Abstract
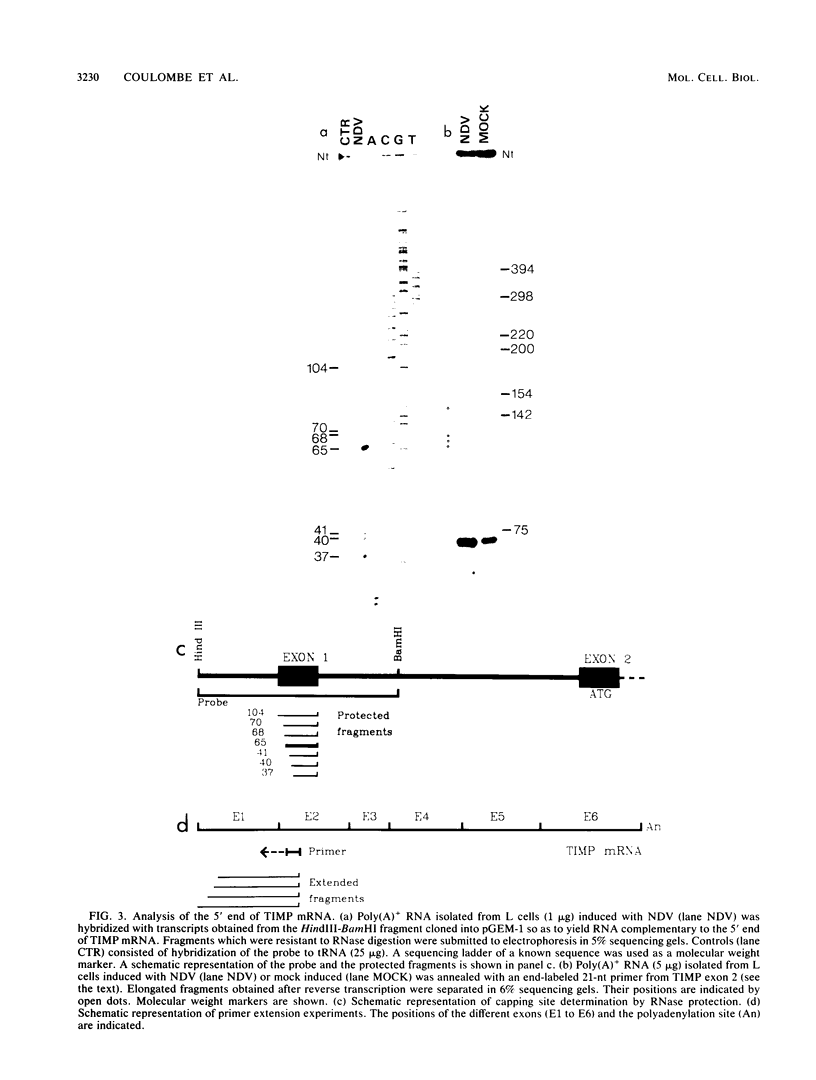
The expression of the gene for the murine tissue inhibitor of metalloproteinases (TIMP) is induced in response to viruses, growth factors, and phorbol esters. In this report we show that the accumulation of TIMP mRNA after Newcastle disease virus induction is caused by transcriptional activation of the gene. Comparison of the sequences of cDNA and genomic clones along with RNase protection and primer extension analyses revealed that the murine TIMP gene possesses multiple cap sites and that the exon 1 consists exclusively of 5'-noncoding sequences. We observed that DNA regions analogous to those found upstream of the virus-inducible interferon genes are present within intron 1 of the TIMP gene. To investigate the possible role of TIMP intron 1 in gene expression, we used a functional assay based on the transfection of plasmids in which the DNA segment to be tested is placed in proximity to a marker gene driven by the heterologous herpes simplex virus thymidine kinase promoter. Our results indicate that TIMP intron 1 contains DNA sequence elements capable of modulating the activity of a heterologous promoter in two different ways: (i) by enhancing constitutive expression and (ii) by conferring virus inducibility. These results suggest that intron 1 may be involved in the transcriptional regulation of TIMP gene expression.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belhumeur P., Paterno G. D., Boileau G., Claverie J. M., Skup D. Isolation and characterisation of a murine cDNA clone highly homologous to the yeast L29 ribosomal protein gene. Nucleic Acids Res. 1987 Feb 11;15(3):1019–1029. doi: 10.1093/nar/15.3.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe B., Skup D. Expression of a synthetic human interferon-alpha 1 gene with modified nucleotide sequence in mammalian cells. Gene. 1986;46(1):89–95. doi: 10.1016/0378-1119(86)90170-8. [DOI] [PubMed] [Google Scholar]
- Coulombe B., Skup D. In vitro synthesis of the active tissue inhibitor of metalloproteinases encoded by a complementary DNA from virus-infected murine fibroblasts. J Biol Chem. 1988 Jan 25;263(3):1439–1443. [PubMed] [Google Scholar]
- Edwards D. R., Waterhouse P., Holman M. L., Denhardt D. T. A growth-responsive gene (16C8) in normal mouse fibroblasts homologous to a human collagenase inhibitor with erythroid-potentiating activity: evidence for inducible and constitutive transcripts. Nucleic Acids Res. 1986 Nov 25;14(22):8863–8878. doi: 10.1093/nar/14.22.8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Ohno S., Yasumitsu H., Taniguchi T. Delimitation and properties of DNA sequences required for the regulated expression of human interferon-beta gene. Cell. 1985 Jun;41(2):489–496. doi: 10.1016/s0092-8674(85)80022-2. [DOI] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Hotta H., Yamanishi K., Taniguchi T. Interferon-beta gene regulation: tandemly repeated sequences of a synthetic 6 bp oligomer function as a virus-inducible enhancer. Cell. 1987 May 8;49(3):357–367. doi: 10.1016/0092-8674(87)90288-1. [DOI] [PubMed] [Google Scholar]
- Gewert D. R., Coulombe B., Castelino M., Skup D., Williams B. R. Characterization and expression of a murine gene homologous to human EPA/TIMP: a virus-induced gene in the mouse. EMBO J. 1987 Mar;6(3):651–657. doi: 10.1002/j.1460-2075.1987.tb04804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Zinn K., Maniatis T. Human beta-interferon gene expression is regulated by an inducible enhancer element. Cell. 1985 Jun;41(2):509–520. doi: 10.1016/s0092-8674(85)80024-6. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan G., Williams B. R. Transcriptional regulation of interferon-responsive genes is closely linked to interferon receptor occupancy. EMBO J. 1986 Jul;5(7):1607–1613. doi: 10.1002/j.1460-2075.1986.tb04403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper E. Collagenases. Annu Rev Biochem. 1980;49:1063–1078. doi: 10.1146/annurev.bi.49.070180.005215. [DOI] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- McKnight S., Tjian R. Transcriptional selectivity of viral genes in mammalian cells. Cell. 1986 Sep 12;46(6):795–805. doi: 10.1016/0092-8674(86)90061-9. [DOI] [PubMed] [Google Scholar]
- Mosca J. D., Pitha P. M. Transcriptional and posttranscriptional regulation of exogenous human beta interferon gene in simian cells defective in interferon synthesis. Mol Cell Biol. 1986 Jun;6(6):2279–2283. doi: 10.1128/mcb.6.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D., Brickell P. M., Latchman D. S., Willison K., Rigby P. W. Transcripts regulated during normal embryonic development and oncogenic transformation share a repetitive element. Cell. 1983 Dec;35(3 Pt 2):865–871. doi: 10.1016/0092-8674(83)90119-8. [DOI] [PubMed] [Google Scholar]
- Ragg H., Weissmann C. Not more than 117 base pairs of 5'-flanking sequence are required for inducible expression of a human IFN-alpha gene. Nature. 1983 Jun 2;303(5916):439–442. doi: 10.1038/303439a0. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Fernie B. F., Pitha P. M. Correlation between the induction of mouse interferon and the amount of its mRNA. Eur J Biochem. 1979 Jul;98(1):215–221. doi: 10.1111/j.1432-1033.1979.tb13179.x. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Analysis of interferon mRNA in human fibroblast cells induced to produce interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7426–7430. doi: 10.1073/pnas.78.12.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Synthesis of new proteins associated with the induction of interferon in human fibroblast cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4918–4922. doi: 10.1073/pnas.77.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Two levels of regulation of beta-interferon gene expression in human cells. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3923–3927. doi: 10.1073/pnas.80.13.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Skup D., Windass J. D., Sor F., George H., Williams B. R., Fukuhara H., De Maeyer-Guignard J., De Maeyer E. Molecular cloning of partial cDNA copies of two distinct mouse IFN-beta mRNAs. Nucleic Acids Res. 1982 May 25;10(10):3069–3084. doi: 10.1093/nar/10.10.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Zinn K., Maniatis T. Detection of factors that interact with the human beta-interferon regulatory region in vivo by DNAase I footprinting. Cell. 1986 May 23;45(4):611–618. doi: 10.1016/0092-8674(86)90293-x. [DOI] [PubMed] [Google Scholar]