Abstract
Aims
Aldosterone antagonists (AldoAs) have been used to treat severe chronic heart failure (CHF).There is uncertainty regarding the efficacy of using AldoAs in mild to moderate CHF with New York Heart Association (NYHA) classifications of I to II. This study summarizes the evidence for the efficacy of spironolactone (SP), eplerenone (EP) and canrenone in mild to moderate CHF patients.
Methods
PubMed, MEDLINE, EMBASE and OVID databases were searched before June 2012 for randomized and quasi-randomized controlled trials assessing AldoA treatment in CHF patients with NYHA classes I to II. Data concerning the study's design, patients' characteristics and outcomes were extracted. Risk ratio (RR) and weighted mean differences (WMD) or standardized mean difference were calculated using either fixed or random effects models.
Results
Eight trials involving 3929 CHF patients were included. AldoAs were superior to the control in all cause mortality (RR 0.79, 95% CI 0.66, 0.95) and in re-hospitalization for cardiac causes (RR 0.62, 95% CI 0.52, 0.74), the left ventricular ejection fraction was improved by AldoA treatment (WMD 2.94%, P = 0.52). Moreover, AldoA therapy decreased the left ventricular end-diastolic volume (WMD −14.04 ml, P < 0.00001),the left ventricular end-systolic volume (WMD −14.09 ml, P < 0.00001). A stratified analysis showed a statistical superiority in the benefits of SP over EP in reducing LVEDV and LVESV. AldoAs reduced B-type natriuretic peptide concentrations (WMD −37.76 pg ml−1, P < 0.00001), increased serum creatinine (WMD 8.69 μmol l−1, P = 0.0003) and occurrence of hyperkalaemia (RR 1.78, 95% CI 1.43, 2.23).
Conclusions
Additional use of AldoAs in CHF patients may decrease mortality and re-hospitalization for cardiac reasons, improve cardiac function and simultaneously ameliorate LV reverse remodelling.
Keywords: aldosterone antagonist, cardiac function, chronic heart failure, meta-analysis, prognosis
Introduction
Heart failure contributes to nearly 1 million hospitalizations annually in the USA, and the mortality rate among patients with chronic heart failure (CHF) remains notably high despite treatment with angiotensin-converting enzyme inhibitors (ACEIs) and angiotension receptor blockers (ARBs) or β-adrenoceptor blockers [1, 2]. Published guidelines for the standardized therapy for CHF recommend that treatment for NYHA classes I and II at baseline treatment includes an ACEI or an ARB plus a β-adrenoceptor blocker and, for classes III and IV, treatment includes an ACEI or ARB and a β-adrenoceptor blocker plus an aldosterone antagonist (AldoA). Recent studies also recommended the use of AldoAs in CHF patients with a low EF (less than 35%) and NYHA classes III and IV.
AldoAs are not recommended by the majority of the guidelines for standardized therapy in CHF patients with NYHA classes I to II. To date, some trials indicated a role for AldoAs in mild to moderate CHF patients [3–5].
Aldosterone has been shown to cause coronary inflammation, cardiac hypertrophy, myocardial fibrosis, ventricular arrhythmias and ischaemic and necrotic lesions. AldoAs compete with aldosterone to bind at the mineralocorticoid receptor. RAAS blockade by ACEIs slows the progression of CHF and improves survival [6]. However, ACEIs do not block the mineralocorticoid receptors, which are usually over-expressed in patients with CHF and long term ACEI therapy can cause a so-called ‘aldosterone escape’. Increased plasma aldosterone concentrations are associated with a poor prognosis. AldoAs act as a neurohormonal blockade of the final effector of the renin-angiotensin axis, which has been confirmed by clinical trials to play an important role in the treatment of NYHA classes III and IV CHF. This function was demonstrated by the RALES trial, in the case of SP. The result of this trial was a reduction in the relative risk of mortality [7]. AldoA is also effective in treating patients after acute myocardial infarction (MI) and heart failure, as demonstrated by the EPHESUS trial for eplerenone [8–10].
Recently, the EMPHASIS-HF mortality trial has documented the efficacy of EP therapy, even in milder stages of CHF with a NYHA classification of II [11, 12]. AldoAs may be an appropriate initial therapy for CHF and may improve prognosis and quality of life in mild to moderate CHF. In this study, we sought to clarify the efficacy of AldoAs in CHF patients with NYHA classes I to II, including their effects on the LVEF, B-type natriuretic, LV function, re-hospitalization, mortality and adverse effects of AldoAs, by discussing the existing clinical evidence supporting the use of AldoAs, expanding the application of AldoAs in CHF and by providing better guidelines for clinical medication.
Methods
Search strategy
We searched the review databases of PubMed, MEDLINE, EMBASE and OVID for studies published before June 2012, using the items ‘heart failure’, ‘aldosterone antagonist’, ‘spironolactone’, ‘eplerenone’, ‘canrenone’ or ‘NYHA class’. We investigated additional studies by reviewing the reference lists of included articles, conference abstracts and the bibliographies of expert advisors.
Selection criteria
Li-jun Hu and Yun-qing Chen reviewed all of the citations and retrieved the literature by titles or abstracts and subsequently by full texts. The studies were selected if they met the following set of inclusion criteria: the studies were either randomized or quasi-randomized and published in the English language, the patients included in the studies had New York Heart Association (NYHA) functional classes I to II HF symptoms with an EF of no higher than 45%, the studies compared the AldoAs (spironolactone or eplerenone or canrenone) with standard CHF therapy (ACEIs and ARB or β-adrenoceptor blocker), the studies used randomized allocation, the studies used parallel group design, the studies were blinded studies and included a treatment durations of a minimum of 12 weeks. The papers reported the sizes of the samples, source of controls, or other information that might help to infer the results. When multiple publications reported the same or overlapping data, we used the most recent or largest population. The Jadad scale was used to evaluate the overall quality of the articles.
Data exclusion
The exclusion criteria were an acute MI, severe CHF with an NYHA class III or IV, no standard HF therapy (ACEIs and ARBs or β-adrenoceptor blocker), a high serum potassium concentration and a severely low GFR.
Data extraction
As required by Cochrane's reviewer's handbook, data were independently extracted from each study by two reviewers according to the pre-specified selection criteria. The extraction forms of the two reviewers were compared. Disagreements were discussed and decisions were made through consensus. A third party was involved when necessary. The following information was extracted from the studies: the first author, year of publication, baseline patient characteristics, intervention, outcomes, source of control and study conclusions.
Data synthesis and analysis
Dichotomous data were analyzed using a risk ratio (RR) with 95% confidence intervals (CIs), whereas continuous variables (changing from baseline to follow-up) were analyzed using either weighted mean differences (WMD) or standardized mean differences (SMD). Pooled analyses were calculated using fixed effect models, whereas random effects models were applied in cases of significant cross study
heterogeneity across studies. Statistical heterogeneity was measured using the I2 statistic and heterogeneity was not considered as significant when I2 < 50%. Sensitivity analyses (excluding one study at a time) were performed to determine the stability of the overall treatment effects. The Cochrane Collaboration meta-analysis software Review Manager 5.1 was used for the meta-analysis. A P value of 0.05 for any test or model was considered to be statistically significant.
Results
Eligible studies
The process of selecting studies for the meta-analysis is shown in Figure 1. Briefly, of the initial 625 hits, 45 articles were retrieved for a detailed evaluation and eight studies published between 2003 and 2011 satisfied the inclusion criteria (four for SP, two for EP and two for CAN) and were finally analyzed in the meta-analysis.
Figure 1.
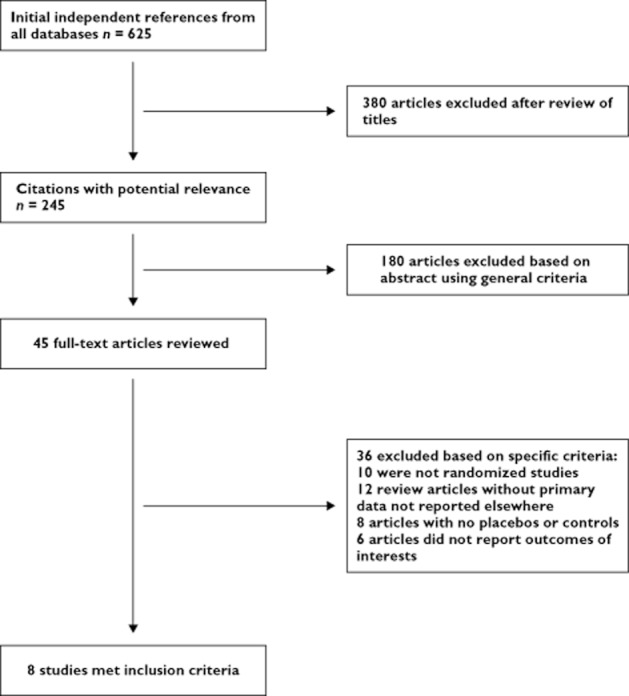
Study flow chart for the process of selecting the final eight trials
Table 1 shows the baseline characteristics of the patients in each study group included in our meta-analysis. Specifically, 1968 patients were assigned to an AldoA group, whereas 1961 subjects were assigned to the control group. Patients who withdrew from the trials were not counted in the total. All patients who had a reduced LVEF were enrolled in the trials, which had clearly stated methods of recruitment, total sample numbers, characteristics of participants and inclusion criteria.
Table 1.
Characteristics of the eight trials included in the meta-analysis
| Trials | Patients | Total cases | Treatment | Mean follow-up | ||
|---|---|---|---|---|---|---|
| Aldo | Acontrol cases | AldoA | Drug control | |||
| Zannad et al. 2011 [12] | NYHA II LVEF ≤ 35% | 1364 | 1373 | Eplerenone 50 mg once daily | Placebo | 21 months |
| Boccanelli et al. 2009 [13] | NYHA II LVEF ≤ 45% | 188 | 193 | Canrenone 25–50 mg once daily | Placebo | 12 months |
| de Simone et al 2010 [14] | NYHA II LVEF ≤ 45% | 146 | 146 | Canrenone 25–50 mg once daily | Placebo | 12 months |
| Udelson et al. 2010 [15] | NYHA I-II LVEF ≤ 35% | 116 | 109 | Eplerenone 50 mg once daily | Placebo | 9 months |
| Vizzardi et al. 2010 [16] | NYHA I-II LVEF ≤ 40% | 79 | 79 | Spironolactone 25–100 mg once daily | Placebo | 6 months |
| Feola et al. 2003 [17] | NYHA I-II LVEF ≤ 40% | 36 | 16 | Spironolactone 25–50 mg once daily | Placebo | 6 months |
| Chan et al. 2007 [18] | NYHA I-II LVEF ≤ 40% | 20 | 21 | Spironolactone 25 mg once daily | Placebo | 12 months |
| Berry et al. 2006 [19] | NYHA I-II LVEF ≤ 40% | 19 | 17 | Spironolactone 25 mg once daily | Placebo | 3 months |
| Trials | With standard HF therapy ACEI/ARB/β-adrenoceptor blocker | Outcomes | ||||
|---|---|---|---|---|---|---|
| Zannad et al. 2011 [12] | Yes, ACEI/AR or both and β-adrenoceptor blocker | Primary: cardiovascular mortality, rehospitalization for HF. Secondary: all cause mortality or rehospitalization, adverse events. | ||||
| Boccanelli et al. 2009 [13] | Yes, ACEI/ARB or both and β-adrenoceptor blocker | Primary: BNP, EF, EDV, ESV, LVM, PIIINP. Secondary: LV mechanics, clinical event. | ||||
| de Simone et al. 2010 [14] | Yes, ACEI/ARB or both and β-adrenoceptor blocker | Primary: EF, BNP, PIIINP, E : A, LVM. Secondary: ESV, NYHA class. | ||||
| Udelson et al. 2010 [15] | Yes, ACEI/ARB and β-adrenoceptor blocker | EF, EDV, ESV, BNP, PIIINP, adverse events. | ||||
| Vizzardi et al. 2010 [16] | Yes, ACEI/ARB and β-adrenoceptor blocker | EF, EDV, ESV, CP exercise, LVM, adverse events, tolerability. | ||||
| Feola et al. 2003 [17] | Yes, ACEI/ARB and β-adrenoceptor blocker | EF, EDV, ESD, BNP, CP exercise, adverse events. | ||||
| Chan et al. 2007 [18] | Yes, ARB | EF, LVM, EDV, ESV, E : A, LV reverse remodelling, adverse events. | ||||
| Berry et al. 2006 [19] | Yes, ACEI and β-adrenoceptor blocker | BNP, PIIINP, safety. | ||||
All cause mortality and re-hospitalization for cardiac causes
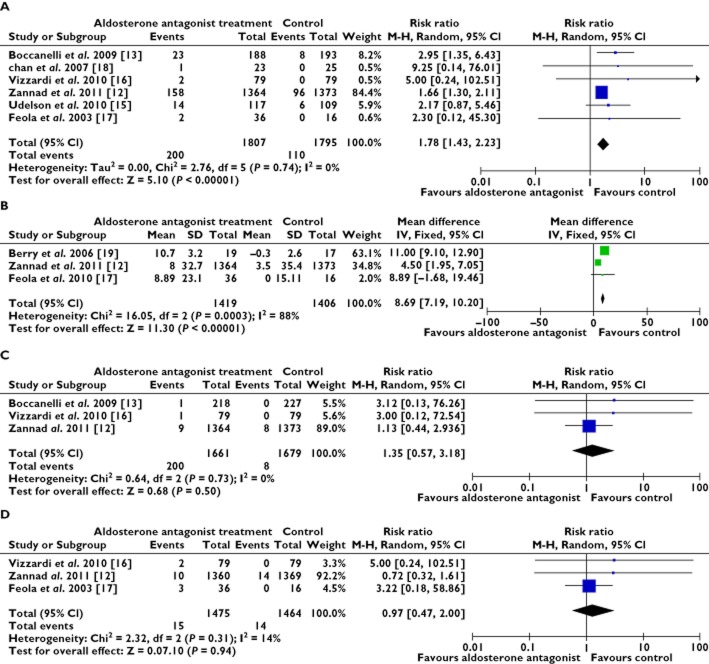
The results of the meta-analysis showed that the all cause mortality in the AldoA group was lower than in the control group (RR 0.79, 95% CI 0.66, 0.95, P = 0.65) [12, 13, 17], 178 in 1630 vs. 225 in 1617 were recorded (first event). Of the 1718 patients with AldoA treatment, 178 required re-hospitalization for worsening heart failure or cardiac causes, which was significantly fewer than the 287 of the 1730 patients assigned to the control group (RR 0.62, 95% CI 0.52, 0.74, P = 0.54). This effect was reported in only four trials and heterogeneity was observed in the trials. Clearly, the AldoA treatment showed a statistically significant benefit in the reduction of re-hospitalization [12, 13, 15, 18] (Figure 2A, B).
Figure 2.
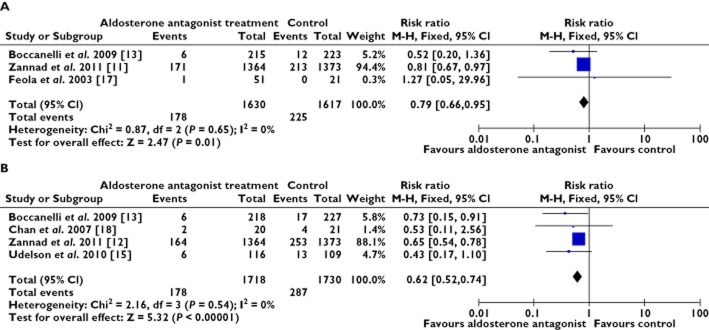
Forest plots for all cause mortality and re-hospitalization for worsening heart failure. (A) all cause mortality and (B) re-hospitalization for worsening heart failure. CI = confidence intervals; M-H = Mantel-Haenszel; RR = relative risk
Left ventricular structure and function
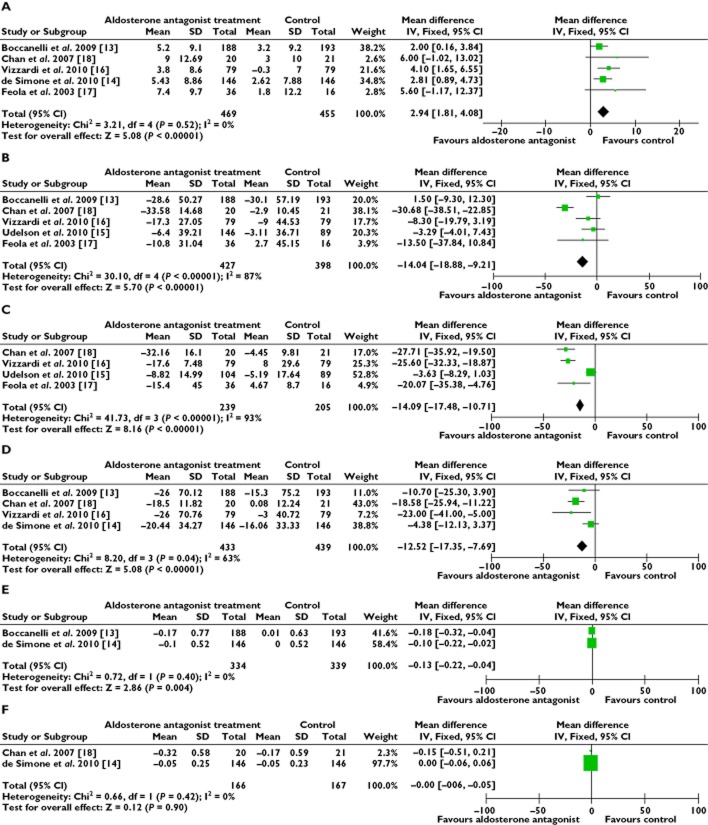
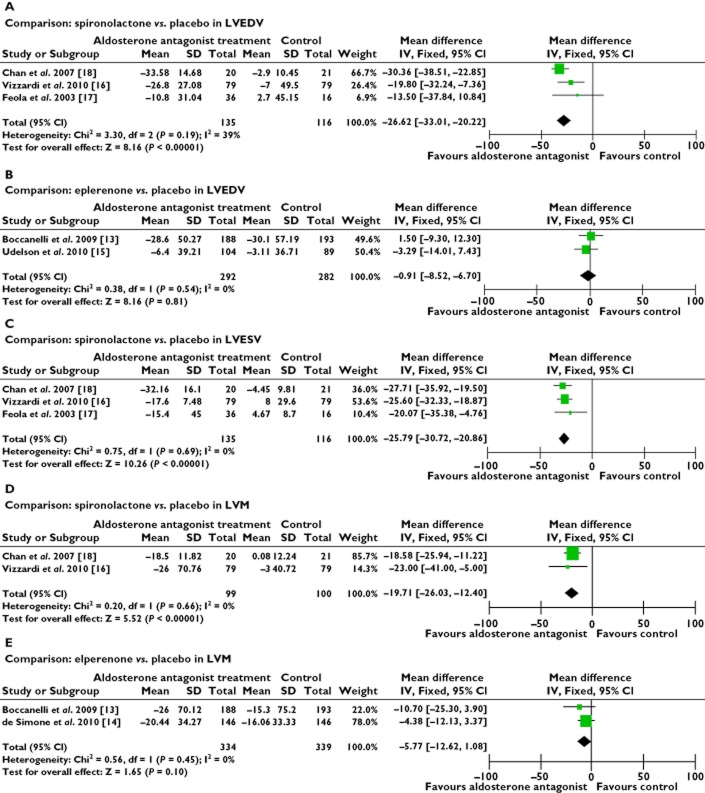
The results showed that the additional AldoA therapy was superior to the standard CHF therapy in LVEF improvement (WMD 2.94%, P = 0.52) (Figure 3A), by modifying the functional parameters of the left ventricle. Among the eight trials, only six trials recorded the LVEF from the baseline to the end of the study [13–18]. The sensitivity analyses suggested this beneficial effect when the study by James et al. was omitted [15] (WMD 1.61%, P = 0.02). AldoA therapy was similarly found to reduce LVESV (WMD −14.04 ml, P < 0.00001) (Figure 3B) and LVEDV (WMD −14.09 ml, P < 0.00001) (Figure 3C). We found that the data collected were heterogeneous (P < 0.00001) and we selected random effect models for statistical analysis which were also heterogeneous (P < 0.00001), we used the fixed effect model. The results showed a decrease of LVEDV and LVESV with AldoA therapy, compared with the control group. We considered that the different types of AldoAs could cause varying degrees of effect. The heterogeneity observed in our primary analysis may be explained by the trial designs, the difference in the types of AldoAs used and the variation in the duration of treatment. Analyses stratified by the types of AldoAs reduced the observed heterogeneity (Figure 4A, B). The analysis showed that CHF patients were more likely to receive the beneficial effects from the SP treatment with a reduced LVEDV (WMD −26.62 ml, P = 0.19) [16–18], as well as the EP group (WMD −0.91 ml, P = 0.54) [13, 15]. This effect was also similar to the LVESV results. Analysis of the SP trials showed a beneficial effect in LVESV (WMD −25.79 ml, P = 0.69) (Figure 4C) [16–18]. The substitution of a fixed model for a random effects model did not change our initial qualitative interpretation of the pooled treatment effect on LVEDV and LVESV. AldoA therapy also tended to reduce the LVM (WMD −12.52 g, P = 0.04) (Figure 3D). AldoAs showed a statistically significant benefit in improving LVEF and in reducing LVEDV and LVESV. Heterogeneity was also reduced when the analysis was stratified by specific treatment comparisons in an LVM analysis, with either SP intervention only (WMD −19.21 ml, P = 0.66) or EP intervention (WMD −5.77 ml, P = 0.43) in the trials showing a beneficial effect (Figure 4D, E).
Figure 3.
Forest plots for left ventricular structure and function. (A) left ventricular ejection fraction, (B) left ventricular end-diastolic volume, (C) left ventricular end-systolic volume, (D) left ventricular mass, (E) left atrial dimension and (F) E : A ratio. Stratified comparison of the effect: (A) comparison : spironolactone vs. placebo in LVEDV; (B) comparison : eplerenone vs. placebo in LVEDV; (C) comparison : spironolactone vs. placebo in LVESV; (D) comparison : spironolactone vs. placebo in LVM and (E) comparison : eplerenone vs. placebo in LVM
Figure 4.
Forest plots for comparison of AldoAs vs. controls on left ventricular structure. (A) and (B) left ventricular end-diastolic volume, (C) left ventricular end-systolic volume and (D) and (E) left ventricular mass
Serum markers
The B-type natriuretic peptide (BNP) concentration was down-regulated by the AldoA treatment (WMD −37.36 pg ml−1, P < 0.00001) [13–15, 17, 19]. We found that the data collected were heterogeneous and, therefore, a random effect model was used, which showed that the BNP concentration was significantly lower in the AldoA treatment group than the control group. We excluded one study at a time, which did not significantly alter the results. The sensitivity analyses predominantly discussed the direction and magnitude of the statistical significance and the statistical analysis of the results. The pro-collagen type III N-terminal amino peptide (PIIINP) concentration was slightly reduced by the AldoA treatment (WMD −0.07 μg l−1, P = 0.52) [13–15, 19] (Figure 5A, B).
Figure 5.
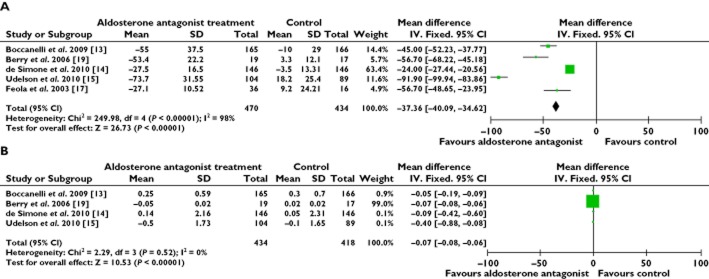
Forest plots for serum markers. (A) B-type natriuretic peptide (BNP) and (B) pro-collagen type III N-terminal amino peptide (PIIINP)
Other adverse events
As shown in Figure 6A, B, AldoA treatment increased the occurrence of hyperkalaemia more than was observed in the control group (RR 1.78, 95% CI 1.43, 2.23, P = 0.74). The associated serum creatinine increased more in the AldoA treatment group (WMD 8.69 μmol l−1, P = 0.0003). As shown in Figures 6C, D, there is no significant difference in the pooled worsening the renal function results (RR 1.35, 95% CI 0.57, 3.18, P = 0.73) and gynaecomastia (RR 0.97, 95% CI 0.47, 2.00, P = 0.31) between the AldoA and the control therapy.
Figure 6.
Forest plots for adverse events. (A) hyperkalaemia, (B) serum creatinine, (C) worsening renal function and (D) gynaecomastia
Discussion
Aldosterone antagonists play an important role in the treatment of NYHA classes III and IV CHF. We evaluated the effect of adding AldoAs (primarily within the low dose range of 25 to 50 mg day−1) to the recommended standard CHF treatment for NYHA functional classes I to II [12–19]. The accumulated data showed that the addition of the AldoAs could provide further benefit, improve prognosis and enhance the quality of life in mild to moderate CHF patients.
In our findings, we demonstrated a reduction in all cause mortality and re-hospitalization for worsening HF or cardiac causes with the use of AldoAs in mild to moderate CHF patients. An early benefit was observed in the largest trials (EMPHASIS-HF), in which the predominant composite endpoint of the study (a combination of cardiovascular mortality and hospitalization for heart failure) was decreased by as much as 37% and with overall and cardiovascular mortality was reduced by 24% [11, 12], improving the quality of life in mild to moderate CHF patients. Beneficial effects had been demonstrated in the changes of LV function and LV reverse remodelling [13–19]. We found a 2.94% improvement in the LVEF and a reduction in the LVEDV, LVESV and LVM, although these results had some statistical heterogeneity. AldoAs slowed the progression of LV remodelling, improved cardiac function and reduced the occurrence of cardiac death. Additionally, the AldoAs further reduced serum BNP concentrations (−37.36 pg ml−1) considering that the BNP concentration is negatively related with the alteration in cardiac structure and function. Although BNP reduction could improve cardiac function, there was no significant difference in PIIINP, a marker of collagen turnover and a specific marker of connective tissue remodelling that could cause cardiac fibrosis and predict clinical outcomes [20]. In this meta-analysis, among patients receiving AldoAs, there was an increased incidence of hyperkalaemia that increased the death rate from electrolyte disturbances or cardiac arrest [21]. This report described a small sample collection. No differences in worsening renal function or gynaecomastia between the AldoAs therapy and the control therapy were observed.
Because of a high heterogeneity in the LV structure parameters (LVEDV, LVESV, and LVM), we considered whether the different types of AldoAs might cause differently sized effects. Stratified comparisons were performed for further study, which showed good effects in LV remodelling, especially in the SP treatment group.
We grouped similar medications together: spironolactone, the oldest type and a non-selective AldoA, eplerenone, which is derived from SP and designed to avoid side effects by its high selectivity and canrenone, a common active metabolite of SP. These three medications have similar mechanisms of action, and although clinical trials have evaluated the efficacy of each drug, no studies have directly compared SP and EP or CAN. These drugs differ predominantly in their side effects [22, 23]. Because hyperkalaemia may be the main side effect of using AldoAs, potassium monitoring is critical to prevent the development of hyperkalaemia. The incidence of gynaecomastia was negligible in the AldoA-treated group compared with the control group [12, 16, 17], perhaps because of insufficient data in our study. Most CHF patients have chronic renal failure, with AldoAs increasing the risk of increased serum creatinine and worsening renal function, reported during the trials [12, 13, 15–18]. Renal hypoperfusion occurs as a result of reduced cardiac output and this effect may lead to a high risk of cardiac death [22, 25]. However, starting with and maintaining low doses of AldoAs and attention to monitoring may reduce the incidence of renal failure and the death rate [24]. Overall, chronic additive treatment with AldoA therapy is feasible, although several adverse effects were observed. AldoAs could be initiated early in those patients with signs or symptoms of mild to moderate CHF with a low risk of complications.
Clinical studies have suggested that mineralocorticoid-receptor antagonists favourably affect several other important mechanisms known to have a role in the progression of HF. This meta-analysis demonstrated that AldoAs may play important roles in mild to moderate CHF patients with NYHA classes I to II. It seems plausible that the AldoAs improved LV reverse modelling metabolism in the cardiomyocytes, ultimately contributing to improvements in cardiac function and clinical symptoms [6]. Early benefits were observed in the largest EMPHASIS-HF trial [11, 12].This trial was designed to detect morbidity and mortality endpoints related to the excess of benefit in reducing the risk of cardiovascular death or hospitalization for CHF obtained with anti-aldosterone therapy. There were no data to verify the changes in the status of symptoms or in the NYHA functional class or gender proportion in our study, which may be due to insufficiencies in the trials that were included.
Heterogeneity of effect
The heterogeneity observed in the pooled estimate in our primary analysis may be explained by the design of the trials and the different types of AldoAs. Analysis stratified by the specific type of AldoA vs. the controls did reduce the observed heterogeneity. Our present analysis cannot fully adjust for differences in the duration of treatment between trials.
Study limitations
Our analysis is limited by a relatively small sample size and a scarcity of data on certain outcomes. A degree of heterogeneity was found to exist, and there is a heavily weighted study (EMPHASIS-HF). Bias may have been introduced by using combined results of a large scale randomized controlled trial (EMPHASIS-HF) with small size studies. Furthermore, given the limited evidence available in the study, this article was restricted to provide more parameters of cardiac function improvement. We cannot distinguish the specific effects of each type of medication.
Conclusions
The additional use of AldoAs in mild to moderate CHF patients may exert beneficial effects, not only in ameliorating clinical symptoms and LV structure and function, but also in reducing cardiac death and re-hospitalization for cardiac causes. Our study indicates that an AldoA may be an additional therapeutic agent for patients with NYHA classes I to II CHF.
Acknowledgments
We thank the National Natural Science Foundation of China (NSFC. no. 30971213, 81100088, 81070140), the 2010 Key Projects of Chongqing Medical University Foundation, (XBZD.201010), and the Project of Chongqing Health Administration (No.2009-1-13) for providing foundation support.
Competing Interests
All authors of the paper have completed the Unified Competing Interest form and declare no support from any organization for the submitted manuscript. There are no financial relationships with any organizations that might have an interest in the submitted paper in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 2.Ezekowitz JA, van Walraven C, McAlister FA, Armstrong PW, Kaul P. Impact of specialist follow-up in outpatients with congestive heart failure. CMAJ. 2005;172:189–194. doi: 10.1503/cmaj.1032017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMurray JJ, Swedberg K. Treatment of chronic heart failure: a comparison between the major guidelines. Eur Heart J. 2006;27:1773–1777. doi: 10.1093/eurheartj/ehl123. [DOI] [PubMed] [Google Scholar]
- 4.Smolle E, Riegelnik V, Oberschneider C, Fruhwald F. Guideline adherence in the treatment of chronic heart failure. Data from the Euroheart failure survey – Pilot phase at the university heart failure clinic Graz. J Kardiologie. 2011;18:5–6. [Google Scholar]
- 5.Caccamo MA, Eckman PM. Pharmacologic therapy for New York Heart Association class IV heart failure. Congest Heart Fail. 2011;17:213–219. doi: 10.1111/j.1751-7133.2011.00235.x. [DOI] [PubMed] [Google Scholar]
- 6.Francis GS. The relationship of the sympathetic nervous system and the renin angiotensin system in congestive heart failure. Am Heart J. 1989;118:642–648. doi: 10.1016/0002-8703(89)90291-3. [DOI] [PubMed] [Google Scholar]
- 7.Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the Randomized Aldactone Evaluation Study (RALES) Circulation. 2000;102:2700–2706. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 8.Pitt B, Bakris G, Ruilope LM, Carlo LD. Serum potassium and clinical outcomes in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) Circulation. 2008;118:1643–1650. doi: 10.1161/CIRCULATIONAHA.108.778811. [DOI] [PubMed] [Google Scholar]
- 9.Adamopoulos C, Ahmed A, Fay R, Angioi M. Timing of eplerenone initiation and outcomes in patients with heart failure after myocardial infarction complicated by left ventricular systolic dysfunction: insights from the EPHESUS trial. Eur J Heart Fail. 2009;11:1099–1105. doi: 10.1093/eurjhf/hfp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction (EPHESUS) N Engl J Med. 2003;14:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 11.Zannad F, McMurray JJ, Drexler H, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pitt B. Rationale and design of the Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure (EMPHASIS-HF) Eur J Heart Fail. 2010;12:617–622. doi: 10.1093/eurjhf/hfq049. [DOI] [PubMed] [Google Scholar]
- 12.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 13.Boccanelli A, Mureddu GF, Cacciatore G, Clemenza F, Lenard AD. Anti-remodelling effect of canrenone in patients with mild chronic heart failure (AREA IN-CHF study): final results. Eur J Heart Fail. 2009;11:68–76. doi: 10.1093/eurjhf/hfn015. [DOI] [PubMed] [Google Scholar]
- 14.de Simone G, Chinali M, Mureddu GF, Cacciatore G, Lucci D, Latini R, Masson S, Vanasia M, Maggioni AP, Boccanelli A. Effect of canrenone on left ventricular mechanics in patients with mild systolic heart failure and metabolic syndrome: the AREA-in-CHF study. Nutr Metab Cardiovasc Dis. 2011;21:783–791. doi: 10.1016/j.numecd.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Udelson JE, Feldman AM, Greenberg B, Pitt B, Mukherjee R, Solomon HA, Konstam MA. Randomized, double-blind, multicenter, placebo-controlled study evaluating the effect of aldosterone antagonism with eplerenone on ventricular remodeling in patients with mild-to-moderate heart failure and left ventricular systolic dysfunction. Circ Heart Fail. 2010;3:347–353. doi: 10.1161/CIRCHEARTFAILURE.109.906909. [DOI] [PubMed] [Google Scholar]
- 16.Vizzardi E, D'Aloia A, Giubbini R, Bordonali T, Bugatti S, Pezzali N, Romeo A, Dei Cas A, Metra M, Dei Cas L. Effect of spironolactone on left ventricular ejection fraction and volumes in patients with class I or II heart failure. Am J Cardiol. 2010;106:1292–1296. doi: 10.1016/j.amjcard.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 17.Feola M, Menardi E. Effects of the addition of a low dose of spironolactone on brain natriuretic peptide plasma level and cardiopulmonary function in patients with moderate congestive heart failure. Med Sci Monit. 2003;9:341–344. [PubMed] [Google Scholar]
- 18.Chan AKY, Sanderson JE, Wang T. Aldosterone receptor antagonism induces reverse remodeling when added to angiotensin receptor blockade in chronic heart failure. J Am Coll Cardiol. 2007;7:591–596. doi: 10.1016/j.jacc.2007.03.062. [DOI] [PubMed] [Google Scholar]
- 19.Berry C, Murphy NF, De Vito G, Galloway S, Seed A, Fishe C, Sattar N, Vallance P, Hillis WS, McMurray J. Effects of aldosterone receptor blockade in patients with mild-moderate heart failure taking a beta-blocker. Eur J Heart Fail. 2007;9:429–434. doi: 10.1016/j.ejheart.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Cicoira M, Rossi A, Bonapace S. Independent and additional prognostic value of aminoterminal propeptide of type III procollagen circulating levels in patients with chronic heart failure. J Card Fail. 2004;10:403–411. doi: 10.1016/j.cardfail.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Schepkens H, Vanholder R, Billiouw JM, Lamiere N. Life-threatening hyperkalaemia during combined therapy with angiotensin-converting enzyme inhibitors and spironolactone: an analysis of 25 cases. Am J Med. 2001;110:438–441. doi: 10.1016/s0002-9343(01)00642-8. [DOI] [PubMed] [Google Scholar]
- 22.Berry C, McMurray JJV. Serious adverse events experienced by patients with chronic heart failure taking spironolactone. Heart. 2001;85:8–9. doi: 10.1136/heart.85.4.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masoudi FA, Gross CP. Adoption of spironolactone therapy for older patients with heart failure and left ventricular systolic dysfunction in the United States. Circulation. 2010;104:527–549. doi: 10.1161/CIRCULATIONAHA.104.527549. [DOI] [PubMed] [Google Scholar]
- 24.Skvortsov AA, Mareev VY, Chelmakina SM, Baklanova NA, Belenkov IN. Efficacy and safety of long-term application of spironolactone in patients with moderate and severe chronic heart failure receiving optimal therapy. Kardiologiia. 2007;10:12–23. [PubMed] [Google Scholar]
- 25.Lopes R, Lourenco P, Mascarenhas J, Azevedo A, Bettencourt P. Is spironolactone therapy feasible in heart failure patients with chronic renal failure? Eur J Heart Fail. 2009;2:113–124. [Google Scholar]