Abstract
Aims
No pharmacokinetic data exist on doses of ropivacaine larger than 300 mg for peripheral nerve block in man, although in clinical practice higher doses are frequently used. The purpose of the present study was to describe the pharmacokinetic profile in serum of 450 mg ropivacaine with and without epinephrine in patients undergoing anterior cruciate ligament reconstruction.
Methods
Twelve patients were randomly allocated to receive a single shot combined sciatic/femoral nerve block with 60 ml of either ropivacaine 0.75% alone (group R, n = 6) or ropivacaine 0.75% plus epinephrine 5 μg ml−1 (group RE, n = 6). Venous blood samples for total and free ropivacaine serum concentrations were obtained during 48 h following block placement. Pharmacokinetic parameters were calculated using a non-compartmental approach.
Results
Results are given as mean (SD) for group R vs. group RE (95% CI of the difference). Total Cmax was 2.81 (0.94) μg ml−1 vs. 2.16 (0.21) μg ml−1 (95% CI −0.23, 1.53). tmax was 1.17 (0.30) h vs. 1.67 (0.94) h (95% CI −1.40, 0.40). The highest free ropivacaine concentration per patient was 0.16 (0.08) μg ml−1 vs. 0.12 (0.04) μg ml−1 (95% CI −0.04, 0.12). t1/2 was 6.82 (2.26) h vs. 5.48 (1.69) h (95% CI −1.23, 3.91). AUC was 28.35 (5.92) μg ml−1 h vs. 29.12 (7.34) μg ml−1 h (95% CI −9.35, 7.81).
Conclusions
Free serum concentrations of ropivacaine with and without epinephrine remained well below the assumed threshold of 0.56 μg ml−1 for systemic toxicity. Changes in pharmacokinetics with epinephrine co-administration did not reach statistical significance.
Keywords: anaesthetic techniques, anaesthetics local, pharmacokinetics, regional, ropivacaine
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
For peripheral nerve blocks, larger doses of local anaesthetic than recommended are frequently used. In the absence of pharmacokinetic data, using higher than recommended doses may pose a medico-legal problem in cases of local anaesthetic systemic toxicity.
WHAT THIS STUDY ADDS
This is the only study describing the pharmacokinetic profile in serum of 450 mg ropivacaine with and without epinephrine for combined sciatic/femoral nerve block. Free serum concentrations of ropivacaine in both groups remained well below the assumed threshold of 0.56 μg ml−1 for systemic toxicity.
Introduction
In recent years, peripheral nerve block (PNB) has rapidly gained popularity as an anaesthetic technique for upper and lower extremity surgery. Compared with general anaesthesia or central neuraxis blockade, interference of PNB with vital functions is minimal and postoperative analgesia is excellent.
For lower extremity surgery, a combination of two or three PNBs is usually necessary, especially when surgery is performed under exsanguination and the use of a tourniquet.
With the combination of several PNBs, larger doses than recommended [1] are frequently used.
Despite a plea for abandoning the practice of stating blanket maximum recommended doses for local anaesthetics [2] and a widespread consensus that maximum recommended doses are not evidence-based, they continue to be mentioned in textbooks and by manufacturers. In the absence of pharmacokinetic data, using higher than recommended doses may pose a medico-legal problem in case of local anaesthetic systemic toxicity.
Ropivacaine is a long acting amide local anaesthetic. It is structurally closely related to bupivacaine, but has a better safety profile with regard to central nervous system and cardiotoxicity [3]. Unlike bupivacaine, which is a racemate, ropivacaine is a single S(–)-enantiomer [4].
Several authors advocate the addition of epinephrine to large doses of local anaesthetics in order to reduce the maximum concentration [1] or to act as a marker for intravascular injection [5]. However, the literature is inconclusive whether the addition of epinephrine 5 μg ml−1 (1:200 000) offers pharmacokinetic advantages over ropivacaine alone. Some studies did find an advantage [3, 6–8], whereas others did not [9, 10].
The purpose of the present study is to describe the pharmacokinetic profile in serum of 450 mg ropivacaine with and without epinephrine, in patients undergoing anterior cruciate ligament reconstruction under single shot combined sciatic/femoral nerve block.
Methods
Patients
This study was approved by the Independent Review Board Nijmegen and was registered at http://www.trialregister.nl (NTR1973) before onset of participant enrolment. Patients scheduled for anterior cruciate ligament repair were assessed for eligibility during the pre-operative screening visit. Patients were informed about the study verbally and in writing and written informed consent was obtained from all patients. The study was conducted at the Sint Maartenskliniek Nijmegen, the Netherlands according to the Declaration of Helsinki and later revisions thereof and in accordance with the ICH guidelines for Good Clinical Practice.
Twelve patients (aged 18 to 60 years, body weight >70 kg and ASA physical health classification I–III) were included. Exclusion criteria included contra-indications for regional anaesthesia (infection at the injection site, coagulopathy), known hypersensitivity to amide-type local anaesthetics, known history of peripheral neuropathy, known history of hepatic or renal insufficiency or use of fluvoxamine, ciprofloxacin, ketoconazole, erythromycin, clarithromycin, itraconazole or rifampicin because of their effect on ropivacaine clearance [1].
Anaesthetic procedure
Using a computer-generated sequence of random numbers and a sealed envelope technique, patients were randomly allocated to receive a combined sciatic/femoral nerve block with 60 ml of either ropivacaine 0.75% (Naropin® AstraZeneca Sweden) alone (group R, n = 6) or ropivacaine 0.75% plus 5 μg ml−1 (1:200 000) epinephrine (group RE, n = 6). Blinded syringes containing the appropriate study solution were prepared by an anaesthesia nurse not involved in the block procedure or the subsequent care of the patient. After establishing intravenous access and routine monitoring (ECG, non-invasive blood pressure and peripheral oxygen saturation), an indwelling venous catheter was placed in the contralateral fossa cubiti for venous blood sampling.
The sciatic nerve block was carried out with the patient in the lateral decubitus position using the parasacral approach [11], a 10 cm stimulating needle and a nerve stimulator. Upon request, the patients received mild sedation during the block procedure with 20 mg propofol i.v and 0.5 mg alfentanil i.v. With the nerve stimulator set to deliver 100 nC (1 mA, 0.1 ms) at 2 Hz, the sciatic nerve was located by either plantar or dorsiflexion of the foot. Needle position was optimized by reducing the current while maintaining the appropriate motor response, until the threshold was between 20 and 40 nC. After negative aspiration, 20 ml of local anaesthetic was injected over the course of 1 min in fractionated doses with intermittent aspiration after each 5 ml. Upon completion of the injection, time was designated as t = 0.
Immediately following the sciatic nerve block the patient was turned supine and the femoral nerve block was performed with a 10 cm stimulating needle under ultrasound guidance using a short-axis view and an in-plane technique. As an additional aid to identify the femoral nerve, the needle was connected to a nerve stimulator set to deliver 100 nC (1 mA, 0.1 ms) at 2 Hz. Upon correct needle position as confirmed by ultrasound visualization and a motor response of the quadriceps muscle and after negative aspiration, 40 ml of local anaesthetic was injected over the course of 1 min in fractionated doses with intermittent aspiration after each 5 ml. A dose of 40 ml was chosen because it is our experience that this higher dose is associated with a higher incidence of complete 3-in-1 block. The total dose of both injections adds up to 450 mg ropivacaine with or without 300 μg epinephrine.
Surgery was performed under regional anaesthesia. In case of patient discomfort or upon patient request, sedation was provided with propofol and remifentanil. There are no known interactions of the comedications used (propofol, alfentanil, remifentanil) with ropivacaine pharmacokinetics [4].
Clinical assessments
During the first 120 min, patients were monitored and observed continuously for signs of systemic local anaesthetic toxicity.
Sensory block was assessed 15 and 30 min after block placement in femoral and sciatic nerve supply areas. In case of inadequate anaesthesia, intravenous sedation with propofol (25–60 μg kg−1 min−1) and remifentanil (0.01–0.05 μg kg−1 min−1) was initiated before surgical incision.
Blood sampling and assays
Venous blood samples of 5 ml were taken by the investigator (KS) before the first injection of local anaesthetic (−0.25 h), and at times 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 2.5, 3, 3.5, 4, 6, 12, 18, 24, 36 and 48 h.
Samples were centrifuged within 3 h of collection. Serum samples were stored at −40°C until assay. After determining total ropivacaine concentrations in serum, unbound ropivacaine concentrations were determined in ultrafiltrate in three samples per patient: the sample with the highest total concentration as well as the samples taken immediately before and after. The highest concentration of these three measurements was taken as Cumax for each individual patient.
Analysis of ropivacaine in serum and in ultrafiltrate (free concentration) was performed by the Laboratory for Toxicology, Therapeutic Drug Monitoring and Pharmaceutical analysis of the Department of Hospital Pharmacy at the University Medical Center Groningen, Groningen, the Netherlands. A triple quadrupole Quantum LC/MS/MS system with a Surveyor MS pump and a Surveyor Plus autosampler (Thermo Scientific, Breda, the Netherlands) was used.
To obtain protein-free ultrafiltrate, 300 μl serum was added to a Centrifree ultrafiltration device (Millipore, Amsterdam, the Netherlands) and centrifuged at 1000 g in a 33° fixed angle centrifuge for 10 min.
A 10 μl aliquot of serum or ultrafiltrate was transferred into an autosampler vial and 750 μl precipitation reagent (methanol 160 ml l−1, ACN 840 ml l−1 and cyanoimipramine 0.04 mg l−1) with internal standard was added. The vials were then vortexed for 1 min and stored at −20°C for 30 min to promote protein precipitation. The vials were centrifuged at 11,000 g for 5 min and 5 μl of the clear upper layer was injected onto a 50 × 2.1 mm HyPURITY Aquastar C18 analytical column (Interscience, Breda, the Netherlands). The mobile phase consisted of a gradient mixture of an acid buffer pH = 3.5 (containing ammonium acetate 5 g l−1, acetic acid 35 mg l−1 and trifluoroacetic anhydride 2 ml l−1 of water), water and acetonitrile. Acetonitrile for LC-MS, trifluoroacetic anhydride for LC-MS and water for LC-MS were purchased by BioSolve (Valkenswaard, the Netherlands). Methanol Lichrosolv and formic acid were from Merck KGaA (Darmstadt, Germany). Acetic acid 100%, ammonium acetate and ammonium formate (98–100%) were from Acros Organics (Geel, Belgium). All reagents were of suitable analytical grade. Ultra pure water was obtained from a Milli-Q water purifying system (Millipore Corporation, Billerica, MA, USA). The flow rate was 300 μl min−1.
The mass selective detector was operated in electrospray-positive ionization mode and performed selected reaction monitoring. High purity nitrogen was used as the sheath gas and auxiliary gas, and argon was used as the collision gas. Sample analysis was performed using the following transitions: ropivacaine m/z 275.0 > 126.2 (collision energy 22 eV) and the internal standard (cyanoimipramine) m/z 306.2 > 218.0 (collision energy 39 eV).
The lower limit of quantification (LLOQ) of ropivacaine was set at 50 μg l−1 of serum (CV = 7.1%; n = 15) and 5 μg l−1 of ultrafiltrate (CV = 6.8%; n = 15).
Pharmacokinetic analysis
Because of extravascular administration of ropivacaine at two different injection sites, a non-compartmental approach was used for the description of the ropivacaine pharmacokinetic parameters using MWPharm® (Medi-Ware BV, Zuidhorn, the Netherlands) [12]. Peak serum concentrations (Cmax) and time to reach peak serum concentrations (tmax) were obtained directly from the measured serum concentration–time curves. The slope of the terminal log-linear portion of the serum concentration–time curve was determined by least-square regression to find the terminal elimination rate constant (λz). The terminal elimination half-life (t1/2) was calculated as 0.693/λz. The area under the serum concentration-time curve from time zero to the time of the last quantifiable concentration (AUC(0,tlast)) was calculated using the linear trapezoidal rule. The area under the serum concentration–time curve from time zero to infinity (AUC(0,∞)) was calculated as AUC(0,∞) = AUC(0,tlast) + Clast/λz. The Cumax is the highest of three measured free ropivacaine concentrations per patient. The percent free concentration of ropivacaine was calculated as (Cu/total ropivacaine concentration in the corresponding sample) × 100%.
Sample size and statistics
The aim of this study was to describe the pharmacokinetic profile of 450 mg ropivacaine with and without epinephrine including the highest free ropivacaine concentrations. We decided to study two groups of six patients each. Randomization and blinding was performed to eliminate potential preference of anaesthesiologists for the addition of epinephrine. The GraphPad InStat v.3.10 package (GraphPad Software Inc, San Diego, California) was used to perform descriptive statistics and to compare pharmacokinetic parameters using point estimates and 95% confidence intervals of the differences.
All data are presented as mean (SD) (range) or proportions.
Results
Twelve patients completed the study protocol, six in each group (Table 1).
Table 1.
Patient characteristics
| Group R | Group RE | |
|---|---|---|
| Gender (M/F) | 5/1 | 4/2 |
| Age (years) | 27 (8) | 31 (13) |
| Weight (kg) | 90 (21) | 81 (12) |
| Height (m) | 1.82 (0.10) | 1.77 (0.06) |
| BMI (kg m−2) | 27 (3) | 26 (3) |
Group R: Ropivacaine 450 mg without epinephrine. Group RE: Ropivacaine 450 mg with epinephrine 5 μg ml. Values are proportions or mean (SD).
Clinical outcome measures
Signs for systemic toxicity of local anaesthetics were not observed.
The time to complete the femoral nerve block after completion of the sciatic nerve block was 6.2 (1.6) (3–8) min. Nine patients underwent surgery without need for additional sedation (five (R) and four (RE) patients), whereas three patients received sedation at any time during surgery due to patient discomfort (one (R) and two (RE) patients). Sedation was provided with a short acting sedative (propofol) and a short acting opioid drug (remifentanil) and was mild, patients maintaining spontaneous respiration at all times. None of the patients required conversion to general anaesthesia.
Pharmacokinetics
Figure 1A and 1 show the individual and average concentration–time curves. Figure 2 shows these curves in detail during the first 6 h. Total ropivacaine concentrations became <LLOQ (0.050 μg ml−1) in all patients 35–50 h after administration.
Figure 1.
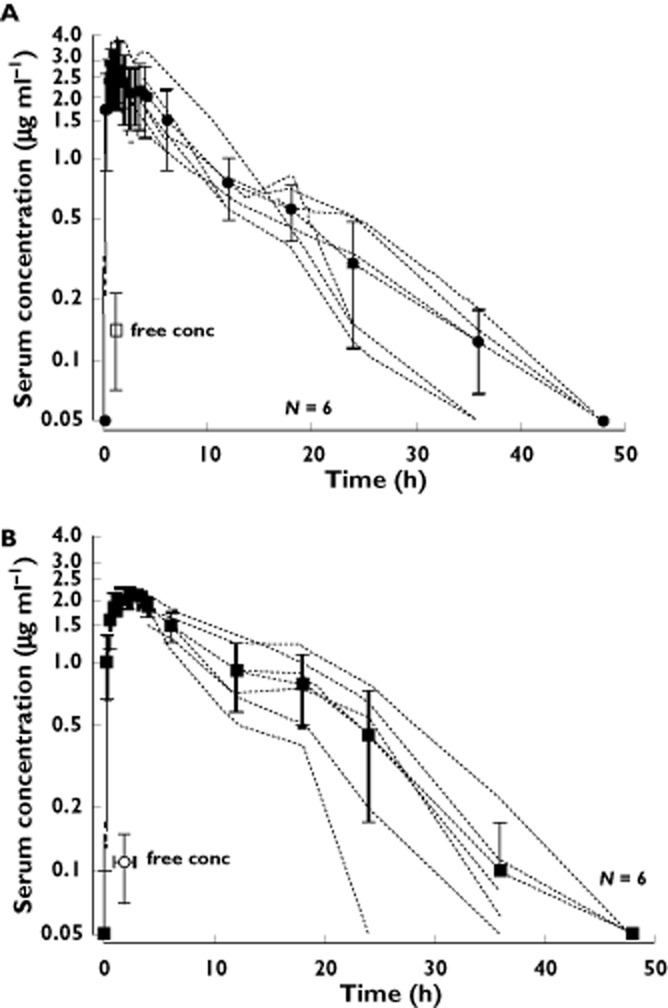
Individual (dotted lines) and mean (solid lines) serum concentration–time curves of ropivacaine 450 mg without (A) and with (B) epinephrine 5 μg ml−1. Mean (SD), free concentration added. LLOQ = 0.050 μg ml−1
Figure 2.
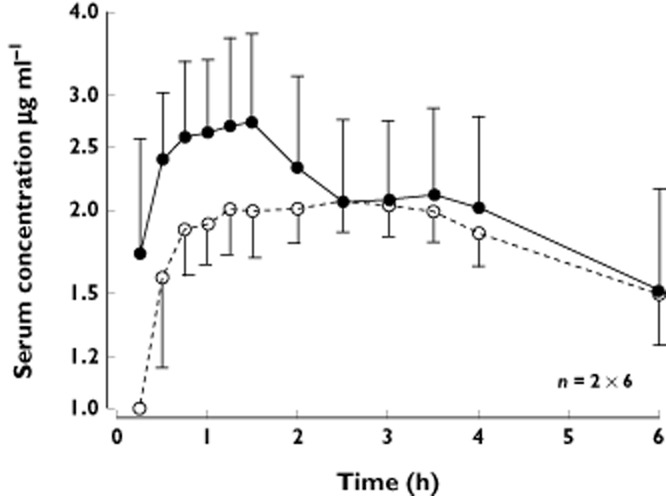
Mean (SD) serum concentration–time curves during the first 6 h of ropivacaine 450 mg without (•) and with (○) epinephrine 5 μg ml−1. LLOQ = 0.050 μg ml−1
Table 2 shows data on Cmax, tmax and other pharmacokinetic parameters, as well as the 95% confidence intervals for their differences. The measured maximum total concentration (Cmax) in group R was 2.81 (0.94) μg ml−1 and 2.16 (0.21) μg ml−1 in group RE. The 95% CI for the difference was from −0.23 to 1.53 μg ml−1 (P = 0.13). The measured time to reach the maximum concentration (tmax) was 1.17 (0.3) h in group R vs. 1.67 (0.94) h in group RE. The 95% CI for the difference was from −1.40 to 0.40 h (P = 0.25). Elimination half-life (t1/2) was 6.82 (2.26) h in group R and 5.48 (1.69) h in group RE.
Table 2.
Pharmacokinetic parameters
| Group R | Group RE | Difference group R vs. RE, (95% CI) | |
|---|---|---|---|
| Cmax (μg ml−1) | 2.81 (0.94) (1.94–3.82) | 2.16 (0.21) (1.68–2.29) | 0.65 (−0.23, 1.53) |
| tmax (h) | 1.17 (0.30) (0.43–1.26) | 1.67 (0.94) (1.25–3.35) | −0.50 (−1.40, 0.40) |
| Cumax (μg ml−1) | 0.16 (0.08) (0.07–0.30) | 0.12 (0.04) (0.05–0.17) | 0.04 (−0.04, 0.12) |
| AUC (μg ml−1 h) | 28.35 (5.92) (22.49–39.23) | 29.12 (7.34) (19.83–41.08) | −0.77 (−9.35, 7.81) |
| t1/2 (h) | 6.82 (2.26) (4.71–9.73) | 5.48 (1.69) (3.02–8.07) | 1.34 (−1.23, 3.91) |
Groups as defined in Table 1. Values are mean (SD) (range). Cmax = maximum total ropivacaine concentration; tmax = time to Cmax; Cumax = maximum free ropivacaine concentration; AUC = area under the serum concentration–time curve from time zero to infinity; t1/2 = elimination half-life.
Table 3 presents raw data on the highest free concentration per patient. The measured highest free ropivacaine concentration (Cumax) was 0.16 (0.08) μg ml−1 in group R and 0.12 (0.04) μg ml−1 in group RE. The 95% CI for the difference was from −0.12 to 0.04 μg ml−1 (P = 0.31). The percentage of free ropivacaine calculated from all samples was 5.1% (1.6) (2.2%−8.0%) in group R and 5.2% (1.7) (2.4%−8.5%) in group RE. Free ropivacaine concentrations are also shown in Figure 1A and 1B.
Table 3.
Highest measured free ropivacaine serum concentration per patient and corresponding total ropivacaine serum concentration
| Patient | Time (h) | Group R | Patient | Time (h) | Group RE | ||
|---|---|---|---|---|---|---|---|
| Total concentration (μg ml−1 ) | Free concentration (μg ml−1 ) | Total concentration (μg ml−1 ) | Free concentration (μg ml−1 ) | ||||
| 2 | 1.5 | 4.09 | 0.21 | 1 | 1.5 | 1.99 | 0.17 |
| 3 | 1.25 | 1.75 | 0.14 | 4 | 0.75 | 2.33 | 0.15 |
| 5 | 0.75 | 2.25 | 0.13 | 6 | 1.5 | 1.79 | 0.09 |
| 8 | 1.0 | 2.10 | 0.07 | 7 | 1.25 | 2.12 | 0.05 |
| 9 | 1.25 | 2.40 | 0.13 | 10 | 3.0 | 2.10 | 0.14 |
| 11 | 1.5 | 3.89 | 0.30 | 12 | 1.25 | 2.37 | 0.14 |
Groups as defined in Table 1. Time points are at Cumax.
Discussion
This is the first study describing the pharmacokinetic profile in serum of 450 mg ropivacaine with and without epinephrine for combined sciatic/femoral nerve block.
In the current literature, several studies report pharmacokinetic data on ropivacaine doses of 300 mg. Cuvillon and colleagues [13] and Vanterpool and colleagues [14] used 300 mg ropivacaine with epinephrine (1:200 000 and 1:400 000, respectively) in combined lumbar plexus/sciatic nerve block and found a Cmax for total ropivacaine of 1.84 (0.59) μg ml−1 and 1.56 (0.35) μg ml−1 respectively and a tmax less than 1 h. In these studies, samples were only taken until 90 min [13] and 4 h [14] after injection and therefore they do not provide data on elimination half-lives. Wank and colleagues [15] and Pere and colleagues [16] studied the pharmacokinetic profile of 300 mg ropivacaine without epinephrine in axillary brachial plexus block and found a Cmax of 2.3 (0.8) μg ml−1 and 1.8 (0.5) μg ml−1 respectively. In both studies, tmax was less than 1 h and elimination half-lives were 6.1 (1.8) h [15] and 8.4 (10.5) h [16]. Although these studies differ from ours with respect to dose and site of injection, the tmax and t1/2 are comparable with the data obtained in our group R (tmax = 1.17 (0.3) h, t1/2 = 6.82 (2.26) h).
The major concern when using high doses of local anaesthetic is systemic toxicity. If and when systemic toxicity occurs, depends on the free ropivacaine serum concentration exceeding the toxic threshold. Ropivacaine in serum is mainly bound to α1-acid glycoprotein (AAG) and the % free concentration is approximately 6% 17. In a study in healthy volunteers receiving i.v. ropivacaine, Knudsen and colleagues [18] defined the toxic threshold for free ropivacaine in arterial samples as 0.56 (range 0.34–0.85) μg ml−1. In our study we took venous samples. However, when absorption into the central compartment is slow (as is the case with perineural injection), arterial and venous concentrations will be comparable. The highest free ropivacaine serum concentration we found in our study was well below the threshold of 0.56 μg ml−1. Systemic toxicity may occur directly in relation with the injection of local anaesthetic, or after a delay. Direct systemic toxicity occurs in case of accidental i.v. injection when the serum concentration rises rapidly and the interval between injection and the onset of symptoms is characteristically short (1–2 min). Excluding accidental i.v. injection, the free ropivacaine serum concentration may still rise above the toxic threshold and result in systemic toxicity. In the latter case, the concentration–time profile is determined by total dose and absorption from the site of injection into the central compartment, as well as by distribution and elimination. The delay between the injection and the onset of symptoms is much longer (>15 min).
A limitation of the present study is the small numbers in each group. Our study groups contained six patients each and authorative statements about safety obviously require larger numbers. However, in the past 10 years, more than 5000 patients at our institution have received a combined sciatic/femoral nerve block with 450 mg ropivacaine. During this period, we have observed only one patient with mild signs of delayed systemic toxicity (anxiety and restlessness, 45 min after injection) that resolved within minutes without treatment.
A second limitation of this study is the relatively high total dose of ropivacaine. With the introduction of ultrasound, there has been a rapid refinement in block placement techniques. The use of ultrasound allows a reduction in local anaesthetic volume and dose without compromising block efficacy, reducing the need for large doses as described in this study. However, doses exceeding the maximum recommended dose of 300 mg ropivacaine still occur regularly. As such there remains a need for pharmacokinetic data on doses of ropivacaine for peripheral nerve block that are higher than the maximum recommended dose.
The rationale for adding epinephrine to reduce the maximum plasma concentration is local vasoconstriction at the site of injection [19], thereby slowing absorption. Several studies found a decrease in Cmax and an increase in tmax as a result of adding epinephrine to ropivacaine for epidural [7, 8], caudal [20] or regional [6] (thoracic paravertebral block) anaesthesia. On the other hand, for perivascular subclavian block, Hickey et al. [9] found no effect on pharmacokinetics (Cmax, tmax or AUC) after the addition of epinephrine to ropivacaine.
In our study, the addition of epinephrine shows a trend for a lower Cmax and an increase in tmax. However, the differences were not statistically significant, possibly due to the small number of subjects in each group. To document the pharmacokinetic safety of higher than recommended doses of ropivacaine, as well as to study the effect of adding epinephrine, adequately powered studies are necessary. The results of this study may serve as a basis for such studies.
In conclusion, this is the first study describing the pharmacokinetic profile in serum of 450 mg ropivacaine with and without epinephrine for combined sciatic/femoral nerve block. Free serum concentrations of ropivacaine in both groups remained well below the assumed threshold of 0.56 μg ml−1 for systemic toxicity.
Acknowledgments
We gratefully acknowledge the help of Jos Lerou, M.D., Ph.D. with the revision of this manuscript.
Competing Interests
The authors declare that no competing interests exist.
This work was supported entirely by internal funds of the Department of Anaesthesiology, Sint Maartenskliniek, Nijmegen, the Netherlands.
References
- 1.Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: a multifactorial concept. Reg Anesth Pain Med. 2004;29:564–575. doi: 10.1016/j.rapm.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Heavner JE. Let's abandon blanket maximum recommended doses of local anesthetics. Reg Anesth Pain Med. 2004;29:524. doi: 10.1016/j.rapm.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Leone S, Di Cianni S, Casati A, Fanelli G. Pharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine. Acta Biomed. 2008;79:92–105. [PubMed] [Google Scholar]
- 4.Simpson D, Curran MP, Oldfield V, Keating GM. Ropivacaine: a review of its use in regional anaesthesia and acute pain management. Drugs. 2005;65:2675–2717. doi: 10.2165/00003495-200565180-00013. [DOI] [PubMed] [Google Scholar]
- 5.Mulroy MF, Hejtmanek MR. Prevention of local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:177–180. doi: 10.1097/AAP.0b013e3181d26068. [DOI] [PubMed] [Google Scholar]
- 6.Karmakar MK, Ho AM, Law BK, Wong AS, Shafer SL, Gin T. Arterial and venous pharmacokinetics of ropivacaine with and without epinephrine after thoracic paravertebral block. Anesthesiology. 2005;103:704–711. doi: 10.1097/00000542-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Lee BB, Ngan Kee WD, Plummer JL, Karmakar MK, Wong AS. The effect of the addition of epinephrine on early systemic absorption of epidural ropivacaine in humans. Anesth Analg. 2002;95:1402–1407. doi: 10.1097/00000539-200211000-00055. [DOI] [PubMed] [Google Scholar]
- 8.Ratajczak-Enselme M, Estebe JP, Rose FX, Wodey E, Malinovsky JM, Chevanne G, Dollo C, Ecoffey C, Le Corre P. Effect of epinephrine on epidural, intrathecal, and plasma pharmacokinetics of ropivacaine and bupivacaine in sheep. Br J Anaesth. 2007;99:881–890. doi: 10.1093/bja/aem291. [DOI] [PubMed] [Google Scholar]
- 9.Hickey R, Blanchard J, Hoffman J, Sjovall J, Ramamurthy S. Plasma concentrations of ropivacaine given with or without epinephrine for brachial plexus block. Can J Anaesth. 1990;37:878–882. doi: 10.1007/BF03006624. [DOI] [PubMed] [Google Scholar]
- 10.Weber A, Fournier R, Van Gessel E, Riand N, Gamulin Z. Epinephrine does not prolong the analgesia of 20 ml ropivacaine 0.5% or 0.2% in a femoral three-in-one block. Anesth Analg. 2001;93:1327–1331. doi: 10.1097/00000539-200111000-00060. [DOI] [PubMed] [Google Scholar]
- 11.Mansour NY. Reevaluating the sciatic nerve block: another landmark for consideration. Reg Anesth. 1993;18:322–323. [PubMed] [Google Scholar]
- 12.Proost JH, Meijer DK. MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput Biol Med. 1992;22:155–163. doi: 10.1016/0010-4825(92)90011-b. [DOI] [PubMed] [Google Scholar]
- 13.Cuvillon P, Nouvellon E, Ripart J, Boyer JC, Dehour L, Mahamat A, L'Hermite J, Boisson C, Vialles N, Lefrant JY, De la Coussaye JE. A comparison of the pharmacodynamics and pharmacokinetics of bupivacaine, ropivacaine (with epinephrine) and their equal volume mixtures with lidocaine used for femoral and sciatic nerve blocks: a double-blind randomized study. Anesth Analg. 2009;108:641–649. doi: 10.1213/ane.0b013e31819237f8. [DOI] [PubMed] [Google Scholar]
- 14.Vanterpool S, Steele SM, Nielsen KC, Tucker M, Klein SM. Combined lumbar-plexus and sciatic-nerve blocks: an analysis of plasma ropivacaine concentrations. Reg Anesth Pain Med. 2006;31:417–421. doi: 10.1016/j.rapm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Wank W, Büttner J, Maier KR, Emanuelson BM, Selander D. Pharmacokinetics and efficacy of 40 ml ropivacaine 7.5 mg/ml (300 mg), for axillary brachial plexus block – an open pilot study. Eur J Drug Metab Pharmacokinet. 2002;27:53–59. doi: 10.1007/BF03190406. [DOI] [PubMed] [Google Scholar]
- 16.Pere P, Salonen M, Jokinen M, Rosenberg PH, Neuvonen PJ, Haasio J. Pharmacokinetics of ropivacaine in uremic and nonuremic patients after axillary brachial plexus block. Anesth Analg. 2003;96:563–569. doi: 10.1097/00000539-200302000-00048. [DOI] [PubMed] [Google Scholar]
- 17. SPC Naropin 7.5 mg/ml. Available at http://www.medicines.org.uk/emc/medicine/7289 (last accessed 14 December 2011)
- 18.Knudsen K, Beckman Suurkula M, Blomberg S, Sjovall J, Edvardsson N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth. 1997;78:507–514. doi: 10.1093/bja/78.5.507. [DOI] [PubMed] [Google Scholar]
- 19.Bernards CM, Kopacz DJ. Effect of epinephrine on lidocaine clearance in vivo: a microdialysis study in humans. Anesthesiology. 1999;91:962–968. doi: 10.1097/00000542-199910000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Van Obbergh LJ, Roelants FA, Veyckemans F, Verbeeck RK. In children, the addition of epinephrine modifies the pharmacokinetics of ropivacaine injected caudally. Can J Anaesth. 2003;50:593–598. doi: 10.1007/BF03018647. [DOI] [PubMed] [Google Scholar]


