Abstract
Aim
It has been suggested that there is an inverse association between breastfeeding and the risk of childhood cancer. We investigated the association between full breastfeeding and paediatric cancer (PC) in a case control study in Spain.
Methods
Maternal reports of full breastfeeding, collected through personal interviews using the Paediatric Environmental History, were compared among 187 children 6 months of age or older who had PC and 187 age-matched control siblings.
Results
The mean duration of full breastfeeding for cases were 8.43 and 11.25 weeks for controls. Cases had been significantly more often bottle-fed than controls (odds ratio (OR) 1.8; 95% confidence interval (CI) 1.1–2.8). Cases were significantly less breastfed for at least 2 months (OR 0.5; 95% CI 0.3–0.8), for at least 4 months (OR 0.5; 95% CI 0.3–0.8), and for 24 weeks or more (OR 0.5; 95% CI 0.2–0.9).
Conclusions
Breastfeeding was inversely associated with PC, the protection increasing with the duration of full breastfeeding. Additional research on possible mechanisms of this association may be warranted. Meanwhile, breastfeeding should be encouraged among mothers.
Keywords: full breastfeeding, paediatric oncology, risk factor
The positive effects that breastfeeding has on the prevention of infectious diseases and in strengthening the immune system are well-known.1 Breastfeeding has many advantages, whether practised for a short or a long term.2,3 Antineoplasic protection has been suggested as one of the advantages of breastfeeding for both mother and child.4 Previous published studies have focused on the long-term effects of breastfeeding on cancer protection in both children and mothers.5 The low prevalence of PC, long latency periods (including the pregestational period) and the difficulty of epidemiological research design encourage the use of observational case control studies to investigate the risk factors (RF) implicated with paediatric cancer (PC).6
At every age, cancer results from the interaction of both genetic (endogenous) and environmental (exogenous) determinants. Concurrently, each determinant comprises a range of carcinogenic RF, most of which remain unknown.7 Furthermore, each RF can be comprised of diverse carcinogenic agents. Such is the case with tobacco smoke, which contains more than 55 different carcinogens. Environmental factors have been associated with 98–99% of all known cancers and with 85–96% of PCs.8,9
The primary approach towards PC prevention relies upon research of the RF associated with PC.7,10,11 Primary prevention is the most effective and beneficial form of prevention method in sanitary, financial and socio-cultural terms. The objective of the study was to analyse the preventive effect of full breastfeeding (FB) on PC.
MACAPE (Medio Ambiente y Cáncer Pediátrico – the Environment and Paediatric Cancer Group) is a project for the development of the Paediatric Environmental History (PEH) in children with cancer in the United States, Argentina and Spain. As part of a larger and ongoing study on the determinants of PC that uses the PEH and in which data are still being collected in Spain, we analyse here the role of breastfeeding, examining the association between various PCs and the duration of FB.12–14
Methods
Study population
We conducted a case control study in Spain in 2007. Cases were all children aged 6 months to 16 years newly diagnosed with cancer from 1 August 2005 to 1 August 2006 at any of the six collaborating hospitals. Exclusion criteria included children who were born before gestation week 33, children with a second cancer, and newborns who were hospitalised for longer than 3 days following birth. The study was approved by the hospital network ethics committees and the institutional review boards.
In Spain about 900 children are diagnosed with cancer every year. In our study network 220 children with cancer were identified (24% of all Spanish incident PCs that year), of which 33 had some exclusion criteria. Centralised care in reference units of PC in Spain facilitated access to medical records in the hospitals of the network.
Families were contacted by telephone to set the interviews. Completion of the PEH questionnaire lasted 2–3 h. The interview was conducted in person, with one or both parents present. Informed consents were administered to all parents. Children more than 12 years of age were besides offered assent forms. One paediatrician conducted all the interviews at the collaborating hospitals and at the sites of the local parent associations of children with cancer. The paediatrician has expertise on environmental health and oncology and experience to interact with PC patients and their families.
As controls, 187 siblings of affected children were recruited, and matched with the cases on age, seeking a difference of no more than 25% age difference. When a case was a single child or a control of a suitable age could not be found, siblings of a different case and with the same postal code were used as controls (17% of cases).
Exposure data: PEH
The PEH in paediatric oncology used in the study includes a series of concise and basic questions through which the paediatrician identifies environmental exposures in PC. The PEH documents human carcinogens characterised by the International Agency for Research on Cancer and by the US National Toxicology Program.15,16 The PEH included information on birth-weight, gestational age, delivery type, socio-economic status (net family income), mother’s educational level, mother’s smoking habits during pregnancy, and mother’s current working status (employed or unemployed).
Data were collected on FB, as defined by the World Health Organization recommendations: ‘Full breastfeeding is defined as exclusive (no other liquid or solid is given to the infant) or almost exclusive (vitamins, mineral water, juice, or ritualistic feeds are given infrequently in addition to breastfeeds).17,18 The duration of FB and bottle-feeding were noted along with the date bottle-feeding was first introduced. In the analyses the duration of FB was used as a continuous quantitative variable (indicated by the number of weeks of lactation). The duration of breastfeeding was also grouped in intervals.
SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Odds ratios (ORs) and 95% confidence intervals (CIs) were employed to evaluate the association between the duration of FB and PC. Analyses were done for all PCs. A logistic multivariable regression model was used to control for possible confounding factors, such as age, birthweight, gestation period, birthing technique, socio-economic status, mother’s educational level, mother’s smoking habits during pregnancy, and mother’s current working status. Effects were considered statistically significant with P-value < 0.05 and ORs with a 95% CI that did not include 1.
Results
In total, 187 cases and 187 controls were analysed. Participation reached 100% of the cases and controls originally identified in our study. Figure 1 shows the distribution of tumour types of cases. Paediatric acute lymphoblastic leukemia, central nervous system tumours and lymphomas comprised 34%, 14% and 12% of the cases, respectively. The mean age of children at diagnsis was 6.5 years.
Fig. 1.
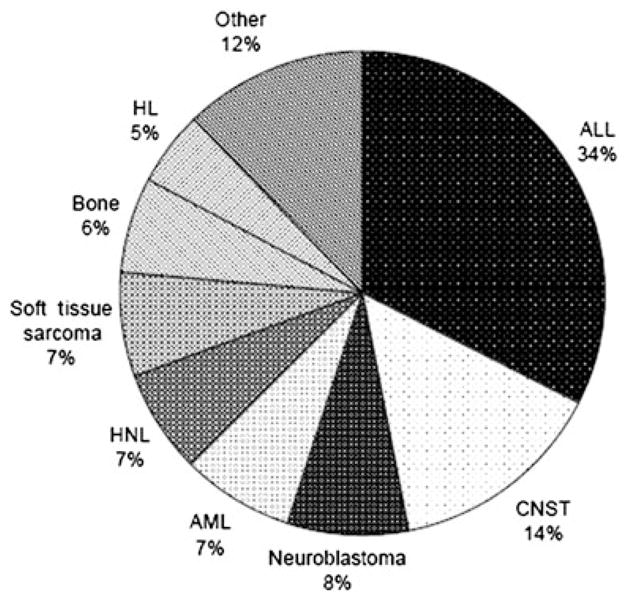
Cases grouped by cancer types. The proportion of ‘Other’ includes renal tumours 6, hepatoblastoma 4, histiocitosis 4, teratoma 3, vascular 2, carcinoma 1, melanoma 1, ovarian tumour 1, thyroid 1. ALL, acute lymphoblastic leukemia; AML, acute myeloblastic leukemia; CNST, central nervous system tumour; HL, Hodgkin’s lymphoma; NHL, non-Hodgkin lymphoma.
Table 1 shows the main characteristics and differences between cases and controls. Mean age of the mother, gestational age, birthweights and Apgar scores did not differ between the two groups. There were no significant differences between cases and controls in socio-demographic variables.
Table 1.
Socio-demographic variables
| Cases (n) | Controls (n) | Cases (Mean) | Controls (Mean) | |
|---|---|---|---|---|
| Newborn’s gender | ||||
| Male | 113 | 111 | ||
| Female | 74 | 76 | ||
| Mother’s age at pregnancy | 29.6 | 30.1 | ||
| Mean gestational age | 38.9 | 39.3 | ||
| Newborn weight | 3387 | 3279 | ||
| Apgar store (at 1 and 5 min) | >7/>8 | >7/>8 | ||
| Mother’s employment | ||||
| No | 112 | 117 | ||
| Yes | 75 | 70 | ||
| Mother’s educational level | ||||
| None | 19 | 18 | ||
| Primary school | 62 | 60 | ||
| Incomplete secondary | 19 | 18 | ||
| Complete secondary | 40 | 39 | ||
| Incomplete college | 2 | 3 | ||
| Complete college | 45 | 49 | ||
| Smoked during pregnancy? | ||||
| No | 91 | 95 | ||
| Yes | 93 | 89 | ||
| Age when interviewed (year) | ||||
| 0.5–1 | 5 | 5 | ||
| 1–5 | 83 | 83 | ||
| 6–10 | 44 | 43 | ||
| >10 | 55 | 56 | ||
| Delivery type | ||||
| Vaginal | 137 | 138 | ||
| Cesarian | 44 | 40 | ||
| Vacuum | 3 | 5 | ||
| Forceps | 3 | 4 | ||
| Net Income/month (€) | ||||
| <800 | 15 | 16 | ||
| 800–1500 | 41 | 37 | ||
| 1500–2000 | 33 | 34 | ||
| 2000–2500 | 14 | 11 | ||
| 2500–3500 | 25 | 23 | ||
| >3500 | 16 | 17 | ||
There were no significant differences in any of the variables.
The mean and median duration of FB for cases were 8.43 and 4 weeks, respectively, and for controls 11.25 and 8 weeks, respectively. Table 2 shows the ORs between cases and controls of the distribution of the duration of breastfeeding in intervals. Cases had been significantly more often bottle-fed than controls (OR 1.8; 95% CI 1.1–2.8). FB was lower in cases than in controls in all age groups. The odds of being breastfed for at least 2 months and for at least 4 months were, in both age categories, half in cases than in controls (OR 0.5; 95% CI 0.3–0.8). In the stepwise logistic regression analysis, only the duration of FB remained in the model, with an estimated OR decreasing as the number of weeks of FB increased. One extra week of breast-feeding was equivalent to a decrease in the OR of 0.97; 95% CI 0.95–0.99.
Table 2.
Duration of full breastfeeding in weeks in children with paediatric cancer and controls
| Cases
|
Controls
|
% | OR | |||
|---|---|---|---|---|---|---|
| n | % | n | 95% CI | |||
| Bottle-feeding only† | 65 | 34.8 | 43 | 23.0 | 1.8† | 1.1–2.8 |
| Full breastfeeding | ||||||
| Less than 8 weeks | 41 | 21.9 | 40 | 21.4 | 0.7 | 0.4–1.2 |
| 8–15 weeks | 30 | 16.0 | 33 | 17.6 | 0.6 | 0.3–1.1 |
| 16–23 weeks | 28 | 15.0 | 39 | 20.9 | 0.5 | 0.3–0.9 |
| 24 weeks or more | 23 | 12.3 | 32 | 17.1 | 0.5 | 0.2–0.9 |
| At least 2 months | 81 | 43.3 | 104 | 55.6 | 0.5 | 0.3–0.8 |
| At least 4 months | 51 | 27.3 | 71 | 38.0 | 0.5 | 0.3–0.8 |
Odds ratio of bottle-feeding in cases versus controls.
Discussion
Our study suggests that FB has a protective effect for PC. That protective effect may start to show from the first 8 weeks of breastfeeding and increase progressively from then on, at least, during the first 6 months of age. Previous research has demonstrated that this effect continues into the second year of life.19–21
Studies report a diminished risk for children to develop acute leukemia, predominantly acute lymphoblastic leukemia, as well as acute myeloid leukemia, non-Hodgkin lymphoma, Hodgkin’s lymphoma, Wilms tumour and tumours of the sympathetic nervous system if breastfed exclusively.19–25 The protective action of breastfeeding therefore seems to be important not only in a subtype but also in all different histological subtypes. In our study, all PCs were analysed concomitantly because it is plausible that similar antineoplasic protection mechanisms may apply for all childhood tumours.24
Our investigation differs from most studies with a subject pool of n > 100, in that it has been completed by personal interviews conducted by one paediatrician trained to conduct these interviews face to face. We believe that that interview facilitated an accurate collection of data, though at the expense of increasing the economic cost. Personal interviews allowed us to estimate breastfeeding history with a level of detail that would not easily available using other methods for data collection. Consequently, we were able to estimate a clear protective dose–response effect of breastfeeding on PC starting from the first 8 weeks of life.
Siblings of cases were included as controls in our study. The main criterion for selecting appropriate controls in a case control study is to ensure comparability between the two groups. Our controls would be almost certainly included in the cases group if they had developed cancer. Our two groups are highly comparable in all the variables that were measured and presented in our study, as well as in other factors that may not have been measured but might be related to both cancer risk and duration of breastfeeding. Therefore, we believe that our study design has been highly efficient in that it has decreased the possibility of confounding by known or unknown variables.
The main concerns with case control studies are information bias, specially recall bias, and confounding. Recall bias is certainly one of the most serious concerns in case control studies, and our study may not be an exception. Recall bias would be a concern if recall of exposure were differential among cases and controls. There are two reasons why we believe that that concern may have been minimised by our study design. First, we believe that differential recall between cases and controls may have been minimised by the use of a highly qualified interviewer and by having the same parents as sources of information for both cases and controls. Sibling controls guarantees that recalls are done by the same persons in both cases and controls. Therefore, unless there is prior hypothesis held by parents suggesting that breastfeeding may be related to cancer, or if the recall periods were significantly different between cases and controls, we may assume that recall would be similar in the two groups. Mean ages of cases and controls were not significantly different in our study. Therefore, on average recall periods were similar between the two groups. Second, and until now, the association between breastfeeding and cancer is not known by parents, as it is not even known by most clinicians in Spain. Therefore, it is unlikely that parents would make a differential effort to recall breastfeeding history in their children who were cases than in those who were used as controls. Besides, the interviews with parents cover a wide range of exposures with breastfeeding being only one of them. Therefore, it is unlikely that there might be a differential recall in that regards.
Confounding by known or unknown factors is also a concern in case control studies. As mentioned earlier, we believe that our study design may also have minimised the role of confounding in this study. Matching by age in our study was not an attempt to control confounding but rather to ensure comparable numbers in all the strata by age, a possible confounder. Therefore, age was included in the analyses as a possible confounder.
Finally, in our analyses we did not incorporate other environmental exposures related to childhood cancer. Consequently, we have not being able to address here possible interactions between those variables and breastfeeding. These possible interactions may be important and should be fully explored. Our work is part of an ongoing research effort if we expect to be able to address these issues in the future.
Key Points.
The protective effect of breastfeeding is important in overall paediatric cancer.
The degree of protection conferred by breastfeeding increases with the duration of full breastfeeding.
The protective effect of breastfeeding on paediatric cancer is observed even for relatively short periods of breastfeeding.
Acknowledgments
The authors express their gratitude for the support and funding granted by the Scientific Foundation of the AECC (Asociación Española Contra el Cáncer). Additionally, we thank the International Exchange Program for Minority Students funded by National Institutes of Health and the International Training and Research Program in Environmental and Occupational Health at the Mount Sinai Medical Center (New York), funded by the Fogarty. International Center (NIH TW00640). The authors would also like to thank an anonymous reviewer for the thorough and thoughtful comments. Special thanks also go to children as family participants.
References
- 1.Hanson LA, Korotkova M. The role of breastfeeding in prevention of neonatal infection. Semin Neonatol. 2002;7:275–81. doi: 10.1016/s1084-2756(02)90124-7. [DOI] [PubMed] [Google Scholar]
- 2.Villalpando S, Harnost M. Early and late effects of breast feeding: does breastfeeding really matter? Biol Neonate. 1998;74:177–91. doi: 10.1159/000014022. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. A Systematic Review. Geneva: WHO; 2002. The Optimal Duration of Exclusive Breastfeeding. [Google Scholar]
- 4.Martin RM, Gunnell D, Owen CG, Smith GD. Breast-feeding and childhood cancer: a systematic review with metaanalysis. Int J Cancer. 2005;117:1020–31. doi: 10.1002/ijc.21274. [DOI] [PubMed] [Google Scholar]
- 5.Guise JM, Austin D, Morris CD. Review of case-control studies related to breastfeeding and reduced risk of childhood leukemia. Pediatrics. 2005;116:e724–31. doi: 10.1542/peds.2005-0636. [DOI] [PubMed] [Google Scholar]
- 6.Linet MS, Wacholder S, Zahm SH. Interpreting epidemiologic research: lessons from studies of childhood cancer. Pediatrics. 2003;112:218–32. [PubMed] [Google Scholar]
- 7.Saracci R. Neoplasms. In: Detels R, editor. Oxford Textbook of Public Health. 3. New York: Oxford University Press; 1997. pp. 1043–63. [Google Scholar]
- 8.Narod SA, Stiller C, Lenoir GM. An estimate of the heritable fraction of childhood cancer. Br J Cancer. 1991;63:993–9. doi: 10.1038/bjc.1991.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quesnel S, Malkin D. Genetic predisposition to cancer and familial cancer syndromes. Pediatr Clin North Am. 1997;44:791–808. doi: 10.1016/s0031-3955(05)70530-7. [DOI] [PubMed] [Google Scholar]
- 10.Keefe KA, Meyskens FL., Jr . Cancer prevention. In: Abeloff MD, editor. Clinical Oncology. 2. New York: Churchill Livingstone; 2000. pp. 318–65. [Google Scholar]
- 11.Stiller CA. Aetiology and epidemiology. In: Pinkerton CR, editor. Paediatric Oncology. 2. London: Chapman & Hall; 1997. pp. 3–26. [Google Scholar]
- 12.Ferris Tortajada J, Ortega Garcia JA, Marco Macian A, Garcia Castell J. Environment and paediatric cancer. An Pediatr. 2004;61:42–50. doi: 10.1016/s1695-4033(04)78352-6. [DOI] [PubMed] [Google Scholar]
- 13.Kriebel D, Tickner J, Epstein P, et al. The precautionary principle in environmental science. Environ Health Perspect. 2001;109:871–6. doi: 10.1289/ehp.01109871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortega Garcia JA, Ferris i Tortajada J, Claudio Morales L, Berbel Tornero O. Paediatric environmental health specialty units in Europe: from theory to practice. An Pediatr. 2005;63:143–51. doi: 10.1157/13077457. [DOI] [PubMed] [Google Scholar]
- 15.International Agency for Research on Cancer (IARC) Overall Evaluations of Carcinogenicity to Humans. [accessed 15 May 2007];IARC Monographs. 1–82 Available from: http://monographs.iarc.fr/ENG/Classification/crthgr01.php. [Google Scholar]
- 16.Report on Carcinogens. 10. US Department of Health and Human Services, Public Health Service, National Toxicology Program; Dec, 2002. [accessed 20 May 2007]. Available from: http://ehp.niehs.nih.gov/roc/toc10.html. [Google Scholar]
- 17.World Health Organization (WHO/UNICEF) Indicators for Assessing Health Facility Practices That Affect Breastfeeding. Geneva: WHO; 1993. [Google Scholar]
- 18.Labbok MH, Belsey M, Coffin CJ. A call for consistency in defining breast-feeding. Am J Public Health. 1997;87:1060–1. doi: 10.2105/ajph.87.6.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker L. Breast-feeding and cancer prevention. Eur J Cancer. 2001;37:155–8. doi: 10.1016/s0959-8049(00)00381-6. [DOI] [PubMed] [Google Scholar]
- 20.Bener A, Denic S, Galadasi S. Longer breast-feeding and protection against childhood leukaemia and lymphomas. Eur J Cancer. 2001;37:234–8. doi: 10.1016/s0959-8049(00)00339-7. [DOI] [PubMed] [Google Scholar]
- 21.Shu XO, Linet MS, Steinbuch M, et al. Breast-feeding and risk of childhood acute leukaemia. J Natl Cancer Inst. 1999;91:1765–72. doi: 10.1093/jnci/91.20.1765. [DOI] [PubMed] [Google Scholar]
- 22.Saddlemire S, Olshan AF, Daniels JL, Breslow NE, Bunin GR, Ross JA Children’s Oncology Group. Breast-feeding and Wilms tumor: a report from the Children’s Oncology Group. Cancer Causes Control. 2006;17:687–93. doi: 10.1007/s10552-005-0508-y. [DOI] [PubMed] [Google Scholar]
- 23.Daniels JL, Olshan AF, Pollock BH, Shah NR, Stram DO. Breast-feeding and neuroblastoma, USA and Canada. Cancer Causes Control. 2002;13:401–5. doi: 10.1023/a:1015746701922. [DOI] [PubMed] [Google Scholar]
- 24.Infante-Rivard C, Fortier I, Olson E. Markers of infection, breastfeeding and childhood acute lymphoblastic leukaemia. Br J Cancer. 2000;83:1559–64. doi: 10.1054/bjoc.2000.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis MK. Review of the evidence for an association between infant and childhood cancer. Int J Cancer. 1998;11:29–33. [PubMed] [Google Scholar]


