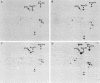
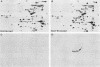
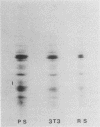
Abstract
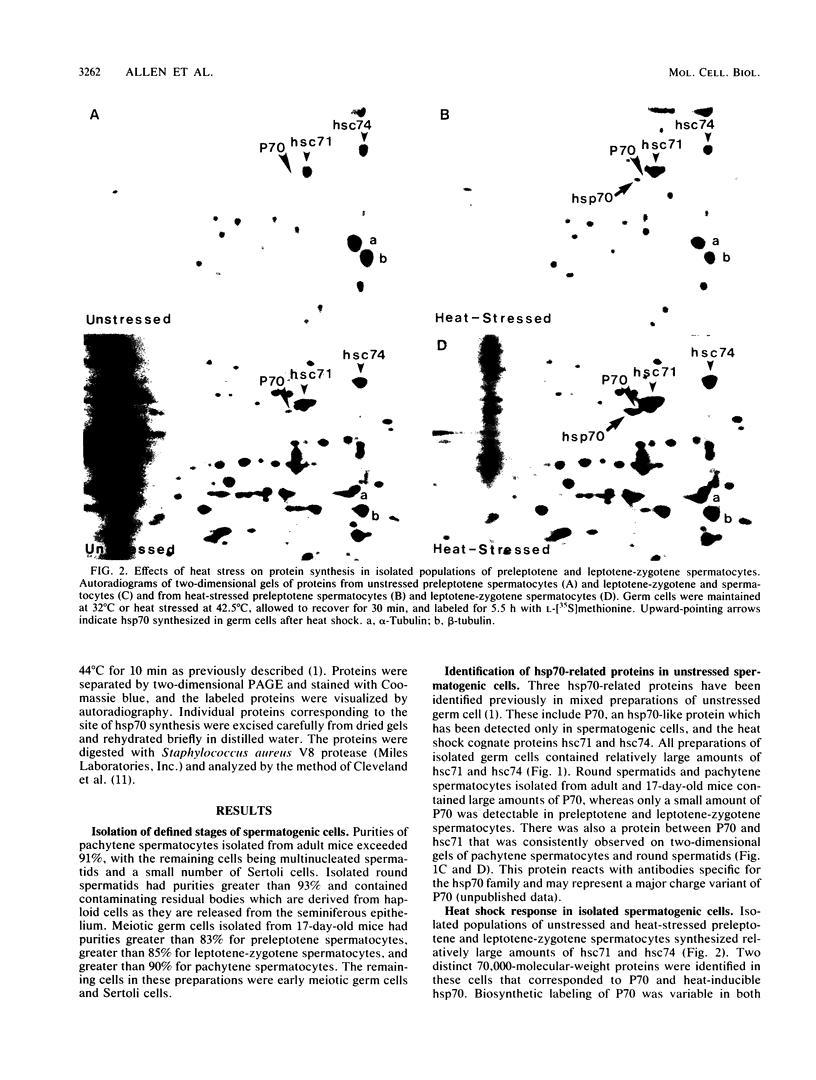
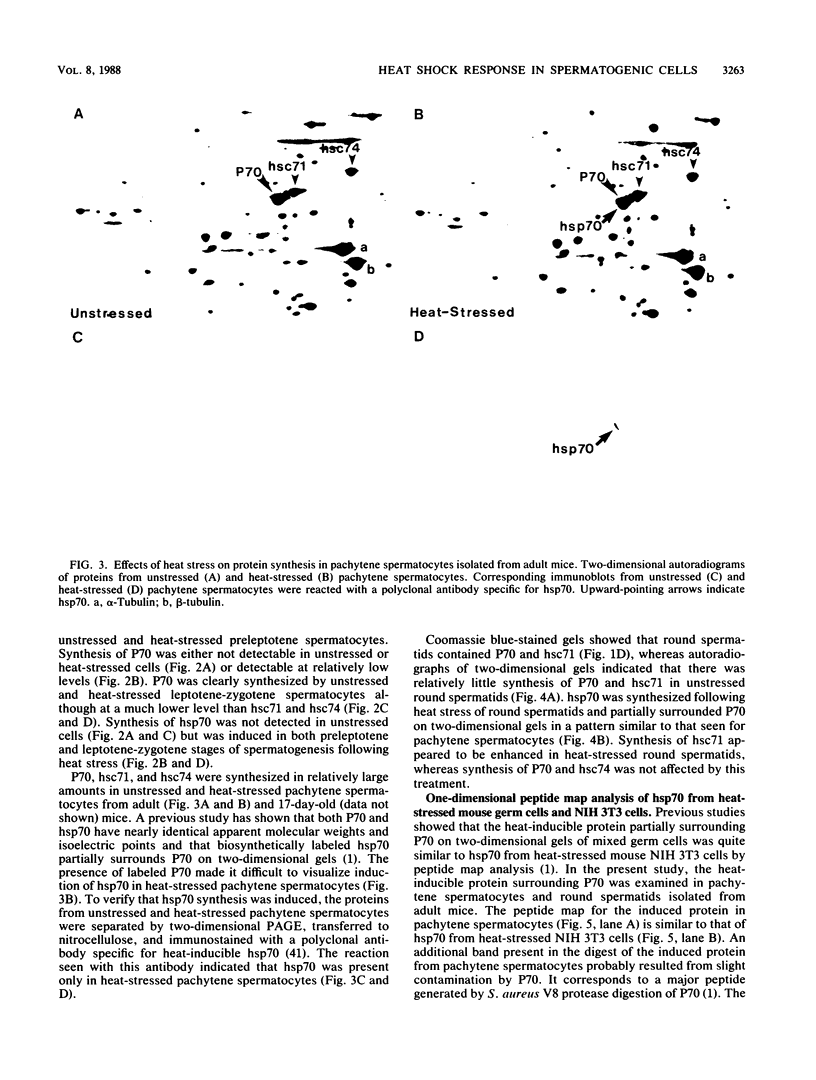
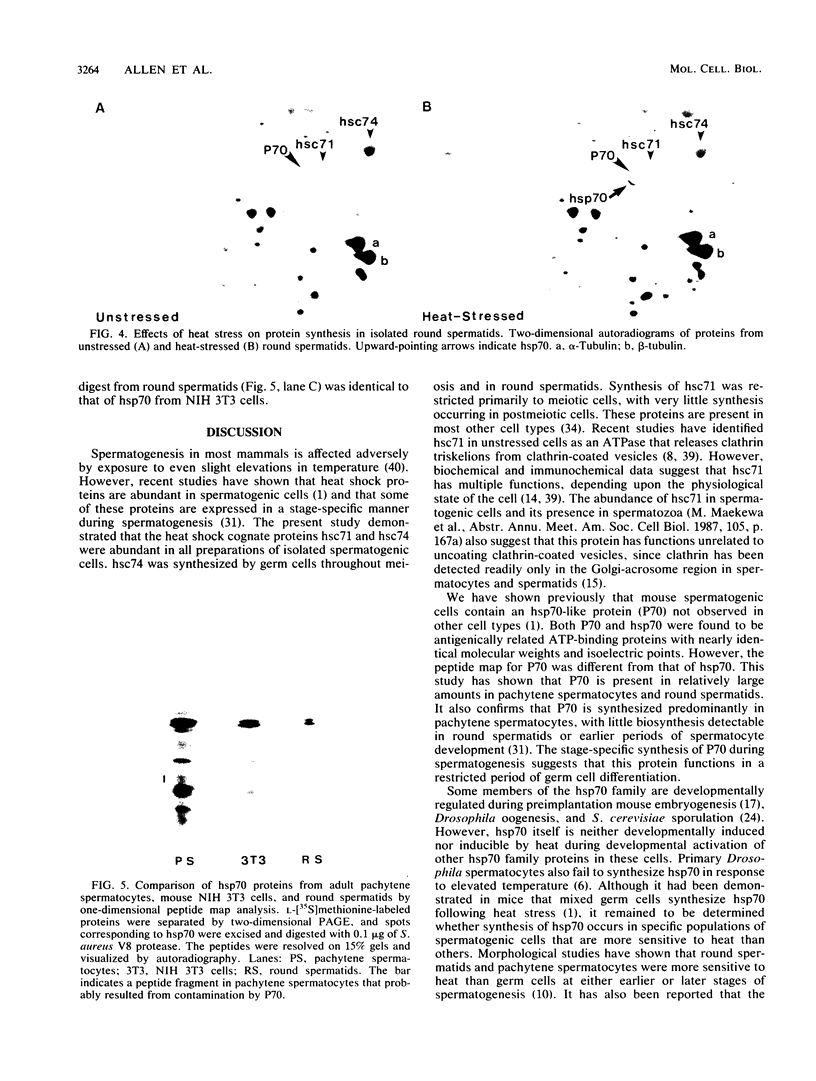
Proteins of the hsp70 family are abundant in mouse spermatogenic cells. These cells also synthesize relatively large amounts of a 70,000-molecular-weight protein (P70) that appears to be a cell-specific isoform of hsp70, the major heat-inducible protein (R.L. Allen, D.A. O'Brien, and E.M. Eddy, Mol. Cell. Biol. 8:828-832, 1988). In this study, proteins of unstressed and heat-stressed spermatogenic cells consisting of purified preparations of preleptotene, leptotene-zygotene, pachytene spermatocytes, and round spermatids were analyzed by two-dimensional polyacrylamide gel electrophoresis. Unstressed preleptotene and leptotene-zygotene spermatocytes contained little P70, whereas relatively large amounts of P70 were present in pachytene spermatocytes and round spermatids. Labeling studies showed that P70 was synthesized primarily in pachytene spermatocytes and that little synthesis occurred in round spermatids or in preleptotene and leptotene-zygotene stages of spermatogenesis. Synthesis of hsp70 was not detectable in unstressed cells but was induced in all stages of isolated germ cells following heat stress. These results indicate that P70 is expressed in a stage-specific manner during cell differentiation, whereas hsp70 is synthesized in response to stress in all populations of isolated spermatogenic cells examined.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. L., O'Brien D. A., Eddy E. M. A novel hsp70-like protein (P70) is present in mouse spermatogenic cells. Mol Cell Biol. 1988 Feb;8(2):828–832. doi: 10.1128/mcb.8.2.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellvé A. R., Millette C. F., Bhatnagar Y. M., O'Brien D. A. Dissociation of the mouse testis and characterization of isolated spermatogenic cells. J Histochem Cytochem. 1977 Jul;25(7):480–494. doi: 10.1177/25.7.893996. [DOI] [PubMed] [Google Scholar]
- Bensaude O., Babinet C., Morange M., Jacob F. Heat shock proteins, first major products of zygotic gene activity in mouse embryo. Nature. 1983 Sep 22;305(5932):331–333. doi: 10.1038/305331a0. [DOI] [PubMed] [Google Scholar]
- Bensaude O., Morange M. Spontaneous high expression of heat-shock proteins in mouse embryonal carcinoma cells and ectoderm from day 8 mouse embryo. EMBO J. 1983;2(2):173–177. doi: 10.1002/j.1460-2075.1983.tb01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M. Developmental control of the heat shock response in Xenopus. Proc Natl Acad Sci U S A. 1984 May;81(10):3138–3142. doi: 10.1073/pnas.81.10.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. J., Parks C., Parker-Thornburg J., Mortin M. A., Pelham H. R. The use of promoter fusions in Drosophila genetics: isolation of mutations affecting the heat shock response. Cell. 1984 Jul;37(3):979–991. doi: 10.1016/0092-8674(84)90432-x. [DOI] [PubMed] [Google Scholar]
- Burdon R. H. Heat shock and the heat shock proteins. Biochem J. 1986 Dec 1;240(2):313–324. doi: 10.1042/bj2400313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOWDHURY A. K., STEINBERGER E. A QUANTITATIVE STUDY OF THE EFFECT OF HEAT ON GERMINAL EPITHELIUM OF RAT TESTES. Am J Anat. 1964 Nov;115:509–524. doi: 10.1002/aja.1001150307. [DOI] [PubMed] [Google Scholar]
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986 Apr 11;45(1):3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- Chowdhury A. K., Steinberger E. Early changes in the germinal epithelium of rat testes following exposure to heat. J Reprod Fertil. 1970 Jul;22(2):205–212. doi: 10.1530/jrf.0.0220205. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Craig E. A., Ingolia T. D., Manseau L. J. Expression of Drosophila heat-shock cognate genes during heat shock and development. Dev Biol. 1983 Oct;99(2):418–426. doi: 10.1016/0012-1606(83)90291-9. [DOI] [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Giebel L. B., Dworniczak B. P., Bautz E. K. Developmental regulation of a constitutively expressed mouse mRNA encoding a 72-kDa heat shock-like protein. Dev Biol. 1988 Jan;125(1):200–207. doi: 10.1016/0012-1606(88)90073-5. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Warren G., Stuhlfauth I., Jockusch B. M. The role of clathrin-coated vesicles in acrosome formation. Eur J Cell Biol. 1981 Dec;26(1):52–60. [PubMed] [Google Scholar]
- Guidon P. T., Jr, Hightower L. E. Purification and initial characterization of the 71-kilodalton rat heat-shock protein and its cognate as fatty acid binding proteins. Biochemistry. 1986 Jun 3;25(11):3231–3239. doi: 10.1021/bi00359a023. [DOI] [PubMed] [Google Scholar]
- Hahnel A. C., Gifford D. J., Heikkila J. J., Schultz G. A. Expression of the major heat shock protein (hsp 70) family during early mouse embryo development. Teratog Carcinog Mutagen. 1986;6(6):493–510. doi: 10.1002/tcm.1770060603. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Drosophila gene related to the major heat shock-induced gene is transcribed at normal temperatures and not induced by heat shock. Proc Natl Acad Sci U S A. 1982 Jan;79(2):525–529. doi: 10.1073/pnas.79.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothary R., Perry M. D., Moran L. A., Rossant J. Cell-lineage-specific expression of the mouse hsp68 gene during embryogenesis. Dev Biol. 1987 Jun;121(2):342–348. doi: 10.1016/0012-1606(87)90170-9. [DOI] [PubMed] [Google Scholar]
- Krawczyk Z., Szymik N., Wiśniewski J. Expression of hsp70-related gene in developing and degenerating rat testis. Mol Biol Rep. 1987;12(1):35–41. doi: 10.1007/BF00580648. [DOI] [PubMed] [Google Scholar]
- Krawczyk Z., Wiśniewski J., Biesiada E. A hsp70-related gene is constitutively highly expressed in testis of rat and mouse. Mol Biol Rep. 1987;12(1):27–34. doi: 10.1007/BF00580647. [DOI] [PubMed] [Google Scholar]
- Kurtz S., Rossi J., Petko L., Lindquist S. An ancient developmental induction: heat-shock proteins induced in sporulation and oogenesis. Science. 1986 Mar 7;231(4742):1154–1157. doi: 10.1126/science.3511530. [DOI] [PubMed] [Google Scholar]
- Lewis M. J., Pelham H. R. Involvement of ATP in the nuclear and nucleolar functions of the 70 kd heat shock protein. EMBO J. 1985 Dec 1;4(12):3137–3143. doi: 10.1002/j.1460-2075.1985.tb04056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. C., Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982 May;79(10):3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lowe D. G., Moran L. A. Proteins related to the mouse L-cell major heat shock protein are synthesized in the absence of heat shock gene expression. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2317–2321. doi: 10.1073/pnas.81.8.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Hall P. F. The influence of temperature upon polysomes of spermatids of rat testes. Biochem Biophys Res Commun. 1978 Nov 29;85(2):756–761. doi: 10.1016/0006-291x(78)91225-1. [DOI] [PubMed] [Google Scholar]
- O'Brien D. A. Stage-specific protein synthesis by isolated spermatogenic cells throughout meiosis and early spermiogenesis in the mouse. Biol Reprod. 1987 Aug;37(1):147–157. doi: 10.1095/biolreprod37.1.147. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Malley K., Mauron A., Barchas J. D., Kedes L. Constitutively expressed rat mRNA encoding a 70-kilodalton heat-shock-like protein. Mol Cell Biol. 1985 Dec;5(12):3476–3483. doi: 10.1128/mcb.5.12.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Petersen N. S., Mitchell H. K. Recovery of protein synthesis after heat shock: prior heat treatment affects the ability of cells to translate mRNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1708–1711. doi: 10.1073/pnas.78.3.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romrell L. J., Bellvé A. R., Fawcett D. W. Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev Biol. 1976 Mar;49(1):119–131. doi: 10.1016/0012-1606(76)90262-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E. The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J. 1985 Dec 16;4(13A):3385–3391. doi: 10.1002/j.1460-2075.1985.tb04094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984 Apr 10;259(7):4501–4513. [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Suhan J. P. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol. 1986 Nov;103(5):2035–2052. doi: 10.1083/jcb.103.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakeri Z. F., Wolgemuth D. J. Developmental-stage-specific expression of the hsp70 gene family during differentiation of the mammalian male germ line. Mol Cell Biol. 1987 May;7(5):1791–1796. doi: 10.1128/mcb.7.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]