Abstract
Projections from neurons of the bed nucleus of the stria terminalis (BST) to the ventral tegmental area (VTA) are crucial to behaviors related to reward and motivation. Over the past few years, we have undertaken a series of studies to understand: 1) how excitatory inputs regulate in vivo excitable properties of BST neurons, and 2) how BST inputs in turn modulate neuronal activity of dopamine neurons in VTA. Using in vivo extracellular recording techniques in anesthetized rats and tract-tracing approaches, we have demonstrated that inputs from the infralimbic cortex and the ventral subiculum exert a strong excitatory influence on BST neurons projecting to the VTA. Thus, the BST is uniquely positioned to receive emotional and learning-associated informations and to integrate these into the reward/motivation circuitry. We will discuss how changes in the activity of BST neurons projecting to the VTA could participate in the development or exacerbation of psychiatric conditions such as drug addiction.
Keywords: Ventral subiculum, Prefrontal cortex, Bed nucleus of the stria terminalis, Ventral Tegmental Area, Dopamine cells, Opiate withdrawal, Reward, Motivation
1. Introduction
The bed nucleus of the stria terminalis (BST), a component of the extended amygdala (ExtA), has been implicated both in rodents and primates as a key mediator of stress and reward interactions (Walker et al., 2003; Burow et al., 2005; Choi et al., 2007; Harris and Aston-Jones, 2007). The mesolimbic dopamine (DA) system is necessary for the processing of naturally rewarding stimuli, as well as for self-administration of drugs of abuse (Grace et al., 2007). Numerous electrophysiological data (Murase et al., 1993; Georges and Aston-Jones, 2002; Floresco et al., 2003; Lodge and Grace, 2006) indicate that potent excitatory afferents of the ventral tegmental area (VTA) arise in the medial prefrontal cortex (mPFC), BST, and laterodorsal and pedunculopontine tegmental nuclei and are essential for burst firing of VTA DA neurons. We previously reported that the BST projects to and exerts a strong excitatory influence on the firing of DA neurons within the VTA (Georges and Aston-Jones, 2001, 2002). This particular excitatory projection has been implicated in physiological and pathological reward-directed behaviors, such as food and cocaine self-administration (Dumont et al., 2005; Grueter et al., 2006) and drug seeking (Aston-Jones and Harris, 2004). Excitatory synaptic inputs are a key component of the regulation of BST cell excitability and are important in the central control of the autonomic system, the actions of many drugs of abuse and the mediation of stress responses (Wilkinson and Pittman, 1995; Dumont and Williams, 2004; Egli et al., 2005; Kash et al., 2007). The BST integrates information from stress input pathways, and subsequently regulates both stress output and reward pathways (Herman and Cullinan, 1997; Georges and Aston-Jones, 2002). The endocannabinoid system participates in these functions, since we have recently reported that the infralimbic cortex (ILCx) glutamatergic projections control mesolimbic DA neurons through the CB1 receptor located in the BST (Massi et al., 2008). Moreover, inhibition of fast excitatory transmission in the BST by injection of an AMPA receptor antagonist blunts anxiety responses (Walker and Davis, 1997), indicating that regulation of glutamatergic transmission in this region is an important target for anxiolytic and anxiogenic stimuli. We will focus here on the function of BST in brain reward circuitry, particularly in terms of its connectivity with the mesolimbic DA system, and the major role it plays in modulating DA neuronal activity. We refer also to an excellent review for studies on the role of BST in the pathophysiology of stress-related psychiatric disorders (Forray and Gysling, 2004).
The anterior part of the BST receives excitatory inputs primarily from the ventral subiculum of the hippocampus (vSUB) and limbic cortical regions (Cullinan et al., 1993; McDonald et al., 1999; Dong et al., 2001). The mPFC and the vSUB play important role in regulating adaptive responses to emotional stress (Spencer et al., 2005; Herman and Mueller, 2006) and reward-directed behaviors (Morgan and LeDoux, 1995; Seamans et al., 1995; Tzschentke and Schmidt, 1999; Vertes, 2006; Grace et al., 2007).
The mPFC is a major component of the motivation network, as it regulates general motivational significance of stimuli and determines the intensity of associated behavioral responses (Goldstein and Volkow, 2002). There is an increasing recognition of the important functional distinctions within discrete subdivisions of the mPFC, namely ILCx, prelimbic (PL) and anterior cingulate (Cg) cortices (Morgan and LeDoux, 1995; Seamans et al., 1995; Tzschentke and Schmidt, 1999; Vertes, 2006; Grace et al., 2007). The ILCx may also have an important role in regulating adaptive responses to emotional stress (Spencer et al., 2005; Radley et al., 2006) as well as in diverse functions including attention, rewarding-directed behavior and working memory, in accordance to its projection to the ExtA (for review: (Uylings et al., 2003). The PL/Cg sends 90 % of the mPFC excitatory projections to the VTA. These synapses primarily connect to GABAergic interneurons, but also to mesocortical DA neurons (Carr and Sesack, 2000; Geisler et al., 2007). In contrast, the ILCx sends only about 10% of the prefrontal excitatory projections to the VTA (Geisler et al., 2007), but little is known regarding a potential implication of the ILCx in the control of the DA neurons activity. In our recent research, we combined tract tracing approaches and in vivo electrophysiological recordings to demonstrate that the BST actively relays the excitatory drive from the ILCx to VTA DA neurons (Massi et al., 2008).
The vSUB plays an important role in the relapse to cocaine seeking behavior (Rogers and See, 2007) and interacts with key limbic structures through its output connections with the PFC, BST, nucleus accumbens (NAc) and the amygdala (Ishikawa and Nakamura, 2006). A serie of seminal studies by Grace and colleagues defined the vSUB as an interface between the hippocampal formation and structures in the brain reward circuitry. Direct stimulation of the vSUB increases the population of active DA neurons in the VTA via polysynaptic pathways relayed through the NAc (Grace et al., 2007). Although the vSUB does not project directly to the VTA DA neurons, it can indirectly activate DA neuronal impulse activity and increase DA levels in the terminal regions (Legault and Wise, 1999; Legault et al., 2000). Pyramidal neurons in the vSUB exhibit transitions between two modes of action potential outputs: bursting and single spiking. The transitions between these two modes is thought to be important in integrating spatial location and context information where reward occurs (Tabuchi et al., 2000).
Some of our experiments demonstrating the prominent role of the BST in the control of DA neurons excitability are summarized below (see also: (Georges and Aston-Jones, 2001, 2002; Massi et al., 2008). These studies were designed to evaluate the influence of the projection of the ILCx and the vSUB to the BST and to further elucidate the interactions between prefrontal and hippocampal regions to control the activity of VTA DA neurons.
2. BST as a hub connecting hippocampus, prefrontal cortex and dopamine system
The anterolateral group of the BST plays a critical role in anxiety and reward related behaviors (Forray and Gysling, 2004; Dumont et al., 2005). It is a complex structure that can be divided into anterolateral, subcommissural (anteroventral), oval, juxta-capsular, fusiform and rhomboid nuclei (Dong et al., 2001). This region of the BST has a large number of GABAergic neurons, mostly classified as medium-sized spiny neurons (McDonald, 1983; Cullinan et al., 1993; Sun and Cassell, 1993). Only recently, physiological properties of neurons from these regions have been studied. It has been reported by our group using in vivo preparations (Georges and Aston-Jones, 2002; Massi et al., 2008) and by others using ex vivo preparations (Rainnie, 1999; Dumont and Williams, 2004; Dumont et al., 2005; Hammack et al., 2007; Kash et al., 2007; Dumont et al., 2008) that there are electrophysiologically distinct populations of neurons in the BST. Specifically, the anterolateral and anteroventral regions of the BST send a monosynaptic excitatory output to DA neurons in the VTA (Georges and Aston-Jones, 2002; Dumont and Williams, 2004; Kash et al., 2007). Our studies demonstrate that a subgroup of BST neurons project to the VTA and potently activate neuronal activity of DA neurons (Georges and Aston-Jones, 2002). However, these data do not allow to conclude formally that the BST contains glutamatergic neurons. But, two converging lines of evidences support the existence of a distinct population of glutamatergic neurons in the anterolateral BST: 1) Using radioactive (Fig. 1A′) or colorimetric (Fig. 1B′ and C′) in situ hybridization techniques, we detect prominent labeled neurons for the glutamate transporter 3 (VGLUT3) mRNA in BST (Fig. 1; (Gras et al., 2002; Schafer et al., 2002; Herzog et al., 2004; Riedel et al., 2008); and 2) Using [H3] D-aspartate retrograde tracing technique, numerous radiolabelled cell bodies were detected in the anterolateral and antero ventral BST (Csaki et al., 2000; Kocsis et al., 2003). This data taken together with our retrograde and anterograde labelings, and antidromic activation of BST neurons by VTA stimulation (Georges and Aston-Jones, 2002), give anatomical and physiological support for a glutamatergic projection from the BST to the VTA.
Figure 1.
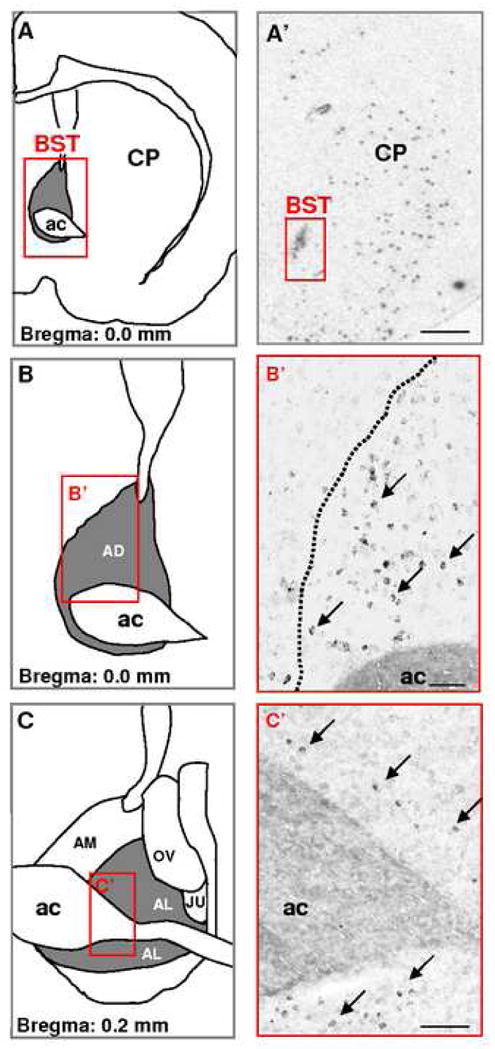
In situ hybridization analysis of VGLUT3 transcript distribution in the rat BST. A–C: Schematics illustrating the anterior region of the BST at two levels (A–B: AP= 0.0 mm from bregma; C: AP= −0.2 mm from bregma. A′– C′: Coronal brain sections were hybridized with antisense 35S-labeled oligonucleotides (A′) or DIC-UTP-labeled cRNA probes (B′ and C′). Neurons expressing VGLUT3 mRNA (arrows) are distributed in the anterior lateral group of the BST. ac, anterior commissure; AD, anterodorsal BST; AL, anterolateral BST; AM, anteromedial BST; OV, oval nucleus; JU, juxtacapsular nucleus. Scale bars: A′= 1.5 mm; B′ and C′= 0.2 mm. These illustrations are issued from experiments published in Gras et al., 2002 and Herzog et al., 2004.
Glutamatergic synapses in the ExtA are involved in behaviors related to stress and the effects of addictive drugs (for review: (Koob, 2003)). Plasticity at excitatory synapses onto the subgroup of VTA-projecting BST neurons correlates with operant learning, suggesting a role of the BST in goal-directed behaviors towards natural and drug associated rewards (Dumont et al., 2005). The vSUB and cortical areas provide the prominent known glutamatergic input into the BST (McDonald et al., 1999; Dong et al., 2001; Herman et al., 2002) and these inputs are topographically organized (McDonald, 1998). Support for this anatomical organization is provided by data from our laboratory (Massi et al., 2008), reporting the first functional evidence of a strong excitatory regulation by ILCx and vSUB of the majority of BST neurons including those projecting to VTA. Considering the short latency excitations for most cells (<15 ms; Table 1), and the distance between the ILCx and the BST (approximately 5 mm) or the vSUB and the BST (approximately 6 mm), we propose that the excitatory responses of BST neurons to ILCx or vSUB stimulations are mediated by direct and monosynaptic glutamatergic inputs. Thus, that study provides the first anatomical and functional evidence (Fig. 2–4 and Table 1) that inputs from the ILCx and vSUB exert a strong excitatory influence on BST neurons projecting to the VTA. We demonstrate that blocking GABAA receptors within the BST by local picrotoxin, enhanced the probability of spike discharge evoked in BST neurons by ILCx stimulation (Massi et al., 2008). These data raise questions about the potential role of feedforward inhibitory circuits, including interneurons within the BST, and/or projections from GABAergic regions like NAc and central nucleus of the amygdala (Le Gal LaSalle et al., 1978; Sun and Cassell, 1993), in the regulation of BST neurons excitability. The BST sends a monosynaptic excitatory output to DA neurons in the VTA (Georges and Aston-Jones, 2002; Dumont and Williams, 2004). However, the majority of BST neurons are GABAergic (70–90%; (Le Gal LaSalle et al., 1978; Sun and Cassell, 1993). Moreover, a particular feature characterizing the BST is its large density of NMDA receptor (subunit: NMDAR1) immunoreactive terminals, forming symmetric synapses and displaying GABA immunoreactivity (Paquet and Smith, 2000). Thus, this population of presynaptic NMDARs, if activated by extrasynaptic glutamate diffusing from neighboring synapses (Kullmann and Asztely, 1998), may modulate the release of GABA. Our data with GABAA antagonist provide further support for the hypothesis that glutamatergic and GABAergic afferent pathways regulate BST neuron population activity (Massi et al., 2008).
Table 1.
Summary of basic electrophysiological properties, onset latencies and durations of excitatory responses of BST and VTA neurons to ILCx and vSUB stimulations. All values are means ± sem. BST neurons projecting to the VTA are those antidromicaly driven by the VTA stimulation. Adapted from Massi and colleagues, 2008.
| BST neurons projecting to the VTA | ILCx stimulation | vSUB stimulation |
|---|---|---|
| Number of neurons | n=32 | n=12 |
| Spontaneous firing rate (Hz) | 10.5 ± 2.6 | 0.73 ± 0.47 |
| Action potential width (ms) | 0.74 ± 0.04 | 0.72 ± 0.02 |
| Neurons responsive to tail pinch (%) | 21.8 | ND |
| Excitatory response to stimulation (% of neurons) | 81.25 | 83.05 |
| Response magnitude of excitation (spikes) | 64.3 ± 12.9 | 96.20 ± 6.2 |
| Onset of excitation (ms) | 9.4 ± 0.7 | 12.8 ± 0.6 |
| Duration of excitation (ms) | 10.5 ± 1.0 | 11.0 ±1.6 |
| Onset of inhibition (ms) | 10.9 ± 3.3 | 15.0 ± 2.2 |
| Duration of inhibition (ms) | 85.0 ± 29.7 | 132.5 ± 11.4 |
Figure 2.
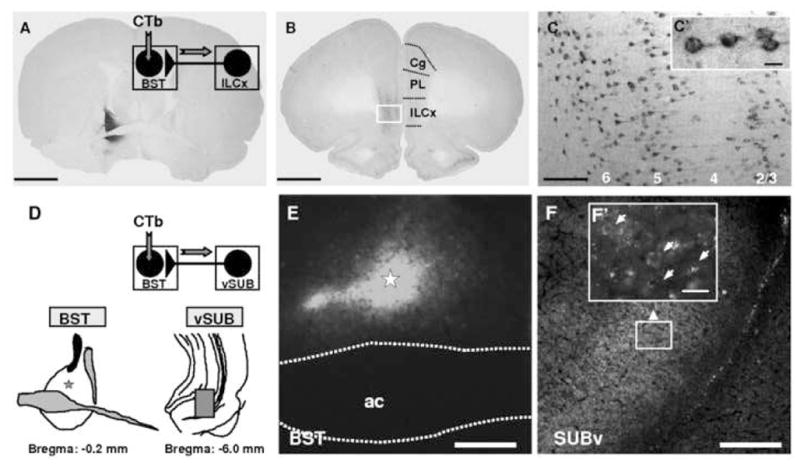
ILCx and vSUB connect directly to BST neurons. A–C: Projections from ILCx to BST revealed by retrograde labeling in ILCx after injection of the subunit b of the cholera toxin (CTb) within the BST. A: Representative photomicrograph of a BST CTb injection (dark labeling). Inset: Diagram of the injection protocol used in this experiment B, C: Bright-field photomicrographs of retrograde labeling in ILCx after BST CTb injection. Injections of CTb into the BST revealed that cortical projections to the BST originate exclusively from the ILCx. In each of the four animals injected with CTb, we observed numerous retrogradely labeled neurons in the ILCx. No anterograde labeling was detected in the ILCx. In sections processed for CTb (dark labeling), cell bodies are observed in ILCx layers 2/3, 5 and 6. Cell bodies retrogradely labeled in the ILCx are shown at higher magnification in C and C′. Scale bar: A and B, 2.0 mm; C, 0.1 mm; C′, 10 μm. D–F: Projection from vSUB to BST revealed by retrograde labeling in vSUB after BST CTb injection. D: Diagram of the injection protocol used in this experiment. BST was injected with Alexa Fluor® 488 conjugated to CTb (Invitrogen; 60 nl). Five rats were sacrified 7 days after receiving CTb into the BST. Below, Anatomical localization on a cartography of the injection site and retrograde labeling represented in E and F. E: Representative photomicrograph of a BST CTb injection (bright labeling show the CTb fluorescence). F: Fluorescent photomicrographs of retrograde labeling in vSUB after BST CTb injection. In each of the five animals injected with CTb, we observed numerous retrogradely labeled neurons in the vSUB. Cell bodies retrogradely labeled in the vSUB are shown at higher magnification in F′. Scale bar: C and D, 0.1 mm; C′, 10 μm; E; F, 0.5 mm and F′, 20 μm. ac, anterior commissure; BST, bed nucleus of the stria terminalis; CTb, Cholera toxin-B subunit; ILCx, infralimbic cortex; PL, prelimbic cortex; Cg, cingular cortex; vSUB, ventral subiculum. Adapted from Massi and colleagues, 2008.
Figure 4.
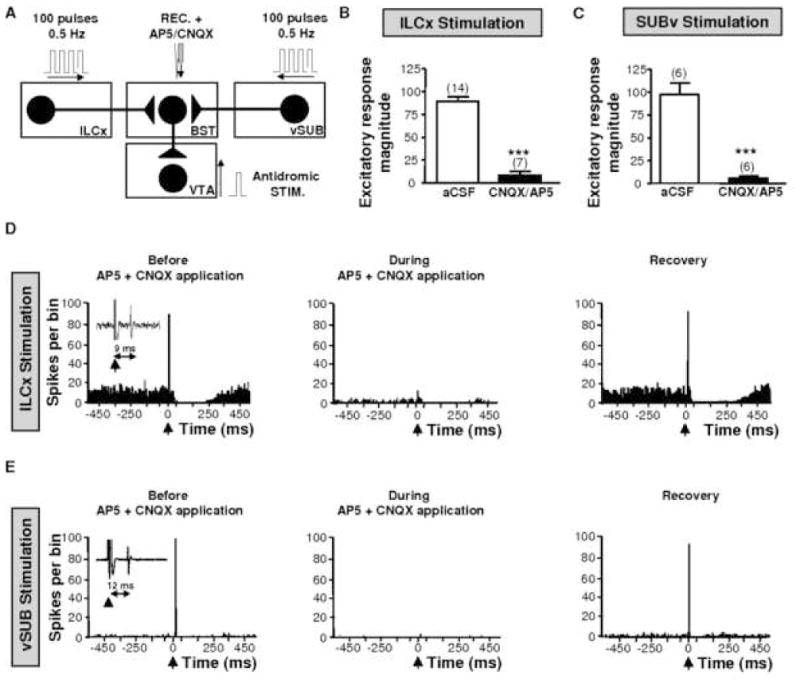
Infusion of glutamatergic receptor antagonists within the BST blocks the excitatory drive from the ILCx or the vSUB on BST neurons that project to the VTA. A, Diagram of the stimulation protocol: ILCx or vSUB was stimulated with a 100-pulse train. A mixture of 100 μM amino-5-phosphonopentanoic acid (AP5) and 50 μM 6-cyano-7nitroquinoxaline-2,3-dione (CNQX) are microinfused through a pipette adjacent to the recording electrode. VTA-projecting BST neurons were identified after antidromic stimulation of the VTA. B and C: Graphs illustrating the effects of ionotropic glutamatergic (black bars) antagonists on excitation of BST neurons projecting to the VTA, after stimulation of the ILCx (B) or the vSUB (C). Scores are percentage ± SEM of baseline response magnitudes for VTA projecting BST neuronal responses evoked by ILCx or vSUB electrical stimulation during microinjection into the BST of aCSF (white bars), the mixture of 50 μM CNQX plus 100 μM AP5 (black bars). Numbers of neurons recorded in each group are mentioned in brackets above each histogram bar. A Student test for pairwise comparisons was performed for excitation.*** p<0.001. D, Effect of the CNQX+AP5 cocktail on a characteristic BST neuron projecting to the VTA during ILCx electrical stimulation. Typical peristimulus time histograms (PSTHs) show ILCx-evoked responses before, during and after (recovery) CNQX+AP5 injection into the BST for the same BST neuron identified as projecting to the VTA. Inset in D shows orthodromic spikes evoked by stimulation of ILCx. Microinjection of CNQX+AP5 prevented the short latency activation of BST neurons evoked by ILCx stimulation, and decreased basal activity but had no effect on the inhibition. E, Effect of the CNQX+AP5 cocktail on a characteristic BST neuron projecting to the VTA during vSUB electrical stimulation. Typical PSTH show vSUB evoked responses before, during and after (recovery) CNQX+AP5 injection into the BST, for the same BST neuron identified as projecting to the VTA. Inset in E shows orthodromic spikes evoked by stimulation of vSUB. Microinjection of CNQX+AP5 prevented the short latency activation of BST neurons evoked by ILCx or vSUB stimulation, and decreased the basal activity but had no effect on the inhibition. BST, bed nucleus of the stria terminalis; ILCx, infralimbic cortex; REC, recording electrode; vSUB, ventral subiculum; VTA, ventral tegmental area; ac, anterior commissure; STIM, stimulating electrode. Adapted from Massi and colleagues 2008.
Our data show that the ILCx and the vSUB exert their powerful phasic control over the activity of BST neurons via ionotropic glutamate receptors (NMDA and AMPA/kainate; Fig. 4). NMDA and AMPA/kainate receptor antagonists dramatically decreased basal-steady state activity suggesting a stimulatory tonic influence of glutamatergic synapses within the BST. Other neurotransmitters may also participate in the ILCx-BST or vSUB-BST pathway. For instance, inhibitory responses (Table 1) may be mediated by co-release of an inhibitory transmitter from the cortical pyramidal neurons projecting from the ILCx to the BST or from cells nearby that are activated at an early latency. The prodynorphin immunoreactivity of pyramidal cells (Evans et al., 2007), together with the presence of kappa opioid receptor on BST neurons, support the role of the endogeneous opioid system in the inhibitory control of BST neurons (Mansour et al., 1996). The ILCx and the vSUB may also send glutamatergic afferents to GABA terminals that make synapses onto BST neurons. Accordingly, anatomical and pharmacological studies suggest that NMDA and non-NMDA receptors may act pre-synaptically to modulate release of GABA (Young and Bradford, 1993; Glitsch and Marty, 1999; Paquet and Smith, 2000). However, such interactions between glutamatergic and GABAergic systems within the BST appears unlikely, as the evoked inhibition of BST neurons by ILCx stimulation is not affected neither by intra-BST infusion of GABAA or NMDA/AMPA/kainate antagonists (Massi et al., 2008). It remains unknown whether external GABAergic projections targeting the BST, GABAB receptors or other neurotransmitter systems like the endocannabionoid system could participate in the inhibitory response evoked by ILCx stimulation. Additional studies are needed to address this issue.
Excitatory afferents to the VTA are of diverse origins (Geisler et al., 2007) and they differentially target subpopulations of VTA DA neurons on the basis of their projections. The mesolimbic DA system represents the vast majority of the VTA DA neurons and projects to limbic structures, including the NAc, the basolateral amygdala (BLA) and the BST (Marinelli et al., 2006). DA projections to these target areas are involved in the rewarding properties of nearly all drugs of abuse (Carboni et al., 2000; Koob and Le Moal, 2008; Wise, 2008). Carr and Sesack revealed that: 1) the majority of VTA DA neurons project to the NAc (65%–85%) and 2) the mPFC send excitatory glutamatergic projection to DA neurons in the VTA, which, in turn are connected in a reciprocal manner to the mPFC (Carr and Sesack, 2000). This reciprocal mesocortical pathway represents 30–40 % of VTA DA neurons. The mPFC powerfully activates the VTA DA neurons by evoking bursting activity (Tong et al., 1996a, b). Although the mPFC has been shown to send an excitatory projection to the VTA, which synapses directly with DA neurons (Sesack and Pickel, 1992), it is not necessarily the case that the mPFC monosynapticaly activates the majority of VTA DA neurons (Tong et al., 1996b). Given the long latency of cortically induced bursts (122 ms), it seems likely that these events were produced by indirect excitatory inputs to the DA neurons (Tong et al., 1996b). We have tested the BST as a glutamatergic relay terminating in the VTA in a recent series of experiments. Our anatomical and in vivo electrophysiological evidence demonstrate that glutamatergic projections arising exclusively from the ILCx converge on BST neurons, which in turn project to and excite DA neurons (Fig. 2 and 3). We demonstrated that BST is necessary for the efficient relay of cortical excitation to DA neurons of the VTA (Massi et al., 2008). Thus, our finding that more than 80 % of the VTA DA cells respond to ILCx stimulation with an increased spiking probability (Massi et al., 2008), strongly suggests that the BST processes, amplifies and actively distributes the excitatory drive from the ILCx to the majority of DA neurons. However, recent evidence with electrical stimulation of the PrL/ILCx region demonstrated a stronger short-latency (<25 sec) excitatory effect on VTA DA neurons than previously reported, indicating that direct effects of mPFC on VTA activity may also exist (Moorman and Aston-Jones, 2007).
Figure 3.
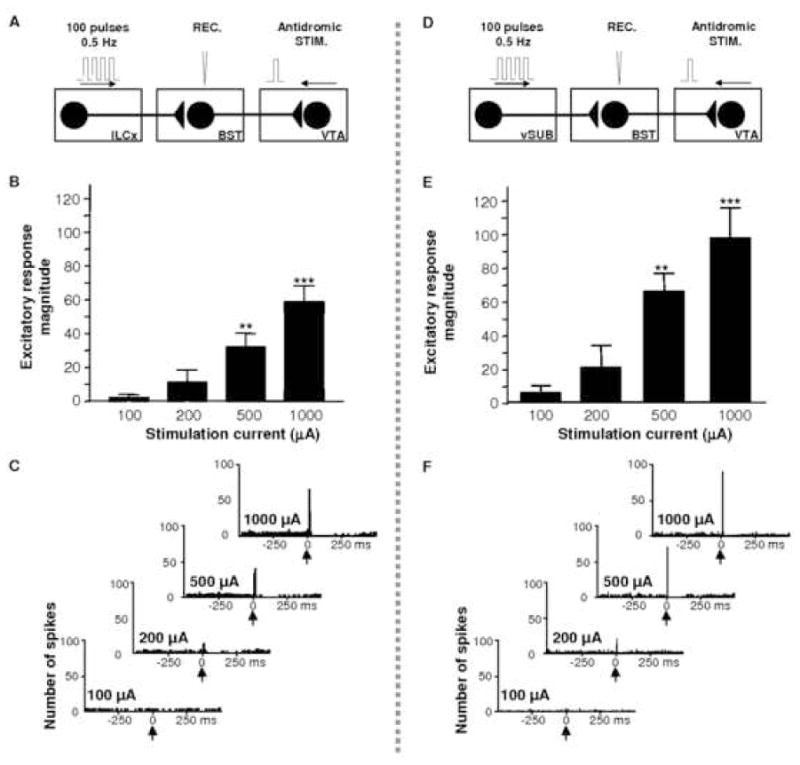
Results of input/ouput tests on BST neurons projecting to the VTA after stimulation of ILCx or the vSUB. A, Diagram of the stimulation protocol used in this experiment. ILCx was stimulated with a 100-pulse train. B, Increasing intensity of stimulation current in ILCx evoked higher response magnitude in VTA-projecting BST neurons. C, Typical post-stimulus time histograms (PSTHs) illustrate activity of VTA-projecting BST neurons in response to ILCx stimulation at increasing intensities (100, 200, 500 and 1000 μA). D, Diagram of the stimulation protocol used in this experiment. Here, the vSUB was stimulated with a 100-pulse train. E, Increasing intensities of vSUB stimulation evoked higher response magnitudes in VTA-projecting BST neurons. C, Typical PSTH illustrate activity of a VTA-projecting BST neuron in response to ILCx stimulation at increasing intensities (100, 200, 500 and 1000 μA). BST neurons projecting to the VTA are those antidromicaly driven by VTA stimulation. The slope of the relationship between injected current and evoked firing rate is similar in VTA-projecting BST neurons after stimulation of the ILCx or vSUB. BST, bed nucleus of the stria terminalis; ILCx, infralimbic cortex; vSUB, ventral subiculum; VTA, ventral tegmental area. Adapted from Massi and colleagues 2008.
The vSUB (Blaha et al., 1997; Legault and Wise, 1999; Legault et al., 2000; Taepavarapruk et al., 2000; Floresco et al., 2001) powerfully activates the DA system. Although the circuitry used by the vSUB to activate the DA system remains to be fully elucidated, the mechanisms proposed to account for this effect involve: 1) an increase of the population of active DA neurons in the VTA through a multisynaptic inhibition of GABAergic inputs to the VTA, and 2) a presynaptic modulation of DA release by the action of glutamatergic inputs arising from the vSUB. Conversely, in a related series of microdialysis and electrophysiological experiments, Wise and colleagues have shown that NMDA injections into the vSUB have a net excitatory effect on VTA dopaminergic transmission, increasing DA neurons firing and increasing both somatodendritic and terminal DA release (Legault and Wise, 1999). Additionally, ionotropic glutamate receptor activation in the VTA is necessary for vSUB stimulation to increase DA efflux in the NAc (Legault et al., 2000). Finally, intra-VTA perfusion of glutamatergic antagonists completely abolished vSUB-evoked DA release in the NAc (Legault et al., 2000). These experiments clearly demonstrated that the vSUB activates VTA DA neurons through a glutamatergic link terminating in the VTA. In light of these data, we propose a possible circuit involving projections from the vSUB to the BST. The BST, in turn, provides well-characterized excitatory inputs to the VTA (Georges and Aston-Jones, 2001, 2002; Massi et al., 2008).
3. Potent regulation of midbrain dopamine neurons by the BST
Midbrain DA neurons are necessary importantly involved in most reward-directed behaviors, motivation and learning (Hyman and Malenka, 2001; Jones and Bonci, 2005; Chen et al., 2008). Furthermore, accumulating evidence indicates that increasing the glutamatergic drive enhances DA neuronal activity and produces the characteristic bursting pattern expressed in vivo (for review: (Marinelli et al., 2006). This increase in bursting activity of VTA DA neurons has been shown to be important in mediating a number of motivated behaviors (Fields et al., 2007). Anatomically, BST neurons are in a good position to control reward-related behaviors. For instance, they innervate the DA-rich VTA (Phillipson, 1979; Georges and Aston-Jones, 2002; Dong and Swanson, 2006) and the NAc (Groenewegen and Russchen, 1984; Dong and Swanson, 2006), structures that drive behaviors motivated by natural or drug rewards. We (Georges and Aston-Jones, 2001, 2002) and others (Rainnie, 1999; Dumont and Williams, 2004; Dumont et al., 2005; Hammack et al., 2007; Kash et al., 2007; Dumont et al., 2008) reported that a sub-population of BST neurons project to the VTA. We used antidromic activation of BST neurons following stimulation of the VTA and retrograde labeling between the BST and VTA (Fig. 5) to demonstrate the monosynaptic input from the BST to the VTA. A recent paper by Dumont and colleagues (Dumont et al., 2005) provides direct and specific evidence that increased glutamate receptor activity on the sub-population of BST neurons projecting to the VTA might be a key component of the limbic circuit underlying operant behaviors in relation to reward. Thus, while at present it remains difficult to predict the net functional and behavioral effects of activation of BST on target brain regions, our recent series of experiments (summarized in Fig. 6) illustrates potential mechanisms by which excitatory input from the BST enhances activity of VTA DA neurons (Georges and Aston-Jones, 2001, 2002).
Figure 5. Projection from the BST to the VTA revealed by antidromic stimulation from the VTA and retrograde labeling in the BST after injection of CTb into the VTA.
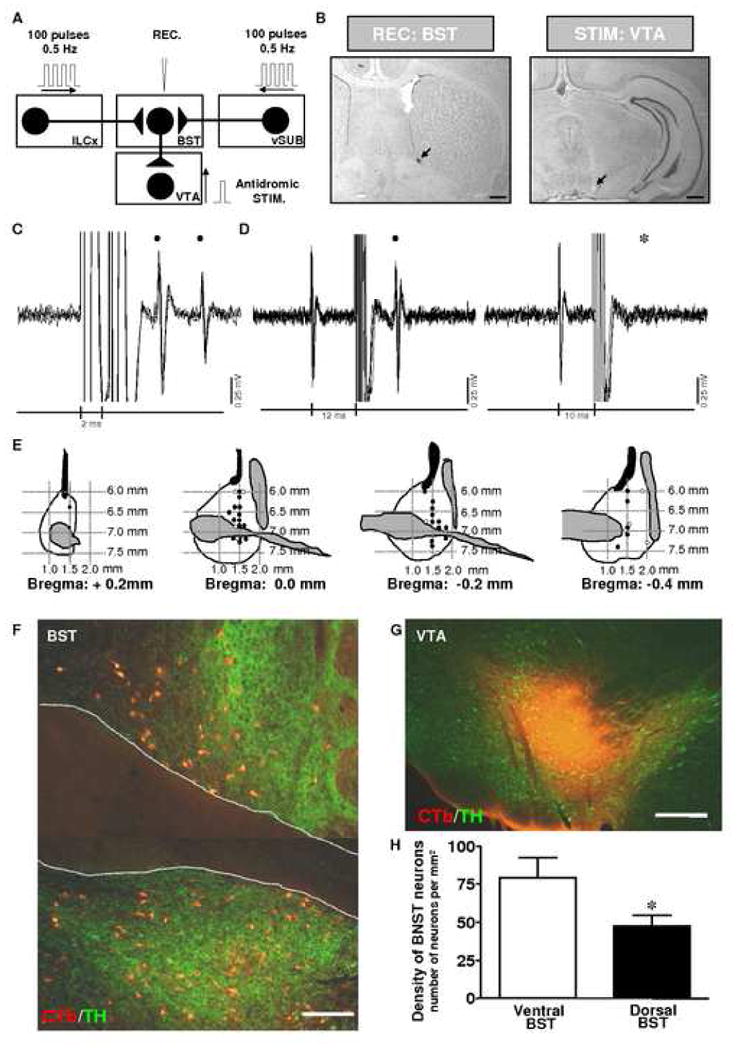
A, Diagram of the stimulation protocol used in this experiment. Here, the ILCx or vSUB are stimulated with a 100-pulse train, and BST neurons projecting to the VTA were identified by antidromic activation from the VTA. B, Left, Recording location for a BST neuron projecting to the VTA (dark spot, black arrow). Right, electrical stimulation site in the VTA (lesioned area, black arrow). Scale bars: 0.5 mm. C and D: Five superimposed traces illustrating high-frequency activation and collision test for a BST cell driven from the VTA. C, Driven spikes (black circles) elicited by each of paired stimuli (vertical lines, 2 ms interpulse interval), indicating frequency following for this cell at 500 Hz. D, Left, Stimulation of the VTA 12 ms after spontaneous spikes (left side of traces) elicit driven spikes (black circle) at 9.5 ms latency. Right, Driven spikes are occluded for similar stimuli delivered 10 ms after spontaneous impulses indicating collision between spontaneous and driven spikes. The asterisk indicates when driven spikes would have occurred in the absence of collision. E, Schematic presentation of neuronal recording sites in the BST of neurons projecting to the VTA. Black circles: locations of synaptically driven BST neurons by single-pulse ILCx electrical stimulation. White circles: locations of synaptically driven BST neurons by single-pulse vSUB electrical stimulation. Numbers refer to stereotaxic coordinates. Note that all the BST neurons projecting to the VTA tested were driven by ILCx or vSUB stimulations. F–H, Projection from the BST to the VTA revealed by retrograde labeling in the BST after injection of CTb into the VTA. F, Epifluorescence photomicrographs illustrating retrograde labeling in the dorsal and ventral parts of the BST after CTb injection into the VTA. G, Photomicrograph illustrating a representative CTb injection site in the VTA. The sections have been processed dually for CTb (red) and tyrosine hydroxylase (TH; green). Scale bars: F, 100 μm; G, 200 μm. H, Bar histograms comparing the density of CTb-immunoreactive neurons in the dorsal and ventral BST after an injection of CTb in the VTA (n=15 rats perfused 7 days after receiving CTb into the VTA). t-test was used to establish statistical differences between the dorsal and the ventral part of the BST. BST, bed nucleus of the stria terminalis; ILCx, infralimbic cortex; REC, recording electrode; vSUB, ventral subiculum; VTA, ventral tegmental area; ac, anterior commissure; STIM, stimulating electrode. Adapted from Massi and colleagues 2008.
Figure 6.
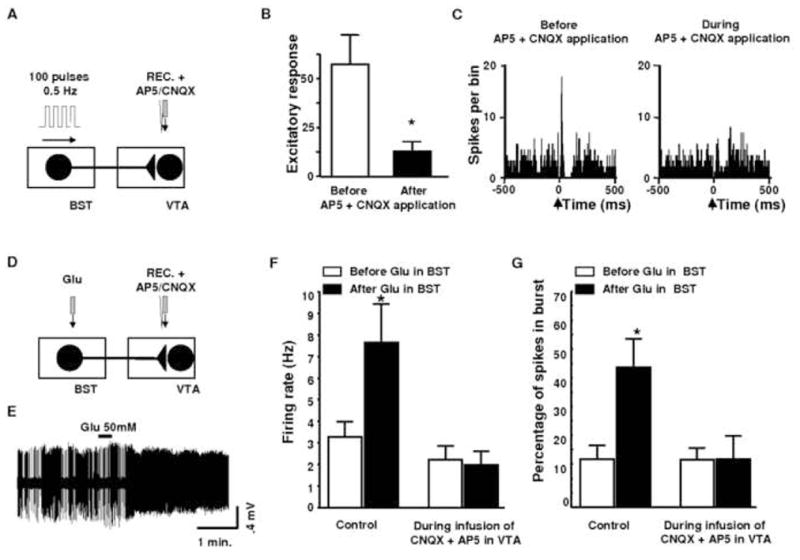
Effects of AP5+CNQX on VTA DA neuronal responses evoked by BST electrical stimulation A, Diagram of the stimulation protocol used in this experiment. Here, the BST stimulated with a 100-pulse train. DA neurons were recorded within the VTA and the CNQX+AP5 cocktail was microinfused through a pipette adjacent to the recording electrode. B, Mean (±SEM) response magnitudes (Rmags) of VTA DA neuronal responses evoked by BST stimulation before (black bars) and during (white bars) microinjection of AP5 (100 μM) + CNQX (50 μM) into the VTA. Microinjection of AP5+CNQX prevented the short latency activation of DA VTA neurons evoked by BST stimulation. The same cells were used before and after drug application. A Student t-test for pairwise comparisons was performed. *p<0.05. C, PSTHs showing VTA evoked responses before and during drug injection into the VTA for a typical DA neurons. Single pulse stimuli (0.5 ms, 0.5/sec) were delivered at time zero. D, Diagram of the stimulation protocol used in this experiment. Here, the BST stimulated with local infusion of glutamate (Glu, 50mM, 60nl). DA neurons were recorded within the VTA and the CNQX+AP5 cocktail was microinfused through a pipette adjacent to the recording electrode. E, Oscilloscope traces of a VTA DA neuron showing the typical firing activity before and after infusion of Glu at 50 mM into the BST. Glu injection is designated by the line above each trace. F and G, Effects of AP5 + CNQX on VTA DA neuronal responses evoked by BST stimulation by Glu microinjection : Graphs comparing firing rate (F) and bursts (G) of VTA DA neurons before (white bars) and during local microinfusion of 50 mM CNQX + 100 mM AP5 into the VTA. Note that the CNQX/AP5 mixture blocked both the increase in bursting as well as the increased in firing rate of VTA DA neurons evoked by chemical stimulation of the BST. BST, bed nucleus of the stria terminalis; Glu, glutamate; REC, recording electrode; VTA, ventral tegmental area; STIM, stimulating electrode. Data were analyzed by two-way ANOVA. * p<0.005. Adapted from Georges and Aston-Jones, 2001, 2002.
The short latency excitations for many cells following BST electrical stimulation (<20ms), and the distance between the BST and the VTA (approximately 6 mm), indicate that at least some of these responses are probably mediated by a direct input to the VTA from the BST (Fig. 6). Our anatomical data demonstrate a direct projection from the BST to the VTA, supporting this hypothesis (Fig. 5). Our results describing a similar activation of VTA DA neurons after microinjections of glutamate in the BST (Fig. 6), indicate that the activation of VTA DA neurons following electrical stimulation of the BST are mediated by activation of BST neurons and not by ≪ en passant ≫ fibers. Our electrophysiological and anatomical results (Fig. 5 and 6) revealed that neurons of the BST project directly to the VTA and exert a strong excitatory influence on DA neurons mediated by NMDA and AMPA glutamatergic receptors (Georges and Aston-Jones, 2001, 2002).
Furthermore, we investigated potential influence of the BST on burst activity of VTA DA neurons. Microinfusion of glutamate into the BST increased burst activity of DA neurons (Fig. 6), whereas microinfusion of GABA into the BST decreased burst activity in DA cells (Georges and Aston-Jones, 2002). These results reveal that the BST can tonically regulate the amount of bursting of VTA DA neurons. Striatal DA efflux has been shown to correlate strongly with DA neuron activity, and is highly dependent on the pattern of discharge (Marinelli et al., 2006). In fact, it has been shown that bursts of action potentials are twice as potent as the same number of regularly spaced spikes to trigger DA release (Suaud-Chagny et al., 1992). Thus, the tonic modulation of burst firing by the BST could be an important element in the regulation of the VTA DA system.
4. Role of BST in reward-related behaviors
Acute opiate withdrawal
The ventral BST (vBST) has a remarkably dense innervation by norepinephrine (NE) fibers (Phelix et al., 1992), which largely originate in the medullary A1 and A2 cell groups (Delfs et al., 2000). This indicated to us that the vBST is a possible site for the ability of NE drugs to alleviate opiate withdrawal. Indeed, we found that systemic administration of the beta NE antagonist propranolol decreased the strong Fos induction in vBST associated with acute morphine withdrawal (Aston-Jones et al., 1999). In addition, microinjections of selective beta NE antagonists, or the alpha2 NE agonist clonidine, into vBST markedly attenuated the aversiveness of acute opiate withdrawal in rats (Delfs et al., 2000). Previous work established a role for BST in anxiety (Walker and Davis, 1997), and specifically for NE innervation in vBST with stress-induced anxiety (Cecchi et al., 2002). This led us to hypothesize that the aversiveness of opiate withdrawal involved, at least in part, anxiety generated by elevated NE inputs (Delfs et al., 2000; Smith and Aston-Jones, 2008). Mechanistically, NE acting in the BST may drive dysphoria by decreasing excitatory projections from the extended amygdala to its efferent targets, including VTA. This effect may be achieved via NE actions to increase GABA and decrease glutamate inputs onto excitatory VTA-projecting neurons in BST. NE application to neurons in ventrolateral BST (with a physiological profile like that of VTA-projecting neurons) was found to cause increased GABA(A)-IPSC frequency during acute opiate withdrawal (Dumont and Williams, 2004). This effect was mediated through alpha-1 and beta adrenoceptor mechanisms, whereas NE effects were modulated only through alpha-1 mechanisms in naive animals (Dumont and Williams, 2004). These findings are consistent with those of Delfs et al. (Delfs et al., 2000) indicating that the aversiveness of opiate withdrawal is due to NE acting at beta adrenoceptors in BST. VTA may be influenced by the BST during acute withdrawal, as indicated by the finding that acute opiate withdrawal inhibited VTA DA neuron firing and decreased extracellular DA in NAc, and that pre-treatment with clonidine prevented these effects (Pothos et al., 1991; Spanagel et al., 1994; Diana et al., 1995; Georges and Aston-Jones, 2003). This inhibition of VTA DA output may contribute to withdrawal effects, as indicated by the finding that D2 agonist injection into NAc decreased opiate withdrawal behaviors (Harris and Aston-Jones, 1994).
Enhanced drug preference during protracted withdrawal
Prolonged exposure to drugs causes neuronal and behavioral changes that exist long after symptoms of acute withdrawal have dissipated. These alterations cause a variety of maladaptive effects, including anxiety, vulnerability to stress-induced relapse, and enhanced drug-seeking. A series of experiments from our laboratory have shown that post-dependent animals process reward-related stimuli in an aberrant manner, such that conditioned place preference (CPP) is increased for stimuli associated with morphine or cocaine, whereas CPP for natural rewards such as food or novel objects is less than normal (Harris et al., 2001; Harris and Aston-Jones, 2003b, 2007).
To determine whether the extended amygdala plays a role in the enhanced preference observed in CPP tests during protracted morphine abstinence, neuronal activity was investigated using immunohistochemistry for the immediate early gene protein Fos. For these experiments, animals were sacrificed two hours following the drug-free CPP test session, so that Fos expression reflects re-exposure to, and conditioned response to, the drug-paired environment. Elevated Fos was observed in anterior cingulate, NAc core and shell, ventral BST, BLA and central nucleus of the amygdala (CeA), lateral hypothalamus and nucleus of the tractus solitatius (NTS) in post-dependent rats after five weeks of protracted abstinence, as compared to non-dependent and non-conditioned rats (Harris and Aston-Jones, 2003b; Aston-Jones and Harris, 2004; Harris and Aston-Jones, 2007). Fos in anterior cingulate and BLA correlated with CPP in all conditioned animals; however, only Fos in BST also correlated with CPP in post-dependent rats (Harris and Aston-Jones, 2003a). This indicates that activation in BST is uniquely associated with the elevated drug preference found during protracted abstinence. These data, in view of the strong NE innervation and effects in BST, led our lab to hypothesize that drug-conditioned stimuli can activate A1 and A2 NE neurons that release NE into BST. This was proposed to cause increased anxiety and produce negative reinforcement for drug rewards given during conditioning sessions. These negatively reinforcing effects were proposed to summate with positively reinforcing effects to increase motivation to seek out drugs (Aston-Jones and Harris, 2004; Smith and Aston-Jones, 2008).
Animals also exhibited decreased CPP for food or novel objects during protracted morphine abstinence. Food CPP in previously morphine-dependent rats was negatively correlated with Fos expression in CeA, ventrolateral BST and NTS (Harris and Aston-Jones, 2007). This, in view of the increased drug preference (described above), indicates that Fos activation in BST and CeA in the extended amygdala, and their major NE afferent in NTS, is associated with enhanced drug preference and decreased food preference. This indicates that increased activity in these stress systems may decrease the rewarding properties of natural rewards, while increasing the reward associated with drug rewards. These findings add to others reviewed above indicating that activation in extended amygdala areas, including the BST, is strongly associated with altered reward processing during protracted abstinence.
5. Potential functional significance
The BST plays critical roles in responses to both stress/anxiety and reward through mechanisms that involve excitatory glutamatergic transmission (Winder et al., 2002; Kash and Winder, 2006). Dumont and collaborators highlighted the role of increased excitatory synaptic transmission in the BST in both cocaine and food self-administration (Dumont et al., 2005). The BST plays an important role in addictive behaviors such as drug-seeking (Harris and Aston-Jones, 2007) and stress-induced relapse to cocaine (Erb et al., 2001). Activation of the BST neurons projecting to the VTA seems to be necessary for learning to associate drug rewards with specific environmental cues (Dumont et al., 2005). Although the DA system is classically associated with reward (Schultz, 2007), goal-directed behaviors can be altered by adding a stressful component (Ghiglieri et al., 1997; Phillips and Barr, 1997; Bowers et al., 1999; Calvo-Torrent et al., 1999). This indicates that final behavioral outcomes result from interactions between stressful and rewarding stimuli. The BST, driven by the ILCx and the vSUB may be involved in processing both emotional- and context-dependent stimuli, which in turn influence motivated-behaviors through the DA system (Fig. 7). Peters and colleagues recently reviewed convincing evidences showing that the ILCx regulates the expression of both fear and drug memories after extinction, through divergent projections to the amygdala and NAc respectively (Peters et al., 2009). We propose that the BST could be part of parallel circuit adjusting the impact of events coming from the ILCx or the vSUB in order to filter and to amplify the excitatory drive on DA neurons. The BST, with its roles in both stress activation and reward based learning and memory, could provide an important target for future pharmacotherapies designed to prevent pathologies associated with reward/motivation circuitry.
Figure 7.
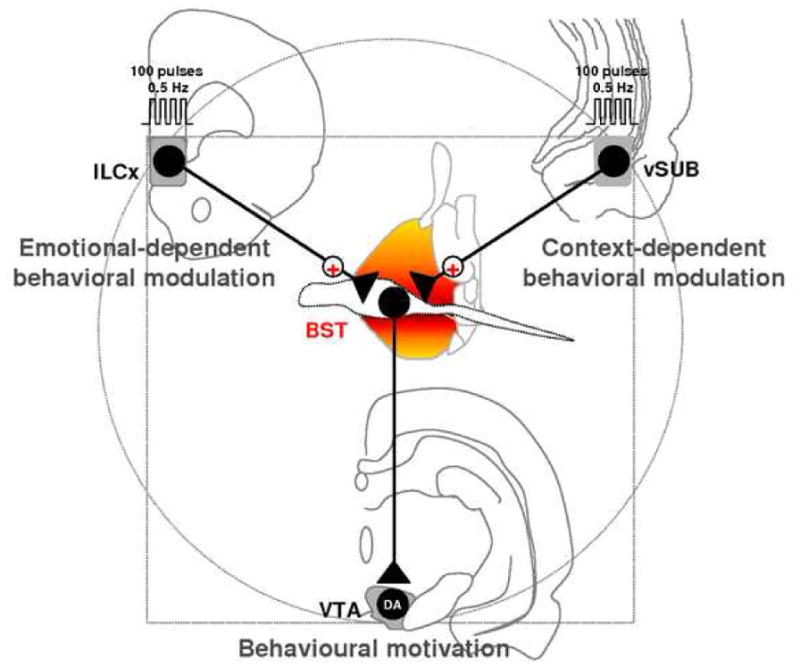
We propose a model to describe the neural circuitries by which the ILCx and vSUB modulate the BST neurons projecting to the VTA. Electrical stimulation of the ILCx and vSUB activate excitatory afferents to the BST wich in turn activates BST neurons projecting to the VTA. As a result, we recently demonstrated that activation of the ILCx produces phasic activation of midbrain DA neurons. In this model we propose that the ILCx-BST and vSUB-BST projections provide sufficient excitatory influence to activate the sub-population of BST neurons projecting to the VTA. BST, bed nucleus of the stria terminalis; ILCx, infralimbic cortex; VTA, ventral tegmental area; DA, dopaminergic neurons.
Acknowledgments
This work was supported by grants from INSERM, MILDT/INCa/ INSERM 2006, Région Aquitaine. We are grateful to our collaborators who made essential contributions to the work presented here (Léma Massi, Izaskun Elezgarai and Leire Reguero and Pedro Grandes). The authors would like to thank Mylène Potier for her editorial assistance.
6. Bibliography
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47:167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci. 1997;9:902–911. doi: 10.1111/j.1460-9568.1997.tb01441.x. [DOI] [PubMed] [Google Scholar]
- Bowers WJ, Attiast E, Amit Z. Stress enhances the response to reward reduction but not food-motivated responding. Physiol Behav. 1999;67:777–782. doi: 10.1016/s0031-9384(99)00129-8. [DOI] [PubMed] [Google Scholar]
- Burow A, Day HE, Campeau S. A detailed characterization of loud noise stress: Intensity analysis of hypothalamo-pituitary-adrenocortical axis and brain activation. Brain Res. 2005;1062:63–73. doi: 10.1016/j.brainres.2005.09.031. Epub 2005 Oct 2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Torrent A, Brain PF, Martinez M. Effect of predatory stress on sucrose intake and behavior on the plus-maze in male mice. Physiol Behav. 1999;67:189–196. doi: 10.1016/s0031-9384(99)00051-7. [DOI] [PubMed] [Google Scholar]
- Carboni E, Rolando MT, Silvagni A, Di Chiara G. Increase of dialysate dopamine in the bed nucleus of stria terminalis by clozapine and related neuroleptics. Neuropsychopharmacology. 2000;22:140–147. doi: 10.1016/S0893-133X(99)00085-8. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csaki A, Kocsis K, Halasz B, Kiss J. Localization of glutamatergic/aspartatergic neurons projecting to the hypothalamic paraventricular nucleus studied by retrograde transport of [3H]D-aspartate autoradiography. Neuroscience. 2000;101:637–655. doi: 10.1016/s0306-4522(00)00411-5. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Diana M, Pistis M, Muntoni A, Gessa G. Profound decrease of mesolimbic dopaminergic neuronal activity in morphine withdrawn rats. J Pharmacol Exp Ther. 1995;272:781–785. [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006;494:142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Mark GP, Mader S, Williams JT. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat Neurosci. 2005;8:413–414. doi: 10.1038/nn1414. Epub 2005 Feb 2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Rycroft BK, Maiz J, Williams JT. Morphine produces circuit-specific neuroplasticity in the bed nucleus of the stria terminalis. Neuroscience. 2008;153:232–239. doi: 10.1016/j.neuroscience.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:657–668. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. Epub 2001 Jan 2019. [DOI] [PubMed] [Google Scholar]
- Evans JM, Bey V, Burkey AR, Commons KG. Organization of endogenous opioids in the rostral agranular insular cortex of the rat. J Comp Neurol. 2007;500:530–541. doi: 10.1002/cne.21197. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Prolonged activation of mesolimbic dopaminergic neurons by morphine withdrawal following clonidine: participation of imidazoline and norepinephrine receptors. Neuropsychopharmacology. 2003;28:1140–1149. doi: 10.1038/sj.npp.1300161. [DOI] [PubMed] [Google Scholar]
- Ghiglieri O, Gambarana C, Scheggi S, Tagliamonte A, Willner P, De Montis MG. Palatable food induces an appetitive behaviour in satiated rats which can be inhibited by chronic stress. Behav Pharmacol. 1997;8:619–628. doi: 10.1097/00008877-199711000-00018. [DOI] [PubMed] [Google Scholar]
- Glitsch M, Marty A. Presynaptic effects of NMDA in cerebellar Purkinje cells and interneurons. J Neurosci. 1999;19:511–519. doi: 10.1523/JNEUROSCI.19-02-00511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. Epub 2007 Apr 2002. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223:347–367. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, Winder DG. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006;26:3210–3219. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Mania I, Rainnie DG. Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. J Neurophysiol. 2007;98:638–656. doi: 10.1152/jn.00382.2007. Epub 2007 May 2030. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Involvement of D2 dopamine receptors in the nucleus accumbens in the opiate withdrawal syndrome [see comments] Nature. 1994;371:155–157. doi: 10.1038/371155a0. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Enhanced morphine preference following prolonged abstinence: association with increased Fos expression in the extended amygdala. Neuropsychopharmacology. 2003a;28:292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment. Neuropsychopharmacology. 2003b;28:73–76. doi: 10.1038/sj.npp.1300011. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behav Brain Res. 2007;176:251–258. doi: 10.1016/j.bbr.2006.10.012. Epub 2006 Nov 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Altomare K, Aston-Jones G. Preference for a cocaine-associated environment is attenuated by augmented accumbal serotonin in cocaine withdrawn rats. Psychopharmacology (Berl) 2001;156:14–22. doi: 10.1007/s002130100693. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol Biochem Behav. 2002;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Herzog E, Gilchrist J, Gras C, Muzerelle A, Ravassard P, Giros B, Gaspar P, El Mestikawy S. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123:983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. J Neurophysiol. 2006;96:2134–2138. doi: 10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2006;51:1013–1022. doi: 10.1016/j.neuropharm.2006.06.011. Epub 2006 Aug 1010. [DOI] [PubMed] [Google Scholar]
- Kash TL, Matthews RT, Winder DG. Alcohol Inhibits NR2B-Containing NMDA Receptors in the Ventral Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology. 2007;11:11. doi: 10.1038/sj.npp.1301504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis K, Kiss J, Csaki A, Halasz B. Location of putative glutamatergic neurons projecting to the medial preoptic area of the rat hypothalamus. Brain Res Bull. 2003;61:459–468. doi: 10.1016/s0361-9230(03)00180-1. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F. Extrasynaptic glutamate spillover in the hippocampus: evidence and implications. Trends Neurosci. 1998;21:8–14. doi: 10.1016/s0166-2236(97)01150-8. [DOI] [PubMed] [Google Scholar]
- Le Gal LaSalle G, Paxinos G, Ben-Ari Y. Neurochemical mapping of GABAergic systems in the amygdaloid complex and bed nucleus of the stria terminalis. Brain Res. 1978;155:397–403. doi: 10.1016/0006-8993(78)91037-5. [DOI] [PubMed] [Google Scholar]
- Legault M, Wise RA. Injections of N-methyl-D-aspartate into the ventral hippocampus increase extracellular dopamine in the ventral tegmental area and nucleus accumbens. Synapse. 1999;31:241–249. doi: 10.1002/(SICI)1098-2396(19990315)31:4<241::AID-SYN1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Legault M, Rompre PP, Wise RA. Chemical stimulation of the ventral hippocampus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. J Neurosci. 2000;20:1635–1642. doi: 10.1523/JNEUROSCI.20-04-01635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. Epub 2006 Mar 5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Burke S, Pavlic RJ, Akil H, Watson SJ. Immunohistochemical localization of the cloned kappa 1 receptor in the rat CNS and pituitary. Neuroscience. 1996;71:671–690. doi: 10.1016/0306-4522(95)00464-5. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Rudick CN, Hu XT, White FJ. Excitability of dopamine neurons: modulation and physiological consequences. CNS Neurol Disord Drug Targets. 2006;5:79–97. doi: 10.2174/187152706784111542. [DOI] [PubMed] [Google Scholar]
- Massi L, Elezgarai I, Puente N, Reguero L, Grandes P, Manzoni OJ, Georges F. Cannabinoid receptors in the bed nucleus of the stria terminalis control cortical excitation of midbrain dopamine cells in vivo. J Neurosci. 2008;28:10496–10508. doi: 10.1523/JNEUROSCI.2291-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the bed nucleus of the stria terminalis: a golgi study in the rat. Brain Res Bull. 1983;10:111–120. doi: 10.1016/0361-9230(83)90082-5. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Modulation of ventral tegmental area neural responses to prefrontal stimulation by local orexin application in vivo. Neuroscience Meeting Planner Online:Program No. 916.912.2007. [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- Paquet M, Smith Y. Presynaptic NMDA receptor subunit immunoreactivity in GABAergic terminals in rat brain. J Comp Neurol. 2000;423:330–347. doi: 10.1002/1096-9861(20000724)423:2<330::aid-cne10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Monoamine innervation of bed nucleus of stria terminalis: an electron microscopic investigation. Brain Res Bull. 1992;28:949–965. doi: 10.1016/0361-9230(92)90218-m. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Barr AM. Effects of chronic mild stress on motivation for sucrose: mixed messages. Psychopharmacology (Berl) 1997;134:361–366. doi: 10.1007/s002130050469. discussion 371–367. [DOI] [PubMed] [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Pothos E, Rada P, Mark GP, Hoebel BG. Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Res. 1991;566:348–350. doi: 10.1016/0006-8993(91)91724-f. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG. Neurons of the bed nucleus of the stria terminalis (BNST). Electrophysiological properties and their response to serotonin. Ann N Y Acad Sci. 1999;877:695–699. doi: 10.1111/j.1749-6632.1999.tb09304.x. [DOI] [PubMed] [Google Scholar]
- Riedel A, Westerholz S, Braun K, Edwards RH, Arendt T, Hartig W. Vesicular glutamate transporter 3-immunoreactive pericellular baskets ensheath a distinct population of neurons in the lateral septum. J Chem Neuroanat. 2008;36:177–190. doi: 10.1016/j.jchemneu.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 2007;87:688–692. doi: 10.1016/j.nlm.2007.01.003. Epub 2007 Mar 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. Epub 2007 Apr 2002. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Almeida OF, Shippenberg TS. Evidence that nor-binaltorphimine can function as an antagonist at multiple opioid receptor subtypes. Eur J Pharmacol. 1994;264:157–162. doi: 10.1016/0014-2999(94)00449-8. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. J Comp Neurol. 2005;481:363–376. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- Suaud-Chagny MF, Chergui K, Chouvet G, Gonon F. Relationship between dopamine release in the rat nucleus accumbens and the discharge activity of dopaminergic neurons during local in vivo application of amino acids in the ventral tegmental area. Neuroscience. 1992;49:63–72. doi: 10.1016/0306-4522(92)90076-e. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Tabuchi ET, Mulder AB, Wiener SI. Position and behavioral modulation of synchronization of hippocampal and accumbens neuronal discharges in freely moving rats. Hippocampus. 2000;10:717–728. doi: 10.1002/1098-1063(2000)10:6<717::AID-HIPO1009>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Taepavarapruk P, Floresco SB, Phillips AG. Hyperlocomotion and increased dopamine efflux in the rat nucleus accumbens evoked by electrical stimulation of the ventral subiculum: role of ionotropic glutamate and dopamine D1 receptors. Psychopharmacology (Berl) 2000;151:242–251. doi: 10.1007/s002130000376. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Stimulation of the prefrontal cortex in the rat induces patterns of activity in midbrain dopaminergic neurons which resemble natural burst events. Synapse. 1996a;22:195–208. doi: 10.1002/(SICI)1098-2396(199603)22:3<195::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Antagonism of NMDA receptors but not AMPA/kainate receptors blocks bursting in dopaminergic neurons induced by electrical stimulation of the prefrontal cortex. J Neural Transm. 1996b;103:889–904. doi: 10.1007/BF01291780. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. Eur J Neurosci. 1999;11:4099–4109. doi: 10.1046/j.1460-9568.1999.00834.x. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. Epub 2006 Aug 2002. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson MF, Pittman QJ. Changes in arterial blood pressure alter activity of electrophysiologically identified single units of the bed nucleus of the stria terminalis. Neuroscience. 1995;64:835–844. doi: 10.1016/0306-4522(94)00438-b. [DOI] [PubMed] [Google Scholar]
- Winder DG, Egli RE, Schramm NL, Matthews RT. Synaptic plasticity in drug reward circuitry. Curr Mol Med. 2002;2:667–676. doi: 10.2174/1566524023361961. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM, Bradford HF. N-methyl-D-aspartate releases gamma-aminobutyric acid from rat striatum in vivo: a microdialysis study using a novel preloading method. J Neurochem. 1993;60:487–492. doi: 10.1111/j.1471-4159.1993.tb03176.x. [DOI] [PubMed] [Google Scholar]


