balajo B, et al. A randomized, placebo-controlled trial of intermittent preventive treatment with sulphadoxine-pyrimethamine in Gambian multigravidae. Trop Med Int Health. 2006;11:992–1002. doi: 10.1111/j.1365-3156.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 20.Gamble C, Ekwaru JP, ter Kuile FO. Insecticide-treated nets for preventing malaria in pregnancy. Cochrane Database Syst Rev. 2006;(2):CD003755. doi: 10.1002/14651858.CD003755.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor SM, Juliano JJ, Trottman PA, et al. High-throughput pooling and real-time PCR-based strategy for malaria detection. J Clin Microbiol. 2010;48:512–9. doi: 10.1128/JCM.01800-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. Making pregnancy safer: Democratic Republic of the Congo country profile. http://www.who.int/making_pregnancy_safer/countries/cod.pdf. Accessed 1 February 2011. [Google Scholar]
- 23.Kassberger F. Cytoadhesion of infected erythrocytes in maternal malaria. Tubingen, Germany: University of Tubingen; 2004. [Google Scholar]
- 24.Kabyemela ER, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Fetal responses during placental malaria modify the risk of low birth weight. Infect Immun. 2008;76:1527–34. doi: 10.1128/IAI.00964-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falade C, Mokuolu O, Okafor H, et al. Epidemiology of congenital malaria in Nigeria: a multi-centre study. Trop Med Int Health. 2007;12:1279–87. doi: 10.1111/j.1365-3156.2007.01931.x. [DOI] [PubMed] [Google Scholar]
- 26.Wort UU, Warsame M, Brabin BJ. Birth outcomes in adolescent pregnancy in an area with intense malaria transmission in Tanzania. Acta Obstet Gyn Scan. 2006;85:949–54. doi: 10.1080/00016340600756870. [DOI] [PubMed] [Google Scholar]
- 27.Ofori M, Ansah E, Agyepong I, Ofori-Adjei D, Hviid L, Akanmori B. Pregnancy-associated malaria in a rural community of Ghana. Ghana Med J. 2009;43:13–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Alusala D. Copenhagen, Denmark: University of Copenhagen & DBL-Institute for Health Research and Development; 2003. Malaria-vitamin A interactions in pregnancy and infancy: predictors of congenital malaria and effects of vitamin A supplementation on infant malaria parasitemia and morbidity. [Google Scholar]
- 29.Malaria in Pregnancy consortium. Malaria in Pregnancy Library. 2011. http://www.update-software.com/Publications/Malaria/. Accessed 26 April 2011. [Google Scholar]
- 30.Guerra CA, Gikandi PW, Tatem AJ, et al. The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Med. 2008;5:e38. doi: 10.1371/journal.pmed.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinney MV, Kerber KJ, Black RE, et al. Sub–Saharan Africa’s mothers, newborns, and children: where and why do they die? PLoS Med. 2010;7:e1000294. doi: 10.1371/journal.pmed.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 33.ter Kuile FO, Parise ME, Verhoeff FH, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub–Saharan Africa. Am J Trop Med Hyg. 2004;71(2 Suppl):41–54. [PubMed] [Google Scholar]
- 34.Whitworth J, Morgan D, Quigley M, et al. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet. 2000;356:1051–6. doi: 10.1016/S0140-6736(00)02727-6. [DOI] [PubMed] [Google Scholar]
- 35.Kapito-Tembo A, Meshnick SR, van Hensbroek MB, Phiri K, Fitzgerald M, Mwapasa V. Marked reduction in prevalence of malaria parasitemia and anemia in HIV-infected pregnant women taking cotrimoxazole with or without sulfadoxine-pyrimethamine intermittent preventive therapy during pregnancy in Malawi. J Infect Dis. 2011;203:464–72. doi: 10.1093/infdis/jiq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guyatt HL, Snow RW. The epidemiology and burden of Plasmodium falciparum–related anemia among pregnant women in sub–Saharan Africa. Am J Trop Med Hyg. 2001;64(1–2 Suppl):36–44. doi: 10.4269/ajtmh.2001.64.36. [DOI] [PubMed] [Google Scholar]
- 37.Mockenhaupt FP, Rong B, Gunther M, et al. Anaemia in pregnant Ghanaian women: importance of malaria, iron deficiency, and haemoglobinopathies. Trans R Soc Trop Med Hyg. 2000;94:477–83. doi: 10.1016/s0035-9203(00)90057-9. [DOI] [PubMed] [Google Scholar]
- 38.Mankhambo L, Kanjala M, Rudman S, Lema VM, Rogerson SJ. Evaluation of the OptiMAL rapid antigen test and species-specific PCR to detect placental Plasmodium falciparum infection at delivery. J Clin Microbiol. 2002;40:155–8. doi: 10.1128/JCM.40.1.155-158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adegnika AA, Verweij JJ, Agnandji ST, et al. Microscopic and sub-microscopic Plasmodium falciparum infection, but not inflammation caused by infection, is associated with low birth weight. Am J Trop Med Hyg. 2006;75:798–803. [PubMed] [Google Scholar]