Abstract
Background
GPs need to recognise significant pigmented skin lesions, given rising UK incidence rates for malignant melanoma. The 7-point checklist (7PCL) has been recommended by NICE (2005) for routine use in UK general practice to identify clinically significant lesions which require urgent referral.
Aim
To validate the Original and Weighted versions of the 7PCL in the primary care setting.
Design and setting
Diagnostic validation study, using data from a SIAscopic diagnostic aid randomised controlled trial in eastern England.
Method
Adults presenting in general practice with a pigmented skin lesion that could not be immediately diagnosed as benign were recruited into the trial. Reference standard diagnoses were histology or dermatology expert opinion; 7PCL scores were calculated blinded to the reference diagnosis. A case was defined as a clinically significant lesion for primary care referral to secondary care (total 1436 lesions: 225 cases, 1211 controls); or melanoma (36).
Results
For diagnosing clinically significant lesions there was a difference between the performance of the Original and Weighted 7PCLs (respectively, area under curve: 0.66, 0.69, difference = 0.03, P<0.001). For the identification of melanoma, similar differences were found. Increasing the Weighted 7PCL’s cut-off score from recommended 3 to 4 improved detection of clinically significant lesions in primary care: sensitivity 73.3%, specificity 57.1%, positive predictive value 24.1%, negative predictive value 92.0%, while maintaining high sensitivity of 91.7% and moderate specificity of 53.4% for melanoma.
Conclusion
The Original and Weighted 7PCLs both performed well in a primary care setting to identify clinically significant lesions as well as melanoma. The Weighted 7PCL, with a revised cut-off score of 4 from 3, performs slightly better and could be applied in general practice to support the recognition of clinically significant lesions and therefore the early identification of melanoma.
Keywords: diagnostic techniques and procedures, general practice, melanoma, pigmented skin lesions
INTRODUCTION
The thickness of a melanoma at diagnosis is a key determinant of patient outcome, therefore early detection and timely referral are crucial steps in the appropriate management of this skin malignancy.1 Melanoma is an important cause of mortality in the UK where there were 11 870 new cases and 2203 deaths in 2010.2 Distinguishing melanoma from other pigmented skin lesions in general practice can be challenging.3 Patients frequently present to their GPs with concerns about pigmented skin lesions (melanocytic naevus, ‘mole’) but few will be diagnosed as melanoma: even among higher risk groups such as males aged >60 years, less than 1 in 33 000 pigmented skin lesions are estimated to become malignant.4 A pigmented skin lesion is extremely common, with most people having between five and 20 which may vary in size, shape and colour, and an increase in number is associated with age, fair skin, and sunlight exposure. The majority of melanomas arise from pre-existing lesions, whereas some melanomas arise spontaneously; patients and their GPs need to be able to distinguish these ‘normal’ changes from ‘abnormal’ changes that may indicate melanoma.
Diagnostic aids are increasingly used in primary care for a variety of conditions.5 The 7-point checklist (7PCL) was developed in Glasgow in the 1980s as guidance to help non-dermatologists (GPs and patients) detect features indicating possible melanoma, with the advice that each feature should score 1 and lesions with scores of ≥3 should be referred for specialist opinion (Original 7PCL).6 The checklist was adopted and widely disseminated by the Cancer Research Campaign to raise public awareness, but there were concerns that it under-identified early and nodular melanomas and over-identified certain benign lesions such as seborrhoeic keratoses.
The 7PCL was revised in 1989 to identify three major signs (change in size, shape and/or colour) and four minor signs (inflammation, crusting/bleeding, sensory change, diameter ≥7 mm) for suspected malignant melanoma; the scoring was weighted (2 for major, 1 for minor signs), and again, any lesion scoring ≥3 warranting referral,7,8 (Box 1 and Figure 1).
Box 1. The 7-point checklist (7PCL): Original and Weighted6,7
|
Original 7PCL (Score of ≥3 suggests referral) |
Weighted 7PCL (Score of ≥3 suggests referral) |
|---|---|
All features (equal weighting)
|
Major features (2 points)
Minor features (1 point)
|
Figure 1.
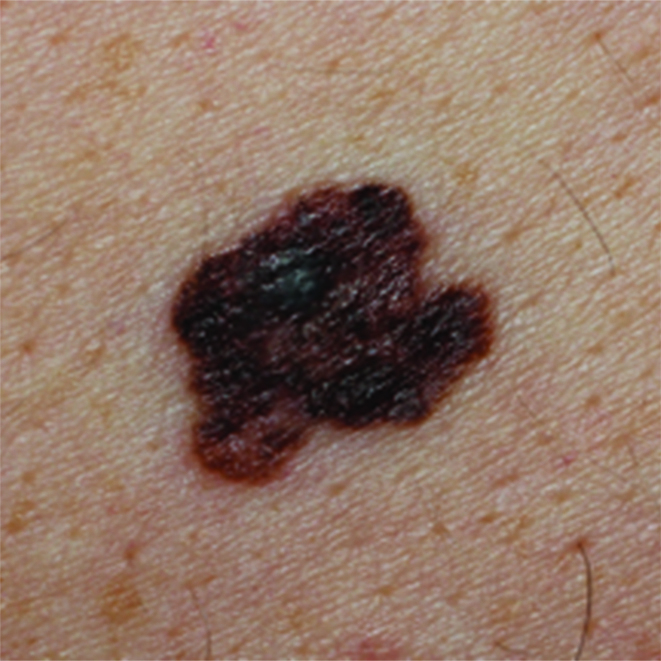
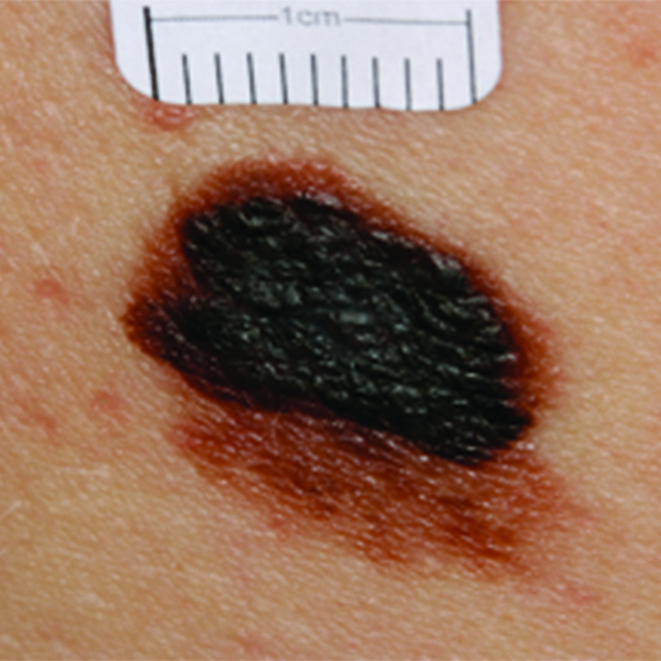
Images of early malignant melanomas to show major features: irregular pigmentation and irregular borders. Left = Early melanoma (0.8mm). Right = Early melanoma (1.0mm)
This Weighted 7PCL was recommended for use by all primary care professionals in the assessment of pigmented skin lesions by the 2005 English National Institute for Health and Clinical Excellence (NICE) guidelines on referral for suspected cancer: all lesions scoring ≥3, suggestive of possible melanoma, should be referred urgently.9 Other diagnostic aids have been developed to assist visual inspection of a pigmented skin lesion, such as the ABCD(E) rule,10,11 commonly used in North America and Australia. However, both the Original and Weighted 7PCLs and ABCD(E) rules were developed and validated using retrospective studies of clinically obvious melanomas or clinically equivocal melanocytic naevi that had been excised. There are no reports of formal validation of the 7PCL in general practice, although an Australian interview study established that the 7PCL’s major criteria were perceived to be more useful than the ABCD(E) rule in differentiating between melanomas and benign pigmented lesions in the hands of patients.12
How this fits in
Distinguishing malignant from benign pigmented skin lesions is challenging in general practice. The 2005 NICE guidelines for referral of suspected cancer recommended use of the Weighted 7-point checklist (7PCL) for assessment of pigmented skin lesions. This is the first study to validate the Original and Weighted 7PCL in primary care, in the context of a SIAscopic diagnostic aid trial. Both checklists perform moderately well in the identification of clinically significant lesions and melanoma. Diagnostic accuracy can be improved using the Weighted 7PCL with the cut-off score revised of 4 from 3. This would result in a diagnostic aid that maintains a very high sensitivity for melanoma while improving the positive predictive value and specificity for clinically significant lesions in primary care, in order not to significantly increase referrals to secondary care.
This study reports the first validation of the 7PCL, according to STAndards for the Reporting of Diagnostic accuracy studies (STARD) criteria,13 from data collected prospectively as part of a randomised controlled trial (RCT) of adding a SIAscopic diagnostic aid to best practice.14 The diagnostic performance of the individual items was examined and the diagnostic accuracy of the different scoring checklists was compared for ‘clinically significant’ (that is, warranting urgent referral from general practice for specialist opinion), and melanoma.
METHOD
The RCT has already been reported.14 Briefly, this study was Registered as an International Standard Randomised Controlled Trial (ISRCTN 79932379), approved by Cambridgeshire 2 Local Research Ethics Committee (reference 07/H0308/167) and set in 15 general practices in eastern England between March 2008 and May 2010. Potential participants were consecutive patients, aged ≥18 years, presenting in general practice, who had a suspicious pigmented lesion, defined as one which could not be immediately diagnosed as benign and the patient reassured. They were referred within the general practice to attend an appointment with the trained lead clinician (GP) within 1 week. The lead clinician randomised participants to either the Best Practice (comparison) group or the MoleMate (intervention) group.
In both groups the lesions were assessed using clinical history, naked eye examination, and completion of the Original 7PCL items. In the MoleMate group lesions were also assessed using the MoleMate system. The trial’s primary outcome was the appropriateness of the primary care decision to refer, defined as the proportion of referred lesions that secondary care experts decided to biopsy or monitor. This was considered a clinically significant lesion and a measure of the diagnostic accuracy of the GP.
Reference standard
A reference standard final diagnosis of clinically significant or ‘clinically benign’ was recorded for all lesions in the trial. Two researchers applied the 7PCL score after completion of the main trial analysis, blinded to the reference diagnosis.
For referred lesions the reference standard was defined by histology or dermatology expert opinion, performed within 2 weeks of collection of the 7PCL data (the RCT’s primary outcome had been the appropriateness of referral, defined as the proportion of referred lesions that secondary care experts decided to take a biopsy from or monitor14). Histology reports for all excised lesions were conducted by the Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust’s histology service. All malignant, premalignant and dysplastic lesions were reviewed by a second pathologist with expertise in dermato-histopathology. Within the clinically significant group, the subgroup with ‘melanoma’ as the reference standard included malignant and premalignant lesions (nodular melanoma, superficial spreading melanoma, lentigo maligna melanoma, melanoma in situ/lentigo maligna). Three consultant dermatologists made the expert diagnoses for all other referred lesions, and were blind to the 7PCL.
For non-referred lesions, the reference standard was defined by two other dermatology experts by review of the clinical history, a digital photograph, and MoleMate image (including dermoscopy image) where available; these data were collected at the same time as the 7PCL data. The few non-referred lesions considered equivocal or possibly clinically significant were recalled for review by the lead clinician.
Analysis
Clinically significant lesions were compared with clinically benign in terms of demographic characteristics using unpaired t-tests and Pearson χ2 tests. The association between each of the seven items with the reference standards were tested using Pearson χ2 tests (for the clinical standard) and Fisher’s Exact test (for the melanoma standard), summarised with estimates of sensitivity and specificity, positive predictive value (PPV), and negative predictive value (NPV). The exact binomial method was used for the calculation of 95% confidence intervals for single proportions. Standard methods of analysis for independent observations were used as the checklist was applied independently for each lesion, and multiple lesions, though present, were rare (mean number of 1.2 lesions per patients), with outcomes from the same patient contributing to both categories of the reference diagnosis.15 The performance of the versions of the checklist was assessed by testing the difference in area under the receiver operating characteristic (ROC) curves using the paired method for ordinal data of Hanley and McNeil.16 McNemar’s test for paired proportions was used to compare two estimates of sensitivity, or specificity, such as arising from a single item compared with a cut-point from a checklist score. Two estimates of PPV were compared using a weighted least squares method.17 All tests were two-sided and assessed at the 5% level of significance, using SPSS software (version 17).
RESULTS
Study sample
There were 1580 lesions on 1297 participants from 15 general practices (range 45–151 patients per practice) recruited, and four participants (with a total of seven lesions) withdrew after randomisation. Of these 1573 lesions, 42 (2.7%) did not have a reference standard assessment, and the 7PCL was not fully completed for a further 95 (6%) lesions. The analyses are therefore based on 1436 lesions from 1182 patients, representing 91% of the trial. The study population’s mean (SD) age was 44.7 (16.6) years, with 35.9% (n = 424) male, and 94.2% (n = 1118) white British. Of the 1436 lesions, 363 (25.3%) lesions were referred of which 225 were clinically significant lesions (biopsy, monitor), and the remaining 1211 were clinically benign. Therefore the prevalence of clinically significant lesions was 15.7% (95% confidence interval (CI) = 13.8% to 17.6%).
There were 36 histologically confirmed melanomas. More than one-half were less than 1 mm thick with a good prognosis, and none were subsequently found to have metastases or lymph node involvement (nodular melanoma = 2; superficial spreading melanoma = 24; lentigo maligna melanoma = 4; melanoma in situ/lentigo maligna = 6). There were six non-melanoma skin cancers detected (basal cell carcinoma = 5; squamous cell carcinoma = 1), and these were not included in the ‘melanoma’ reference standard.
People with clinically significant lesions were older than people with clinically benign lesions (mean age 46.3 years versus 43.4 years, P = 0.02) and more likely to be male (42.2% versus 32.9%, P = 0.007).
Performance of individual 7PCL items
Table 1 shows the association of each 7PCL item individually. For the detection of clinically significant lesions, items 2 (irregular pigmentation) and 3 (irregular border) were the only items to show a strongly significant association with the reference standards (P<0.001), together with reasonable sensitivity and specificity. For the detection of melanoma, items 1 (change in size of lesion) and 6 (lesion larger than others) were also quite strongly associated with detecting melanoma (items 2 and 3, P<0.001; item 1, P = 0.004; item 6, P = 0.001).
Table 1.
Prevalence and performance of individual 7PCL items in detecting (A) clinically significant lesions and (B) melanoma
| 7PCL Item | Item Prevalence | Clinically significant | Melanoma | ||||
|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Test of associationa | Sensitivity | Specificity | Test of associationb | ||
| 1. Change in size of lesion | 48.3% (693/1436) | 55.1% (124/225) | 53.0% (642/1211) | 0.030 | 72.2% (26/36) | 52.4% (733/1400) | 0.004 |
| 2. Irregular pigmentation | 48.9% (702/1436) | 71.1% (160/225) | 55.2% (669/1211) | <0.001 | 86.1% (31/36) | 52.1% (729/1400) | <0.001 |
| 3. Irregular border | 34.3% (492/1436) | 63.6% (143/225) | 71.2% (862/1211) | <0.001 | 69.4% (25/36) | 66.6% (933/1400) | <0.001 |
| 4. Inflammation | 9.2% (132/1436) | 11.6% (26/225) | 91.2% (1105/1211) | 0.180 | 16.7% (6/36) | 91.0% (1274/1400) | 0.150 |
| 5. Itch or altered sensation | 27.6% (397/1436) | 26.7% (60/225) | 72.2% (874/1211) | 0.720 | 25.0% (9/36) | 72.3% (1012/1400) | 0.850 |
| 6. Lesion larger than others | 48.4% (695/1436) | 56.4% (127/225) | 53.1% (643/1211) | 0.010 | 75.0% (27/36) | 52.3% (732/1400) | 0.001 |
| 7. Oozing/crusting of lesion | 10.0% (144/1436) | 11.6% (26/225) | 90.3% (1093/1211) | 0.410 | 19.4% (7/36) | 90.2% (1263/1400) | 0.080 |
P-values are from the χ2 test of association between item and reference standard.
P-values are from Fisher’s exact test of association between item and reference standard
Performance of the Original 7PCL
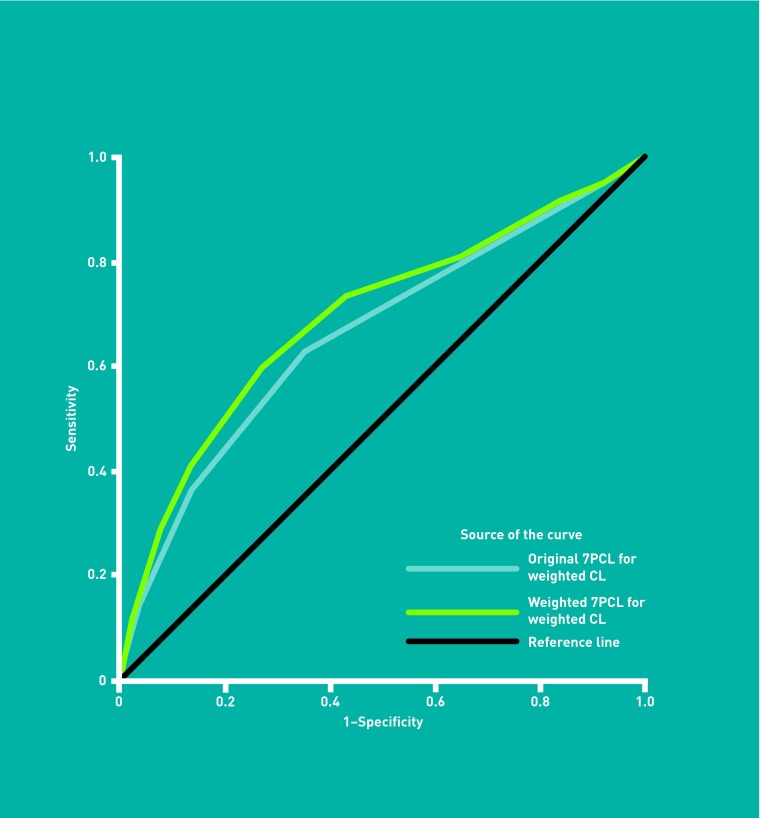
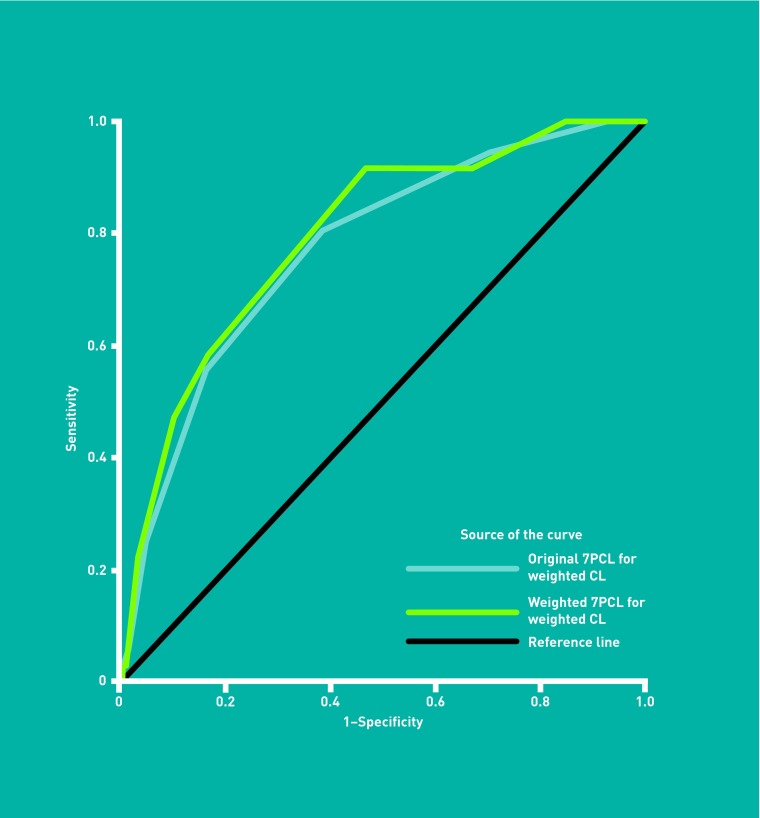
Table 2 shows the performance of discrete cut-points where each item is equally weighted. For clinically significant lesions, a score of ≥3 has sensitivity of 62.7% (56.0% to 69.0%) and specificity of 65.0% (62.2% to 67.7%). For the detection of melanoma, a score of ≥3 has higher sensitivity (80.6%, 64.0% to 91.8%) and similar specificity (61.7%, 59.1% to 64.3%). Figures 2 and 3 show the associated ROC curve for the Original 7PCL detecting clinically significant lesions (AUC = 0.66, 0.62 to 0.70), and melanoma (AUC = 0.77, 0.70 to 0.85).
Table 2.
Original 7PCL with performance for each cut-point in detecting clinically significant lesions and melanoma. (Score of ≥3 used as cut-point in Original 7PCL)
| Score | Clinically significant | Melanoma | ||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Test of associationa | Sensitivity | Specificity | Test of associationb | |
| ≥1 | 95.1% (214/225) | 7.5% (91/1211) | 0.160 | 100% (36/36) | 7.3% (102/1400) | 0.110 |
| ≥2 | 81.8% (184/225) | 31.3% (379/1211) | <0.001 | 94.4% (34/36) | 29.9% (418/1400) | <0.001 |
| ≥3 | 62.7% (141/225) | 65.0% (787/1211) | <0.001 | 80.6% (29/36) | 61.7% (864/1400) | <0.001 |
| ≥4 | 36.4% (82/225) | 86.5% (1048/1211) | <0.001 | 55.6% (20/36) | 83.9% (1175/1400) | <0.001 |
| ≥5 | 14.2% (32/225) | 96.6% (1170/1211) | <0.001 | 25.0% (9/36) | 95.4% (1336/1400) | <0.001 |
| ≥6 | 4.9% (11/225) | 99.3% (1203/1211) | <0.001 | 5.6% (2/36) | 98.8% (1383/1400) | 0.080 |
P-values are from the χ2 test of association between item and reference standard.
P-values are from Fisher’s exact test of association between item and reference standard
Figure 2.
ROC curve for the performance of Original and Weighted 7PCL with the clinically significant standard.
Figure 3.
ROC curve for the performance of Original and Weighted 7PCL with the melanoma standard.
Performance of the Weighted 7PCL
Table 3 and Figures 2 and 3 show the performance of discrete cut-points and the associated ROC curve for the Weighted 7PCL. It has a significantly better overall performance than the Original 7PCL for clinically significant lesions (AUC = 0.69, 0.65 to 0.73; difference in area = 0.03, 0.01 to 0.05, P<0.001) but not for malignancy (AUC = 0.80, 0.73 to 0.87; difference in area = 0.02, −0.01 to 0.06, P = 0.21). As specified for the Weighted 7PCL, a score of ≥3 has high sensitivity of 80.9% (75.1% to 85.8%) but low specificity of 35.0% (32.3% to 37.8%) for clinically significant lesions, with a higher sensitivity (91.7%, 77.5% to 98.3%) and lower specificity (33.1%, 30.7% to 35.7%) for melanoma.
Table 3.
Weighted 7PCL with performance for each cut-point in detecting clinically significant lesions and melanoma. (Score of ≥3 used as cut-point in Weighted 7PCL)
| Score | Clinically significant | Melanoma | ||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Test of associationa | Sensitivity | Specificity | Test of associationb | |
| ≥1 | 95.1% (214/225) | 7.5% (91/1211) | 0.160 | 100.0% (36/36) | 7.3% (102/1400) | 0.110 |
| ≥2 | 91.6% (206/225) | 16.2% (196/1211) | 0.003 | 100.0% (36/36) | 15.3% (215/1400) | 0.004 |
| ≥3 | 80.9% (182/225) | 35.0% (424/1211) | <0.001 | 91.7% (33/36) | 33.1% (464/1400) | 0.001 |
| ≥4 | 73.3% (165/225) | 57.1% (691/1211) | <0.001 | 91.7% (33/36) | 53.4% (748/1400) | <0.001 |
| ≥5 | 59.6% (134/225) | 73.2% (886/1211) | <0.001 | 75.0% (27/36) | 69.1% (968/1400) | <0.001 |
| ≥6 | 40.9% (92/225) | 86.8% (1051/1211) | <0.001 | 58.3% (21/36) | 83.5% (1169/1400) | <0.001 |
| ≥7 | 28.9% (65/225) | 92.5% (1120/1211) | <0.001 | 47.2% (17/36) | 90.1% (1261/1400) | <0.001 |
| ≥8 | 11.6% (26/225) | 97.9% (1186/1211) | <0.001 | 22.2% (8/36) | 96.9% (1357/1400) | <0.001 |
| ≥9 | 3.1% (7/225) | 99.6% (1206/1211) | <0.001 | 2.8% (1/36) | 99.2% (1389/1400) | 0.260 |
P-values are from the χ2 test of association between item and reference standard.
P-values are from Fisher’s exact test of association between item and reference standard
Performance of the single item ‘irregular border’
Item 3 alone (irregular border) has significantly higher specificity than the Original 7PCL for comparable sensitivity to detect both clinically significant lesions (item 3 versus Original 7PCL: sensitivity 63.6% versus 62.7%, P = 0.90; specificity 71.2% versus 65.0%, P<0.001) and melanoma (item 3 versus Original 7PCL: sensitivity 69.4% versus 80.6%, P = 0.34; specificity 66.6% versus 61.7%, P = 0.001).
Comparison of the performances of both checklists and the single item ‘irregular border’
Table 4 presents the performance of both checklists (Original and Weighted 7PCL) and the single item ‘irregular border’ at a number of cut-off scores including the recommended cut-off score of ≥3. Using the Weighted 7PCL with a cut-off score of ≥4 would perform better than current recommendations in terms of maintaining specificity while achieving a higher sensitivity for clinically significant lesions (73.3%) and a very high sensitivity for melanoma (91.7%). The PPV for clinically significant lesions with a cut-off of ≥4 was 24.1% compared to 18.8% for a cut-off score of ≥3, and the NPVs were similar (92.0% and 90.8% respectively). The single item ‘irregular border’ alone performed better than the Weighted 7PCL in terms of its PPV to detect clinically significant lesions (PPV 29.1% versus 24.1% P<0.001) for comparable NPVs (91.3% and 92.0% respectively); however, it had significantly lower sensitivity to detect melanoma (69.4% versus 91.7%, P = 0.008).
Table 4.
Comparison of performance of both checklists and single item ‘irregular border’ in detecting clinically significant lesions and melanoma
| Clinically significant | Melanoma | |||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| Original 7PCL score (0–7) | ||||
| ≥2 | 81.8% (184/225) | 31.3% (379/1211) | 94.4% (34/36) | 29.9% (418/1400) |
| ≥3 | 62.7% (141/225) | 65.0% (787/1211) | 80.6% (29/36) | 61.7% (864/1400) |
| ≥4 | 36.4% (82/225) | 86.5% (1048/1211) | 55.6% (20/36) | 83.9% (1175/1400) |
| Weighted 7PCL score (0–10) | ||||
| ≥3 | 80.9% (182/225) | 35.0% (424/1211) | 91.7% (33/36) | 33.1% (464/1400) |
| ≥4 | 73.3% (165/225) | 57.1% (691/1211) | 91.7% (33/36) | 53.4% (748/1400) |
| ≥ | 59.6% (134/225) | 73.2% (886/1211) | 75.0% (27/36) | 69.1% (968/1400) |
| Single item | ||||
| ‘Irregular border’ present | 63.6% (143/225) | 71.2% (862/1211) | 69.4% (25/36) | 66.6% (933/1400) |
DISCUSSION
Summary
This is the first validation study of the 7PCL conducted in primary care, showing that the Original and Weighted versions of the 7PCL perform moderately well for the identification of clinically significant lesions in primary care and have high sensitivity to detect melanoma. A single item, irregular border, performs surprisingly well on its own. While recognising that these findings ideally require replication, a revision to the Weighted 7PCL is suggested: increasing the cut-off score to 4 from 3 would result in a diagnostic aid with higher specificity for both clinically significant lesions and melanoma, without a reduction in the very high sensitivity for melanoma.
The RCT showed that adding the SIAscopic diagnostic aid (the MoleMate system) to the systematic application of best practice guidelines (including the Weighted 7PCL) did not increase the proportion of appropriately referred lesions; instead, the MoleMate system led to a higher proportion of benign lesions referred. Furthermore, the systematic application of best practice guidelines and the MoleMate system both performed much better than reports of current practice. Therefore, it was concluded that, on current evidence, the systematic application of best practice guidelines (including the Weighted 7PCL) is the paradigm for management of suspicious skin lesions in primary care.
The findings from this study of the 7PCL as a diagnostic aid could have importance in primary care where melanoma is a rare condition, yet the symptoms and signs are commonly presented and represent benign conditions. To ensure timely referral and to improve prognosis, GPs need to differentiate patients with clinically significant lesions who require urgent referral or further investigation, from those who can be reassured, or monitored for change, in primary care.
Strengths and limitations
Although the data were not collected primarily for the purpose of validating the 7PCL, they were obtained during a rigorously conducted RCT set in the east of England.14 Consequently, the quality of the data allows us to analyse and report this diagnostic accuracy study according to all the criteria recommended within the STARD checklist.13 This SIAscopic diagnostic aid trial is the largest primary care RCT ever conducted on skin cancer diagnosis, and has provided a substantial dataset on skin lesions encountered in general practice. All the 7PCL scores were calculated blinded to the reference diagnoses, thereby minimising clinical reviewer bias. The choice of reference standard diagnosis was inevitably pragmatic as, for ethical reasons, it was not possible to obtain histology for every lesion in the trial. This study presents the performance of the score on the basis of the 36 melanomas diagnosed in the trial, aware that, as a primary care-based study, there were inevitably few malignant lesions leading to wide confidence intervals. A reference diagnosis of clinically significant was also deliberately chosen to reflect the purpose of such a diagnostic tool in primary care to inform decisions about referral for possible melanoma. This does not represent every lesion seen in primary care as the inclusion criteria were defined as a pigmented lesion that could not be immediately diagnosed as benign and the patient reassured. However, the included lesions represent those for which there is a degree of clinical uncertainty and for which diagnostic checklists are likely to be of most use in primary care. Finally, although a minority of participants had more than one lesion, the analyses are reported unadjusted for clustering because the main trial analysis showed no differences between the unadjusted and cluster adjusted results.14
Comparison with existing literature
The development and validation of the Original 7PCL and the Weighted 7PCL were conducted with case series of histologically proven melanoma ascertained from skin cancer clinics. A significant strength of the current study is that, for the first time, it validates the diagnostic aid in the population for which it is intended. The sample was of pigmented skin lesions that could not immediately be diagnosed as benign by a GP. These represent a group in whom a diagnostic aid is most likely to be used in general practice, namely where diagnostic uncertainty exists. While the more important outcome is sensitivity and specificity for melanoma diagnosis, the primary reference standard was based on the concept of clinical significance and whether a lesion required referral or could be managed in primary care. Therefore it mimics the intended use of the checklist according to NICE guidelines. Applying this as the primary outcome provided a much greater statistical power to analyse the performance of different cut-off scores than using melanoma.
An English study reported in 1990 that the Original 7PCL had a PPV of 64% and NPV of 99% when used by dermatologists, but a PPV of only 7% and NPV of 99% when used by patients.18 Confirming this study’s findings, this previous study showed that no melanomas were missed by dermatologists when using the irregular border item alone; however, this feature was missed by five of eight patients with melanoma. The Weighted 7PCL has been shown to detect all melanomas in an English dermatology clinic case series, with 62% of the melanomas having more than one major feature compared with only 4% of the referred non-malignant lesions.19 Another evaluation, using all the histologically proven cutaneous melanomas diagnosed in Leicestershire between 1982 and 1996, showed a diagnostic sensitivity of 81.5% for the Weighted 7PCL,20 and a number of similar studies using a referred rather than a primary care population have been reported.21
An evaluation of the diagnostic performance of the 7PCL in a primary care population has not been identified. This work demonstrates for the first time the performance of the checklist and suggests that specificity of referral may be improved further without loss of sensitivity of melanoma diagnosis by altering the cutoff score.
The ABCD(E) rule is the other widely used checklist to aid the diagnosis of melanoma, although it is more specifically directed for use by the public than the 7PCL. The American Cancer Association originally advertised a 4-point checklist (A: asymmetry, B: border irregularity, C: colour irregularity, D: diameter >6 mm), which was then modified to five items to include the importance of change in a pigmented lesion.22 A comparison of the 4-point ABCD against the Original 7PCL conducted in the US showed that the ABCD scale had higher sensitivity and confirmed the importance of irregular border.23 A more recent Australian comparison of patient use of the two updated checklists found that the ABCD(E) rule failed to discriminate between melanoma and benign pigmented lesions. Change in size and change in colour were most useful as discriminators in the hands of patients.12
In this study, irregular border performed well as a single feature, showing good sensitivity and specificity in terms of clinically significant lesions, although this did not translate into such good performance for melanoma diagnosis. An alternative clinical visual aid to diagnosis of melanoma is the so-called ‘ugly duckling sign’, identifying pigmented moles that look different from a person’s other moles.24 Preliminary data suggest that this may be a common feature of melanomas but there are no formal validation studies published on its diagnostic accuracy in primary care.
Implications for research and practice
The 2005 NICE guidelines on referral for suspected cancer recommended the use of the Weighted 7PCL with a cut-off score of ≥3, to inform referrals to urgent skin cancer clinics.9 At the time there were no data based on its application in primary care. This study’s findings demonstrate reasonable performance of these checklists and suggest that the Weighted 7PCL could be improved by revising the cut-off score from 3 to ≥4. This would improve specificity for clinically significant lesions, and therefore reduce referrals of benign lesions, without losing sensitivity for melanoma.
Further research is needed to confirm the performance of the revised Weighted 7PCL and the single item ‘irregular border’ in a separate UK primary care population, ideally with a larger number of melanomas in the dataset. It may also have utility in primary care settings beyond the UK, including in healthcare systems such as Australia where melanoma diagnosis is often made with excisions performed in general practice rather than referral to secondary care. Decision making about whether or not to excise pigmented lesions in such primary care settings as well as rural UK settings could be facilitated with the revised Weighted 7PCL. Additional research is also needed to assess implementation strategies such as embedding the checklist within the electronic medical record, and examining its impact on GP utilisation, referral patterns, stage of melanoma at diagnosis, survival rates, and cost-effectiveness. The number of benign moles excised for each malignant melanoma detected has recently been promoted as a suitable performance indicator for diagnostic aids in this field.25 Furthermore, the role of the revised Weighted 7PCL in helping patients to appraise and monitor their own skin lesions should also be explored. In conclusion, the Original and Weighted 7PCL both performed reasonably well in a primary care setting to identify clinically significant lesions. An irregular border alone is an important distinguishing feature. Changing the cut-off score of the Weighted 7PCL to 4 from 3 could be applied in general practice to support the assessment of pigmented skin lesions and early identification of melanoma.
Acknowledgments
We would like to thank the other members of the MoleMate UK Trial team: Ms Elka Humphrys, Mr Edward Wilson, Mr Joe Walls, Dr Paul Norris, Mrs Margaret Johnson and Mrs Marion Edwards, as well as the trial’s independent chair, Dr Neil Campbell. We also thank Dr Ed Rytina for his validation of the histological diagnoses, and Prof Rona Mackie and Prof Jonathan Mant for their comments on the draft paper. We would particularly like to thank all participating practices and practice teams and especially the patients without whom this research would not have been possible. Initially MoleMateTM, Astron Clinica, SIAscopy, SIAscan and SIAscope were trademarks of Astron Clinica Limited, which kindly supplied the MoleMate systems for this trial. In August 2009, Astron Clinica Ltd was taken over by Biocompatibles International plc, by BTG (British Technology Group) in January 2011 and in June 2011 by MedX Health which now holds these trademarks.
Funding
The Trial was funded by the National Institute for Health Research School for Primary Care Research. The views expressed in this paper are those of the authors and not necessarily those of the Department of Health. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Ethical approval
Not required.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
Per N Hall does consultancy for Lifescan & Health Screen UK. Although Per N Hall has long-standing intellectual involvement with the development of SIAscopy he has had no commercial involvement with Astron Clinica or Biocompatibles plc, or MedX who now own the intellectual property for SIAscopy. John D Emery has received a research grant from Biocompatibles plc. The other authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Research UK http://www.cancerresearchuk.org/home/ (accessed 8 Mar 2013).
- 3.Murchie P, Campbell NC. Pigmented lesions, cutaneous melanoma, and future challenges for primary care. Eur J Gen Pract. 2007;13(3):151–154. doi: 10.1080/13814780701627354. [DOI] [PubMed] [Google Scholar]
- 4.Tsao H, Bevona C, Goggins W, Quinn T. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: a population-based estimate. Arch Dermatol. 2003;139(3):282–288. doi: 10.1001/archderm.139.3.282. [DOI] [PubMed] [Google Scholar]
- 5.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 6.Mackie RM. An illustrated guide to the recognition of malignant melanoma. Glasgow: Glasgow University Department of Dermatology; 1985. [Google Scholar]
- 7.Mackie RM. Malignant melanoma. A guide to early diagnosis. Glasgow: Glasgow University Department of Dermatology; 1989. [Google Scholar]
- 8.MacKie RM. Clinical recognition of early invasive malignant melanoma. BMJ. 1990;301(6759):1005–1006. doi: 10.1136/bmj.301.6759.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute for Health and Clinical Excellence . Referral guidelines for suspected cancer. London: NICE; 2005. Clinical guidline 27. http://publications.nice.org.uk/referral-guidelines-for-suspected-cancer-cg27/guidance (accessed 8 Mar 2013). [Google Scholar]
- 10.Friedman RJ, Rigel D, Kopf AW. Early detection of malignant melanoma: the role of physician examination and self-examination of the skin. Cancer J Clin. 1985;35(3):130–151. doi: 10.3322/canjclin.35.3.130. [DOI] [PubMed] [Google Scholar]
- 11.Abbasi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA. 2004;292(22):2771–2776. doi: 10.1001/jama.292.22.2771. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Hill D, Gibbs AF, et al. What features do patients notice that help to distinguish between benign pigmented lesions and melanomas?: the ABCD(E) rule versus the seven-point checklist. Melanoma Res. 2005;15(6):549–554. doi: 10.1097/00008390-200512000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326(7379):41–44. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter FM, Morris H, Humphrys E, et al. Effect of adding a diagnostic aid to best practice to manage suspicious pigmented skin lesions in primary care: randomsied controlled trial. BMJ. 2012;344:e4110. doi: 10.1136/bmj.e4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marston L, Peacock JL, Yu K, et al. Comparing methods of analysing datasets with small clusters: case studies using four paediatric datasets. Paediatr Perinat Epidemiol. 2009;23(4):380–392. doi: 10.1111/j.1365-3016.2009.01046.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Davis CS, Soong SJ. Comparison of predictive values of two diagnostic tests from the same sample of subjects using weighted least squares. Stat Med. 2006;25(13):2215–2229. doi: 10.1002/sim.2332. [DOI] [PubMed] [Google Scholar]
- 18.Keefe M, Dick DC, Wakeel RA. A study of the value of the seven-point checklist in distinguishing benign pigmented lesions from melanoma. Clin Exp Dermatol. 1990;15(3):167–171. doi: 10.1111/j.1365-2230.1990.tb02064.x. [DOI] [PubMed] [Google Scholar]
- 19.Healsmith MF, Bourke JF, Osborne JE, Graham-Brown RA. An evaluation of the revised seven-point checklist for the early diagnosis of cutaneous malignant melanoma. Br J Dermatol. 1994;130(1):48–50. doi: 10.1111/j.1365-2133.1994.tb06881.x. [DOI] [PubMed] [Google Scholar]
- 20.Osborne JE, Bourke JF, Graham-Brown RA, Hutchinson PE. False negative clinical diagnoses of malignant melanoma. Br J Dermatol. 1999;140(5):902–908. doi: 10.1046/j.1365-2133.1999.02823.x. [DOI] [PubMed] [Google Scholar]
- 21.Jackson AM, Morgan DR, Ellison R. Diagnosis of malignant melanoma by general practitioners and hospital specialists. Postgrad Med J. 2000;76(895):295–298. doi: 10.1136/pmj.76.895.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas L, Tranchand P, Berard F, et al. Semiological value of ABCDE criteria in the diagnosis of cutaneous pigmented tumors. Dermatology. 1998;197(1):11–17. doi: 10.1159/000017969. [DOI] [PubMed] [Google Scholar]
- 23.McGovern TW, Litaker MS. Clinical predictors of malignant pigmented lesions. A comparison of the Glasgow seven-point checklist and the American Cancer Society’s ABCDs of pigmented lesions. J Dermatol Surg Oncol. 1992;18(1):22–26. doi: 10.1111/j.1524-4725.1992.tb03296.x. [DOI] [PubMed] [Google Scholar]
- 24.Scope A, Dusza SW, Halpern AC, Rabinovitz H, et al. The ‘ugly duckling’ sign: agreement between observers. Arch Dermatol. 2008;144(1):58–64. doi: 10.1001/archdermatol.2007.15. [DOI] [PubMed] [Google Scholar]
- 25.Sidhu S, Bodger O, Williams N, Roberts DL. The number of benign moles excised for each malignant melanoma: the number needed to treat. Clin Exp Dermatol. 2012;37(1):6–9. doi: 10.1111/j.1365-2230.2011.04148.x. [DOI] [PubMed] [Google Scholar]