Abstract
Aims
Vitamin D deficiency is prevalent in HIV-infected patients and has been associated with osteopenia and HIV disease progression. Our aims were to investigate the pharmacokinetics of 25-hydroxycholecalciferol [25(OH)D], the effect of antiretroviral treatment (ARV) and others factors that may influence the pharmacokinetics, and to determine a vitamin D3 dosing scheme to reach the 30 ng ml−1 threshold (defined as 25(OH)D sufficiency).
Methods
This monocentric retrospective study included 422 HIV-infected patients aged 16 to 85 years. A total of 723 25(OH)D concentrations were available for pharmacokinetic evaluation and a population pharmacokinetic model was developed with MONOLIX 3.2.
Results
Median 25(OH)D at baseline was 16 ng ml−1 (interquartile range 11–23 ng ml−1) for the total population, 17% of patient had concentrations below 10 ng ml−1, 68% between 10 and 30 ng ml−1 and 15% above 30 ng ml−1. 25(OH)D pharmacokinetics were best described by a one compartment model with an additional endogenous production. The effects of season and skin phototype were significant on production rate. The endogenous production was 20% lower in non-white skin phototype patients and was decreased by 16% during autumn, winter and spring. No significant differences in 25(OH)D concentrations were related to antiretroviral drugs (ARV). To obtain concentrations between 30 and 80 ng ml−1, the dosing recommendation was 100 000 IU every month.
Conclusions
Season and skin phototype had an influence on the endogenous production of 25(OH)D. However no effect of ARV was found. A dosing scheme to reach sufficient 25(OH)D concentrations is proposed.
Keywords: 25-hydroxycholecalciferol, HIV-infected adults patients, population pharmacokinetics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Prevalence of vitamin D deficiency in HIV-infected adult patients has been reported in many studies and shown to be particularly high in this population. Vitamin D deficiency has been shown to be related to a higher risk of mortality and HIV-disease progression.
WHAT THIS STUDY ADDS
This is the first population pharmacokinetics analysis of 25-hydroxycholecalciferol (25(OH)D) in HIV-infected adult patients. Season and skin phototype appear to be the main covariates which influence 25(OH)D concentrations. Thus a vitamin D3 supplementation scheme taking into account these covariates is proposed to reach sufficient 25(OH)D concentrations in HIV-infected adults.
Introduction
The concentration of 25-hydroxycholecalciferol [25(OH)D, calcidiol], reflects vitamin D status, and a cut off of 30 ng ml−1 has been advised to reduce the risk of peripheral fractures, osteopenia and rickets [1–4]. There is no consensus about the vitamin D deficiency cut off, since it could vary from 10 to 20 ng ml−1 [1, 5, 6], whereas vitamin D insufficiency is usually defined as a concentration of less than 30 ng ml−1 [3, 6, 7]. Vitamin D toxicity has been reported only for concentrations above 150 ng ml−1, however the expert panel recommends an upper safety limit of 100 ng ml−1 [3, 8, 9]. Vitamin D is essentially provided by sunlight exposure and to a much lesser extent by food. It has been shown that some factors influence endogenous production such as skin phototype and season. Calcidiol deficiency has been epidemiologically associated with cardiovascular and autoimmune diseases and increased risk of renal failure, cancer and diabetes [3].
Vitamin D deficiency is prevalent among the HIV-infected population [10–17]. Several studies have suggested that 25(OH)D concentrations were not different in HIV-1 infected patients and in the general population [10, 18, 19]. However, 25(OH)D and 1,25-dihydroxycholecalciferol synthesis depends upon complex metabolic pathways in the liver and kidney, respectively [20, 21], and antiretroviral drugs (ARV), i.e. non-nucleosidic reverse transcriptase inhibitors (NNRTI) and protease inhibitors (PI) are known to induce or inhibit CYP P450 enzymes, which could alter vitamin D metabolism [13, 22–24]. The use of efavirenz, a NNRTI, has frequently been proposed as an independent risk factor for lower 25(OH)D concentrations [10, 25–28]. Regarding PIs, one study suggested a protective effect of ritonavir against vitamin D deficiency [10], but most recent papers did not find an association between PIs and vitamin D deficiency [25, 27, 29]. The use of tenofovir, a nucleotide reverse transcriptase inhibitor is associated with an increase in parathyroid hormone concentrations [10, 13, 30]. Furthermore, vitamin D deficiency has been associated with disease progression, complications and all-cause mortality in persons living with HIV [11]. By contrast, hyperlipidaemia is frequent in HIV-infected patients and several studies performed in the general population have shown an increase in 25(OH)D concentrations following the start of some statin drugs (mainly rosuvastatin and atorvastatin) [31–35].
Consequently, the issue of vitamin D3 supplementation in HIV-infected patients is now discussed. The aims of this study were (i) to investigate the population pharmacokinetics of 25(OH)D in HIV-1-infected adult patients, (ii) to investigate the factors that influence 25(OH)D pharmacokinetics in this population and (iii) and to propose a dosing recommendation in order to reach the 25(OH)D target of 30–150 ng ml−1 in a maximum number of patients.
Methods
Patients
Data were retrospectively collected from the electronic files of adult ARV-exposed HIV-1-infected patients treated at the Hôtel-Dieu hospital from 2009 to 2011. Ethics committee approval and patient consent are not required in France in order to use retrospectively therapeutic drug monitoring data, so no informed consent had to be collected. Age, bodyweight (BW), body mass index (BMI), skin phototype, CD4 T cell count, nadir CD4 T cell count, viral load, centres for disease control and prevention (CDC)stage, ARV drugs and statin therapy were recorded at each follow-up visit. Based on statements by the patients that they did not receive any supplementation during the previous year, a 25(OH)D value was collected before the initiation of vitamin D3 to assess the basal concentration. For the entire study, the median (min–max) number of samples per patient was 1 (1–6) and for patients receiving vitamin D3 supplementation the median (min–max) number of samples per patient was 3 (2–6).
Analytical method
Serum 25(OH)D concentrations were measured on real-time (as part of routine blood testing) using the DiaSorin RIA method (Saluggia, Italy). The detection limit was 3 ng ml−1. The measurements were done for all patients in the same laboratory hospital. Vitamin D deficiency was defined as serum 25(OH)D concentrations below 10 ng ml−1, vitamin D insufficiency as concentrations between 10 and 30 ng ml−1 and vitamin D toxicity as concentrations above 150 ng ml−1.
Modelling strategy and data analysis
Different structural models for 25(OH)D pharmacokinetics were investigated: one or two compartments with linear elimination and first order or zero order absorption, with or without a lag time or a transit compartment for absorption. Because vitamin D3 supplementation was exclusively given by the oral route, clearance (CL) and volume of distribution (V) are apparent parameters, V/F and CL/F, where F is the unknown bioavailability fraction.
Data were analyzed using the nonlinear mixed effect modelling software program Monolix version 3.2 (http://wfn.software.monolix.org) [36]. Parameters were estimated by computing the maximum likelihood estimator of the parameters without any approximation of the model (no linearization) using the stochastic approximation expectation maximization (SAEM) algorithm combined with a Markov Chain Monte Carlo (MCMC) procedure. The number of MCMC chains was fixed to 1 for all estimations. Several error models (proportional, additive or mixed) were investigated to describe the residual variability (ε). The between-subject variabilities (η or BSVs) were assumed to be exponential. The Likelihood Ratio Test (LRT) including the log-likelihood, the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) were used to test different hypotheses regarding the final model, covariate effect(s) on pharmacokinetic parameter(s), residual variability model (proportional vs. proportional plus additive error model), and structure of the variance–covariance matrix for the BSV parameters.
The main covariates of interest in the population were age, gender, skin phototype, seasons, body weight (BW), body mass index (BMI) and co-medications. Fat-free mass (FFM), lean body weight (LBW) and fat mass (FM) were calculated according to the Green & Duffull paper [37]. The effect of each patient covariate was systematically tested via the LRT. Continuous covariates (X): age, BW, BMI, FFM, LBW, FM, CD4 T cell count or viral load were tested according to the following equation, using CL for example,
where θCL is the typical value of clearance for a patient with the median covariate value and 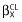 is the estimated influential factor for the continuous covariate. Binary covariates (CAT), skin phototype, gender, statin therapy, antiretroviral family or combined antiretroviral therapies were tested according to the following equation,
is the estimated influential factor for the continuous covariate. Binary covariates (CAT), skin phototype, gender, statin therapy, antiretroviral family or combined antiretroviral therapies were tested according to the following equation,
where  is the estimated influential factor for the binary covariate, CAT = 0 stands for the reference θCL value and CAT = 1 for the CL value in presence of the covariate.
is the estimated influential factor for the binary covariate, CAT = 0 stands for the reference θCL value and CAT = 1 for the CL value in presence of the covariate.
The effects of ARV were investigated as a binary covariate respectively for PI, NNRTI, EFV and TDF. Combined therapies were also tested as a binary covariate respectively for PI + TDF and NNRTI + TDF. The comparison of the effects of different antiretroviral therapies was investigated as a categorical covariate with three different classes for PI, NNRTI and other.
Seasons were firstly tested as four different groups, then non-significantly different groups were combined as long as the BIC value did not increase.
A covariate was finally retained if (i) its effect was biologically plausible, (ii), a reduction in AIC/BIC criteria was observed (LRT) and (iii) it produced a reduction in the variability of the pharmacokinetic parameter, assessed by the associated inter-subject variability. Graphical evaluation of the goodness-of-fit was mainly assessed by observed vs. predicted concentrations (PRED-DV) and weighted residuals vs. time and/or weighted residuals vs. PRED. The final population model was mainly appreciated by the normalized prediction distribution errors (NPDE) metrics [38] and the prediction-corrected visual predictive check (PC VPC) [39]. Diagnostic graphics and distribution statistics were obtained using RfM (link on http://wfn.sourceforge.net) via the R program [40].
Dose simulations
Dose simulations were performed in order to obtain 25(OH)D concentrations between 30 and 80 ng ml−1 during a 12 months follow-up according to the patient 25(OH)D baseline concentrations, i.e. <10 ng ml−1, 10 to 20 ng ml−1 or 20 to 30 ng ml−1. More precisely, the vector of pharmacokinetic parameters from 3000 (proportion of patients in and out the defined thresholds) or 400 (concentration−time profile for 12 months supplementation) replicates of the database was simulated using the final model, including the covariates. Each vector parameter was drawn in a log-normal distribution with a variance corresponding to the BSV previously estimated. A simulated residual error was added to each simulated concentration. The simulations were performed using Monolix.
Results
Demographic data
Data from 422 HIV-1 patients (290 men, 132 women) were collected for pharmacokinetic assessment. The median age was 46 years (range 16–85 years) and the median body weight was 70 kg (range 41–112 kg). A total of 288 patients had a white skin phototype and 134 had a non-white skin phototype. A total of 723 25(OH)D concentrations were available for pharmacokinetic evaluation. Among the 422 patients, 135 received vitamin D3 supplementation with a median (min–max) number of samples per patient of 3 (2–6) and a median prescribed dose of 63 302 (15 508–242 063) IU month−1. The mean follow-up time for the 135 adults (90 men, 45 women) was 11.6 months (range 2–31.7, median 11 months). At baseline 288 patients (68% of the total population) received tenofovir, 127 efavirenz (30%), 59 nevirapine or etravirine (14%), 209 were treated with a PI (50%), 13 with other ARV (3.0%) and 14 patients (3.3%) received no ARV. At baseline 93 patients (22%) received statin therapy and for 37 patients the information was missing (9%). Median 25(OH)D at baseline was 16 ng ml−1 (interquartile range 11–23 ng ml−1) for the total population, 17% of patients had concentrations below 10 ng ml−1, 68% between 10 and 30 ng ml−1 and 15% above 30 ng ml−1. Table 1 summarizes the patients' baseline characteristics.
Table 1.
Characteristics of the 422 patients at baseline
| Baseline | 25(OH)D <10 ng ml−1 | 25(OH)D 10–30 ng ml−1 | 25(OH)D >30 ng ml−1 | All patients |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Gender | ||||
| Male | 46 (16) | 195 (67) | 49 (17) | 290 (69) |
| Female | 25 (19) | 93 (70) | 14 (11) | 132 (31) |
| Ethnic origin | ||||
| White | 44 (15) | 189 (66) | 55 (19) | 288 (68) |
| Other | 27 (20) | 99 (74) | 8 (6) | 134 (32) |
| Season | ||||
| Spring | 31 (22) | 98 (68) | 14 (10) | 143 (34) |
| Summer | 2 (2) | 56 (67) | 26 (31) | 84 (20) |
| Autumn | 15 (16) | 66 (70) | 13 (14) | 94 (22) |
| Winter | 23 (23) | 68 (67) | 10 (10) | 101 (24) |
| Viral load (< 50 copies ml−1) | 54 (16) | 233 (68) | 56 (16) | 343 (81) |
| ARV drug | ||||
| EFV | 24 (19) | 85 (67) | 18 (14) | 127 (30) |
| Others NNRTIs | 10 (17) | 40 (68) | 9 (15) | 59 (14) |
| PI | 31 (15) | 141 (67) | 37 (18) | 209 (50) |
| TDF | 52 (18) | 187 (65) | 49 (17) | 288 (68) |
| No treatment | 2 (14) | 10 (72) | 2 (14) | 14 (3) |
| Combined ARV drug | ||||
| PI + TDF | 21 (16) | 82 (63) | 27 (21) | 130 (31) |
| NNRTI + TDF | 27 (19) | 89 (64) | 24 (17) | 140 (33) |
| Statin treatment | ||||
| Rosuvastatin | 12 (15) | 55 (71) | 11 (14) | 78 (18) |
| Pravastatin | 3 (30) | 6 (60) | 1 (10) | 10 (2) |
| Atorvastatin | 0 | 4 (100) | 0 | 4 (1) |
| Fluvastatin | 1 (100) | 0 | 0 | 1 (0) |
| NA | 6 (16) | 26 (70) | 5 (14) | 37 (9) |
| 25(OH)D (ng ml−1) | ||||
| Median (IQR) | 7 (6–8) | 16 (13–21) | 36 (33–39) | 16 (11–23) |
| Age (years) | ||||
| Median (IQR) | 45 (37–50) | 46 (39–53) | 47 (43–54) | 46 (38–52) |
| Bodyweight (kg) | ||||
| Median (IQR) | 67 (61–79) | 70 (62–81) | 70 (62–78) | 70 (61–80) |
| BMI (kg m−12) | ||||
| Median (IQR) | 23 (20–26) | 23 (21–26) | 23 (21–25) | 23 (21–26) |
| Viral load (log10 copies ml−1) | ||||
| Median (IQR) | 1.7 (1.7-1.7) | 1.6 (1.7-1.7) | 1.6 (1.7-1.7) | 1.6 (1.7-1.7) |
| CD4 (cells mm−3) | ||||
| Median (IQR) | 544 (364–829) | 619 (422–877) | 690 (560–860) | 628 (427–874) |
Population pharmacokinetics
The data were best described by a one compartment model in which the absorption and elimination rate constants were equal. In this model, the dose supplementation, D, is absorbed into the compartment with a constant rate of absorption, whereas the basal concentration of 25(OH)D, reflecting the endogenous production from diet and sun exposure, is described by the parameter C0. The 25(OH)D pharmacokinetic parameters were then the apparent volume of distribution (V/F) and elimination clearance (CL/F) where F is the unknown bioavailability, and C0. Between-subject variability could be estimated for apparent CL and the basal concentration. A proportional model was used to describe the residual variability. The parameter estimates of this basic model were CL/F 2.4 l day−1 (BSV 0.36), V 176 l and C0 16.4 μg l−1 (BSV 0.46).
In a second step, the skin phototype covariate was included in the model on the C0 parameter. This improved the predictive performance of the model and significantly decreased the variability on production from 0.461 to 0.442 and the BIC criteria from 5169 to 5152. In a third step, the season effect coded as summer vs. autumn, winter and spring (non-summer) was also added on the C0 parameter, decreasing the BIC criteria by 8.3 units and the corresponding variability from 0.442 to 0.421. No other covariate effect could be identified on CL/F or C0 parameters. No effect of ARV therapy was found. The mean C0 concentrations among patients taking PIs compared with concentrations in patients not taking PIs were 18.6 vs. 17.2 ng ml−1 (P = 0.14, Wilcoxon Mann-Whitney test). Similarly, for NNRTIs, EFV and TDF these were 17.7 vs. 18.1 ng ml−1 (P = 0.74), 17.5 vs. 18.0 ng ml−1 (P = 0.44) and 18.3 vs. 17.0 ng ml−1 (P = 0.14), respectively.
Table 2 summarizes the final population pharmacokinetic estimates. All parameters were well estimated with relative standard errors (%RSE) lower than 15%. The C0 concentrations were then 20.5 ng ml−1 for a white skin phototype patient in summer. The non-white skin phototype effect had a decreased C0 value, 16.5 ng ml−1 in summer. The difference associated with summer vs. non-summer was for a white adult 17.2 ng ml−1 and 13.8 ng ml−1 for a non white adult. The normalized prediction distribution error performed on the final model showed that the mean and variance were not significantly different from 0 (P = 0.87) and 1 (P = 0.27) and their distribution was not different from a normal one (P = 0.08) (Figure 1). The prediction-corrected visual predictive check shows that the 10th, 50th and 90th percentiles of observed data were well included within the 90% CI of the 10th, 50th and 90th of simulated percentiles (Figure 2).
Table 2.
Population pharmacokinetic parameters of 25(OH)D in 422 patients
| Parameter | Estimate (RSE %) |
|---|---|
| Structural model | |
| CL/F (l day−1) | 2.76 (5) |
| V/F (l) | 178 (5) |
| C0 (ng ml−1)** | 20.5 (7) |
| θNo white | 0.804 (5) |
| θAutumn, spring, winter | 0.839 (4) |
| Statistical model | |
| ω BSV CL/F | 0.35 (15) |
| ω BSV C0 | 0.42 (12) |
| σ proportional | 0.262 (5) |
RSE%, relative standard error (standard error of estimate/estimate × 100); CL/F, apparent elimination clearance; V/F, apparent central volume of distribution; C0, basal 25(OH)D concentration; σ, residual variability estimates and BSV, between-subject variability estimates; θNo white, influential factor on 25(OH)D C0 for a non-white skin phototype patient; θAutumn, spring, winter, influential factor on 25(OH)D C0 for non-summer.
C0 = C0 × (θNo white) × θAutumn, spring, winter). For example, for a non-white patient during winter, mean C0 is 20.5 × 804 × 8.39 = 13.8 ng ml−1.
Figure 1.
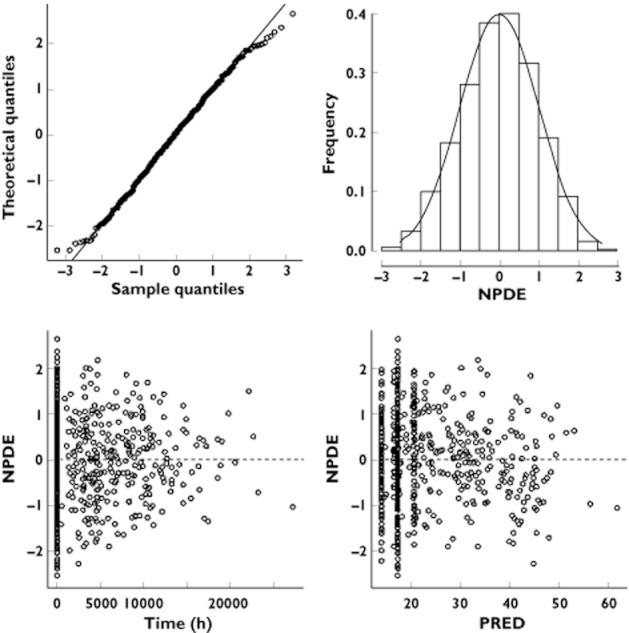
Normalized prediction distribution errors metrics (NPDE)
Figure 2.
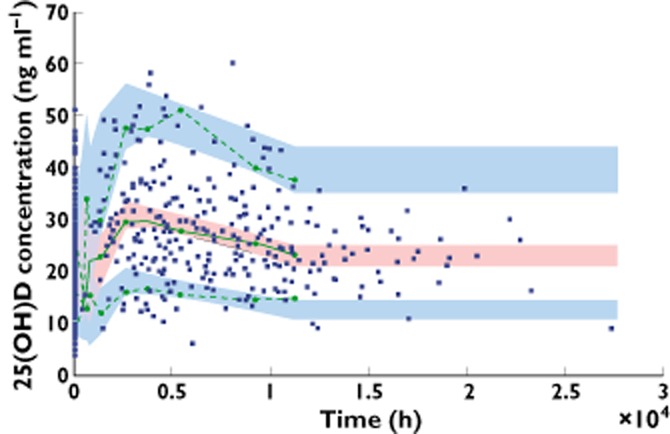
PC VPC (prediction corrected visual predictive check) for 25(OH)D concentrations vs. time. The green lines show the 10th, 50th and 90th percentile of observed data; the areas represent the 90% CI around the simulated percentiles
Dose simulation
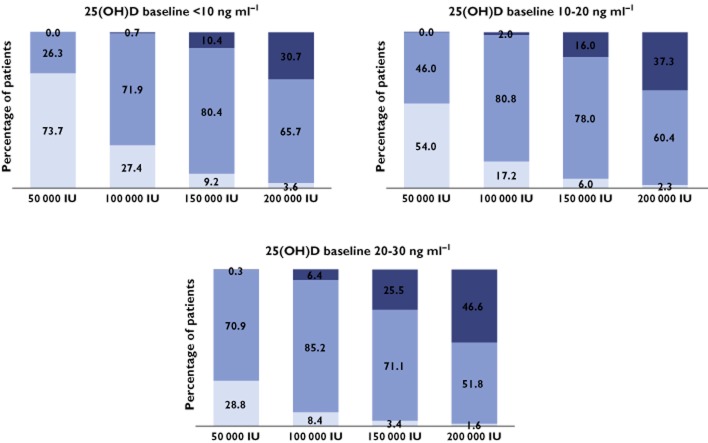
According to the patients’ baseline concentration (<10, between 10 and 20, or between 20 and 30 ng ml−1), different dosing schemes were simulated to obtain 25(OH)D concentrations between 30 and 80 ng ml−1 for 1 year. Figure 3 shows the percentage of patients with 25(OH)D concentrations lower than 30 ng ml−1, between 30 and 80 ng ml−1 (target interval) and higher than 80 ng ml−1 after 12 months of treatment following 50 000, 100 000, 150 000 or 200 000 IU month−1. In order to avoid risk of toxicity, dosing simulations which provided less than 10% of patients above 80 ng ml−1 were retained. Accordingly the dose of 100 000 IU of vitamin D3 month−1 seems to be the more appropriate. Figure 4 shows the final dosing recommendation derived from 400 simulations of the final model. As shown, 4 months after the start of the supplementation, more than 50% of patients reached the 30 ng ml−1 target. The median 25(OH)D (90% CI) concentration was 31.1 ng ml−1 (16, 51). At the end of the first year of treatment the median predicted 25(OH)D concentration (90% CI) was 43.9 ng ml−1 (22, 69).
Figure 3.
After 12 months of treatment, percentage of patients with 25(OH)D concentration lower than 30 ng ml−1, between 30 and 80 ng ml−1 (target interval) and higher than 80 ng ml−1 (related to toxicity); simulations divided in three groups, according to 25(OH)D baseline concentration.  , <30 ng ml−1;
, <30 ng ml−1;  , 30–80 ng ml−1;
, 30–80 ng ml−1;  , >80 ng ml−1
, >80 ng ml−1
Figure 4.
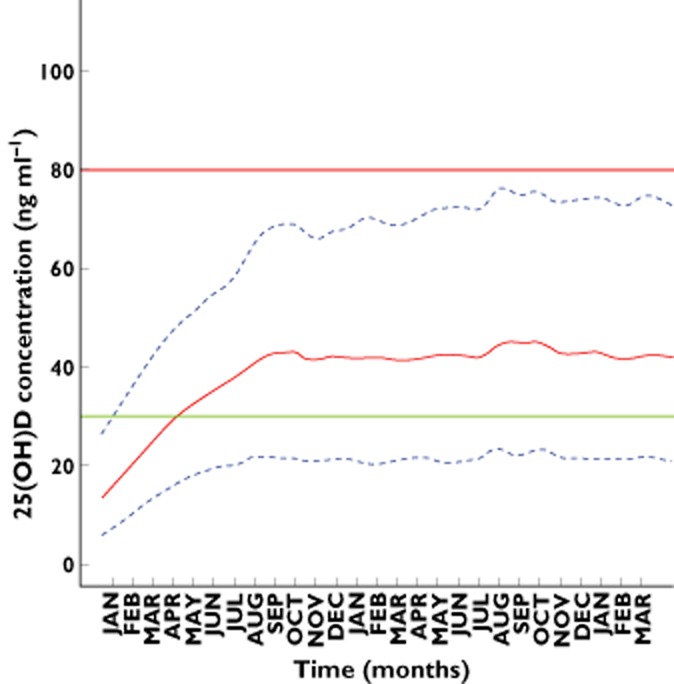
Calculated 25(OH)D concentrations (full line) and 90% CI (dashed lines) vs. time according to the dosing recommendation of this study. The horizontal green and thick red line represent the 30 and 80 ng ml−1 25(OH)D targets for efficacy and toxicity respectively
Discussion
The pharmacokinetics of 25(OH)D were satisfactorily described by a one compartment model. A more physiological model would have considered that vitamin D3 is firstly absorbed towards a storage compartment (mainly adipose tissue) from which it is released before 25(OH)D is formed then cleared. However, the five parameters related to this model could not be identified, probably in part because the absorption rate is very fast relative to the formation and elimination rates, the exponential terms with 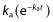 are rapidly close to zero and negligible as compared with the other terms. Therefore, the reduction to a one compartment model is reasonable considering the input as the absorption-formation rate of 25(OH)D (vitamin D3 was considered to be immediately absorbed by the gut in regard of the long half life of 25(OH)D). The classical one compartment model with first order absorption gave close values for the formation (input) and elimination rates, resulting in a flip-flop phenomenon on individual parameters. Thus it was reasonable to fit the data to a model in which these two rate constants were equal. This model was finally retained also on the basis of statistical significance, the BIC decreased by 4.9 units and the RSEs of the estimates were improved.
are rapidly close to zero and negligible as compared with the other terms. Therefore, the reduction to a one compartment model is reasonable considering the input as the absorption-formation rate of 25(OH)D (vitamin D3 was considered to be immediately absorbed by the gut in regard of the long half life of 25(OH)D). The classical one compartment model with first order absorption gave close values for the formation (input) and elimination rates, resulting in a flip-flop phenomenon on individual parameters. Thus it was reasonable to fit the data to a model in which these two rate constants were equal. This model was finally retained also on the basis of statistical significance, the BIC decreased by 4.9 units and the RSEs of the estimates were improved.
An additional basal level parameter represents the endogenous formation of calcidiol provided by food and sunlight exposure. Skin phototype and summer were included in the model on the C0 parameter, decreasing the corresponding BSV. The endogenous production was 20% lower in non-white patients and was decreased by 16% during the autumn, winter and spring. Calcidiol half-life was estimated to be around 40 days which is in agreement with previously reported values, in the range of 15 days to 2 months [5, 41–43].
The antiretroviral treatment status, EFV, NNRTI, TDF or PI use, was systematically tested on both CL/F and C0 parameters. No significant differences were observed. Combined ARV, use of statin, gender, age, viral load, CD4 T cell count, BMI, LBW, FFM, FM and BW did not influence 25(OH)D pharmacokinetics.
Calcidiol insufficiency and deficiency were highly prevalent in these HIV-1 infected patients at baseline: 17% of the patients had a 25(OH)D concentration below 10 ng ml−1, 68% between 10 and 30 ng ml−1 and only 15% above 30 ng ml−1, as previously reported [10, 12, 13, 27]. Due to the possible deleterious effects on HIV disease expected from severe vitamin D deficiency, the question of supplementation is emerging. Therefore, a dosing scheme was optimized in order to obtain 25(OH)D concentrations between 30 and 80 ng ml−1 without exceeding the 150 ng ml−1 toxic value in most patients [8, 9]. A monthly vitamin D3 dosing was chosen in regard to the main available commercial form in France and in order to improve compliance [44]. Dosing simulations were performed according to season and skin phototype effects and did not result in different recommendations between summer vs. non summer or white vs. non-white skin phototype. The recommended dietary allowances in the general population according to Health Canada or the United States Institute of Medicine are of 600 IU day−1 but these recommendations led to vitamin D insufficiency or deficiency in a large part of the general population 45. They also define the tolerable upper limit as 4000 UI day−1. Our dosing recommendation of 100 000 IU month−1 in this HIV population corresponds to 3300 IU day−1, which is in accordance with this tolerable upper limit.
In conclusion, the dosing recommendation was 100 000 IU every month. This dosing scheme resulted in less than 1% of patients with concentrations above 100 ng ml−1 and was in agreement with previously published studies [46].
Acknowledgments
The authors thank the clinical research team of the immunoinfectiology unit of the Hôtel-Dieu hospital (Agnès Cros, Aline Maignan) for patients' file management and data quality control. We acknowledge the Paediatric European Network Treatment AIDS Laboratory Network (PENTA LABNET) for their financial support.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, Norman AW, Scragg R, Whiting SJ, Willett WC, Zittermann A. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 3.Souberbielle J-C, Body J-J, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ, Bischoff-Ferrari HA, Cavalier E, Ebeling PR, Fardellone P, Gandini S, Gruson D, Guérin AP, Heickendorff L, Hollis BW, Ish-Shalom S, Jean G, von Landenberg P, Largura A, Olsson T, Pierrot-Deseilligny C, Pilz S, Tincani A, Valcour A, Zittermann A. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: recommendations for clinical practice. Autoimmun Rev. 2010;9:709–715. doi: 10.1016/j.autrev.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 5.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–586. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 9.Shinchuk L, Holick MF. Vitamin D and rehabilitation: improving functional outcomes. Nutr Clin Pract. 2007;22:297–304. doi: 10.1177/0115426507022003297. [DOI] [PubMed] [Google Scholar]
- 10.Dao CN, Patel P, Overton ET, Rhame F, Pals SL, Johnson C, Bush T, Brooks JT. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D Levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 11.Viard J-P, Souberbielle J-C, Kirk O, Reekie J, Knysz B, Losso M, Gatell J, Pedersen C, Bogner JR, Lundgren JD, Mocroft A. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25:1305–1315. doi: 10.1097/QAD.0b013e328347f6f7. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Gandhi V, Psevdos G, Espinoza F, Park J, Sharp V. Evaluation of Vitamin D Levels among HIV-Infected Patients in New York City. AIDS Research and Human Retroviruses [Internet]. Available at http://www.ncbi.nlm.nih.gov/pubmed/21644847 (last accessed 4 December 2012)
- 13.Mueller NJ, Fux CA, Ledergerber B, Elzi L, Schmid P, Dang T, Magenta L, Calmy A, Vergopoulos A, Bischoff-Ferrari HA. High prevalence of severe vitamin D deficiency in combined antiretroviral therapy-naive and successfully treated Swiss HIV patients. AIDS. 2010;24:1127–1134. doi: 10.1097/QAD.0b013e328337b161. [DOI] [PubMed] [Google Scholar]
- 14.Van Den Bout-Van Den Beukel CJ, Fievez L, Michels M, Sweep FC, Hermus AR, Bosch ME, Burger DM, Bravenboer B, Koopmans PP, Van Der Ven AJ. Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: effects of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:1375–1382. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez M, Daniels B, Gunawardene S, Robbins GK. High frequency of vitamin D deficiency in ambulatory HIV-positive patients. AIDS Res Hum Retroviruses. 2009;25:9–14. doi: 10.1089/aid.2008.0183. [DOI] [PubMed] [Google Scholar]
- 16.Bang UC, Shakar SA, Hitz MF, Jespersen MS, Andersen O, Nielsen SD, Jensen JE. Deficiency of 25-hydroxyvitamin D in male HIV-positive patients: a descriptive cross-sectional study. Scand J Infect Dis. 2010;42:306–310. doi: 10.3109/00365540903463981. [DOI] [PubMed] [Google Scholar]
- 17.Stein EM, Yin MT, McMahon DJ, Shu A, Zhang CA, Ferris DC, Colon I, Dobkin JF, Hammer SM, Shane E. Vitamin D deficiency in HIV-infected postmenopausal Hispanic and African-American women. Osteoporos Int. 2011;22:477–487. doi: 10.1007/s00198-010-1299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milazzo L, Mazzali C, Bestetti G, Longhi E, Foschi A, Viola A, Vago T, Galli M, Parravicini C, Antinori S. Liver-related factors associated with low vitamin D levels in HIV and HIV/HCV coinfected patients and comparison to general population. Curr HIV Res. 2011;9:186–193. doi: 10.2174/157016211795945269. [DOI] [PubMed] [Google Scholar]
- 19.Vescini F, Cozzi-Lepri A, Borderi M, Re MC, Maggiolo F, De Luca A, Cassola G, Vullo V, Carosi G, Antinori A, Tozzi V, Monforte AD. Prevalence of hypovitaminosis D and factors associated with vitamin D deficiency and morbidity among HIV-infected patients enrolled in a large Italian cohort. J Acquir Immune Defic Syndr. 2011;58:163–172. doi: 10.1097/QAI.0b013e31822e57e9. [DOI] [PubMed] [Google Scholar]
- 20.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29:664–673. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Schuster I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys Acta. 2011;1814:186–199. doi: 10.1016/j.bbapap.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Pasquet A, Viget N, Ajana F, de la Tribonniere X, Dubus S, Paccou J, Legroux-Gérot I, Melliez H, Cortet B, Yazdanpanah Y. Vitamin D deficiency in HIV-infected patients: associated with non-nucleoside reverse transcriptase inhibitor or efavirenz use? AIDS. 2011;25:873–874. doi: 10.1097/QAD.0b013e32834542fa. [DOI] [PubMed] [Google Scholar]
- 23.Lattuada E, Lanzafame M, Zoppini G, Concia E, Vento S. No influence of nevirapine on vitamin D deficiency in HIV-infected patients. AIDS Res Hum Retroviruses. 2009;25:849–850. doi: 10.1089/aid.2009.0063. [DOI] [PubMed] [Google Scholar]
- 24.Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS. 2003;17:513–520. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]
- 25.Conesa-Botella A, Florence E, Lynen L, Colebunders R, Menten J, Moreno-Reyes R. Decrease of vitamin D concentration in patients with HIV infection on a non nucleoside reverse transcriptase inhibitor-containing regimen. AIDS Res Ther. 2010;7:40–46. doi: 10.1186/1742-6405-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox J, Peters B, Prakash M, Arribas J, Hill A, Moecklinghoff C. Improvement in vitamin D deficiency following antiretroviral regime change: results from the MONET trial. AIDS Res Hum Retroviruses. 2011;27:29–34. doi: 10.1089/aid.2010.0081. [DOI] [PubMed] [Google Scholar]
- 27.Allavena C, Delpierre C, Cuzin L, Rey D, Viget N, Bernard J, Guillot P, Duvivier C, Billaud E, Raffi F. High frequency of vitamin D deficiency in HIV-infected patients: effects of HIV-related factors and antiretroviral drugs. J Antimicrob Chemother. 2012;67:2222–2230. doi: 10.1093/jac/dks176. [DOI] [PubMed] [Google Scholar]
- 28.Cervero M, Agud JL, García-Lacalle C, Alcázar V, Torres R, Jusdado JJ, et al. Prevalence of vitamin D deficiency and its related risk factor in a spanish cohort of adult HIV-infected patients: effects of antiretroviral therapy. AIDS Res Hum Retroviruses. 2012;28:763–771. doi: 10.1089/AID.2011.0244. [DOI] [PubMed] [Google Scholar]
- 29.Kwan CK, Eckhardt B, Baghdadi J, Aberg JA. Hyperparathyroidism and complications associated with vitamin D deficiency in HIV-infected adults in New York City, New York. AIDS Res Hum Retroviruses. 2012;28:825–832. doi: 10.1089/aid.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Childs KE, Fishman SL, Constable C, Gutierrez JA, Wyatt CM, Dieterich DT, Mullen MP, Branch AD. Short communication: inadequate vitamin D exacerbates parathyroid hormone elevations in tenofovir users. AIDS Res Hum Retroviruses. 2010;26:855–859. doi: 10.1089/aid.2009.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aloia JF, Li-Ng M, Pollack S. Statins and Vitamin D. Am J Cardiol. 2007;100:1329. doi: 10.1016/j.amjcard.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pérez-Castrillón JL, Vega G, Abad L, Sanz A, Chaves J, Hernandez G, Dueñas A. Effects of atorvastatin on vitamin D levels in patients with acute ischemic heart disease. Am J Cardiol. 2007;99:903–905. doi: 10.1016/j.amjcard.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 33.Yavuz B, Ertugrul DT, Cil H, Ata N, Akin KO, Yalcin AA, Kucukazman M, Dal K, Hokkaomeroglu MS, Yavuz BB, Tutal E. Increased levels of 25 hydroxyvitamin D and 1,25-dihydroxyvitamin D after rosuvastatin treatment: a novel pleiotropic effect of statins? Cardiovasc Drugs Ther. 2009;23:295–299. doi: 10.1007/s10557-009-6181-8. [DOI] [PubMed] [Google Scholar]
- 34.Ertugrul DT, Yavuz B, Cil H, Ata N, Akin KO, Kucukazman M, Yalcin AA, Dal K, Yavuz BB, Tutal E. STATIN-D Study: comparison of the influences of rosuvastatin and fluvastatin treatment on the levels of 25 hydroxyvitamin D. Cardiovasc Ther. 2010;29:146–152. doi: 10.1111/j.1755-5922.2010.00141.x. [DOI] [PubMed] [Google Scholar]
- 35.Ware WR. The JUPITER lipid lowering trial and vitamin D: Is there a connection? Dermatoendocrinol. 2010;2:50–54. doi: 10.4161/derm.2.2.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhn E, Lavielle M. Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal. 2005;49:1020–1038. [Google Scholar]
- 37.Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58:119–133. doi: 10.1111/j.1365-2125.2004.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed. 2008;90:154–166. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Team R. others. R: A language and environment for statistical computing. R Foundation for Statistical Computing Vienna Austria. 01/19/2010.
- 41.Mawer EB, Schaefer K, Lumb GA, Stanbury SW. The metabolism of isotopically labelled vitamin D3 in man: the influence of the state of vitamin D nutrition. Clin Sci. 1971;40:39–53. doi: 10.1042/cs0400039. [DOI] [PubMed] [Google Scholar]
- 42.Vieth R. Vitamin D nutrition and its potential health benefits for bone, cancer and other conditions. J Nutr Environ Med. 2001;11:275–291. [Google Scholar]
- 43.Jones KS, Schoenmakers I, Bluck LJC, Ding S, Prentice A. Plasma appearance and disappearance of an oral dose of 25-hydroxyvitamin D2 in healthy adults. Br J Nutr. 2011;7:1–10. doi: 10.1017/S0007114511004132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93:3430–3435. doi: 10.1210/jc.2008-0241. [DOI] [PubMed] [Google Scholar]
- 45.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. 2011. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: National Academies Press. [PubMed]
- 46.Ilahi M, Armas LAG, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr. 2008;87:688–691. doi: 10.1093/ajcn/87.3.688. [DOI] [PubMed] [Google Scholar]