Abstract
Purpose
Androgen replacement therapy has been shown to be safe and effective for most patients with testosterone deficiency. Male partners of infertile couples often report significantly poorer sexual activity and complain androgen deficiency symptoms. We report herein an adverse effect on fertility caused by misusage of androgen replacement therapy in infertile men with hypogonadal symptoms.
Materials and Methods
The study population consisted of 8 male patients referred from a local clinic for azoospermia or severe oligozoospermia between January 2008 and July 2011. After detailed evaluation at our andrology clinic, all patients were diagnosed with iatrogenic hypogonadism associated with external androgen replacement. We evaluated changes in semen parameters and serum hormone level, and fertility status.
Results
All patients had received multiple testosterone undecanoate (NebidoR) injections at local clinic due to androgen deficiency symptoms combined with lower serum testosterone level. The median duration of androgen replacement therapy prior to the development of azoospermia was 8 months (range: 4-12 months). After withdrawal of androgen therapy, sperm concentration and serum follicle-stimulating hormone level returned to normal range at a median 8.5 months (range: 7-10 months).
Conclusion
Misusage of external androgen replacement therapy in infertile men with poor sexual function can cause temporary spermatogenic dysfunction, thus aggravating infertility.
Keywords: Androgen, male infertility, hypogonadism
INTRODUCTION
Infertility, defined as the inability to conceive after 1 year of unprotected intercourse, affects approximately 10% to 15% of reproductive age couples in developed countries such as the United States. Over the past 30 years, the treatment of infertility has been revolutionized, due to the development of new assisted reproductive technologies (ART) such as in vitro fertilization (IVF).1,2 Many young couples, however, have negative feelings about artificial interventions such as IVF and prefer to have children through natural intercourse. It has been known that infertility is associated with decreased sexual activity and male partners of infertile couples report significantly poorer sexual function and satisfaction and increased rates of androgen deficiency complaints than male partners of fertile control couples.3,4 It is reported that in patients with hypogonadism with erectile dysfunction, androgen supplementation may be considered a treatment for erectile dysfunction and is known to improve the therapeutic response to phosphodiesterase type 5 (PDE5)-inhibitors.5 In addition, long-acting injectable testosterone undecanoate (TU, NebidoR, Bayer Health Care AG, Leverkusen, Germany), a new parenteral testosterone preparation, has become available to avoid frequent injections of the conventional injectable esters.6 Here, we report a side effect on fertility caused by misuse of androgen replacement therapy in infertile men.
MATERIALS AND METHODS
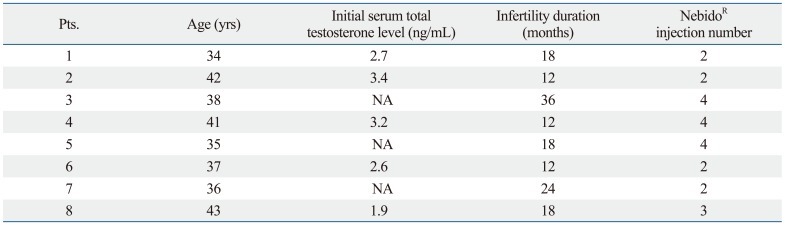
The study population consisted of 8 males who presented for evaluation of male infertility to andrology clinic at CHA Fertility Center, CHA University between January 2008 and July 2011. The median age of the 8 men was 37.5 years (range: 34-43 years), and their median duration of infertility was 18 months (range: 12-36 months) (Table 1). All 8 patients were initially referred from local clinics for further evaluation of azoospermia or severe oligozoospermia. Patient evaluation consisted of a thorough personal and family history, physical examination, semen analyses, and laboratory tests including hormonal profiles. Testis size was measured by orchidometer. Sperm concentration, motility and morphology were assessed as described (WHO, 1999).7 All semen samples were obtained by masturbation into a wide-mouthed plastic container in a separate room after 3 or more days of sexual abstinence and were allowed to liquefy for at least 20 minutes at 37℃ before analysis. If sperm was not detected by conventional microscopic Makler chamber evaluation, the semen sample was centrifuged at 1500 g for 10 min to detect any viable motile sperm. Changes in fertility status and semen analysis during follow-up were evaluated.
Table 1.
Patient Characteristics
NA, non available.
RESULTS
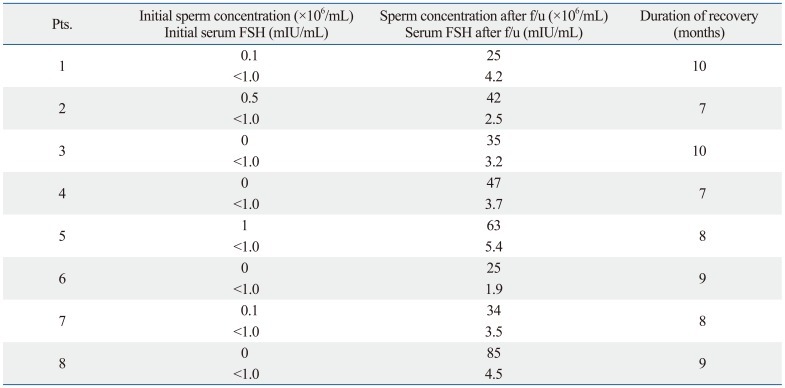
Semen analysis showed complete azoospermia in 4 patients and severe oligozoospermia (<5×106/mL) in 4 patients (Table 2). None of these patients had any previous history of genital infection, varicocele, cryptorchidism, or exposure to gonadotoxin. Physical examination showed that all 8 patients had normal sized testes. Hormonal profile analysis showed that serum follicle-stimulating hormone (FSH) was below the normal range in all 8 patients.
Table 2.
Changes in Sperm Concentration, Serum FSH Level after Follow-Up Period
FSH, follicle-stimulating hormone.
Serum FSH (normal: 1.2-12.4 mIU/mL).
A detailed patient history showed that all 8 patients were treated at primary clinic for erectile dysfunction. Their morning total testosterone level was lower or borderline range at initial test (Table 1). In addition to PDE5-inhibitors, they had undergone androgen replacement therapy (median 8 months, range: 4-12 months) prior to the development of azoospermia, consisting of multiple injections of TU (NebidoR), due to androgen deficiency symptoms combined with decreased sexual function. After being diagnosed as azoospermic, one patient underwent a testicular sperm aspiration procedure at an infertility clinic. The sperm retrieval failed and the couple was told that the husband had nonobstructive type azoospermia, and that they should therefore consider pregnancy with donor sperm.
After detailed evaluation, the patients were told to suffer form iatrogenic hypogonadism associated with external androgen replacement and all were advised to return for repeating semen analysis tests without testicular biopsy or direct ART procedure. The 8 patients underwent regular intermittent semen analysis, and the sperm concentration and serum FSH level of the patients recovered to the normal range during follow-up. Their median time to the recovery of normal sperm concentration (>20×106/mL) was 8.5 months (range: 7-10 months) (Table 2).
DISCUSSION
This study reports about aggravated fertility issue due to misusage of androgen placement in infertile men, and there is a similar case report showing reversible testosterone-induced azoospermia.8 In Korea, the mean ages of marriage and first pregnancy are increasing, along with rapid industrialization and westernization, and low birth rate and infertility have recently become a major social issue. It has been known that male partners of infertile couples often report significantly poorer sexual function and satisfaction compared with fertile control couples, as well as increased rates of androgen deficiency type complaints.3 Infertile men may have feelings of inadequacy, anxiety, guilt and/or depression. These negative emotions contribute to distress and are associated with male sexual dysfunctions.9,10 Hypogonadal men treated with androgen replacement experience improved libido and sexual function, as indicated by frequency of erection and ejaculation.11,12 Although controversy exists about the results of testosterone supplementation on sexual function, the well-known benefits of androgen replacement therapy include increases of body hair, energy, muscle mass and stamina; increased ability to perform more physically demanding tasks; and overall increases in the sense of well-being, confidence, and motivation.13 In addition, androgen replacement therapy with testosterone is safe for most patients. Currently, there is no universal consensus on the hormonal and clinical criteria that should be used to identify who are candidates for androgen therapy. Since androgen replacement therapy is indicated primarily for men at or beyond middle age, fertility issue has not been a concern for most of this patients.14 Ideally, androgen supplementation therapy is indicated in patients with clinical manifestations, supported by a biochemical diagnosis. Measurements of total testosterone are the most widely available and the least expensive of the testosterone assays. When biochemical hormonal measurements are borderline abnormal, patient's complaints may be the deciding factor. Because these somewhat vague indications broadly improve general benefits with minor side effects and it is easily prescribed by primary physician at local clinics, androgen replacement may be prescribed to relatively younger men with moderately decreased libido and sexual dysfunction. All of our patients received TU (NebidoR) injections due to poor sexual function and libido plus hypogonadal symptoms while infertile. However, none of our patients was informed about the possibility that androgen supplementation would reduce their fertility, and all experienced reversible spermatogenic dysfunction.
Maintenance of normal spermatogenesis depends on adequate gonadotrophin and intratesticular testosterone concentrations. Testosterones and more recently used androgen-progestin combinations reversibly suppress spermatogenesis by suppressing the pituitary LH and FSH. Many primary physicians are unaware of this mechanism, by which the male reproductive hormonal system regulates spermatogenesis. In fact, androgens are currently being actively investigated for potential use in male medical contraception.15,16
Since the advent of ART, male infertility has largely been treated by gynecologists, who may be totally untrained in examining and treating infertile male patients. In Korea, many infertile couples are treated at a local infertility clinic by a gynecologist. The imbalance between easy availability of androgen replacement therapy and poor evaluation of infertile males may worsen the problem.
Four of 8 patients in this study were initially diagnosed with azoospermia at a local infertility clinic during infertility evaluation. Generally, azoospermia is classified as non obstructive type or obstructive type, with a testicular biopsy required to differentiate between them. This relatively simple procedure is often used not just for diagnostic purposes but for therapeutic sperm retrieval for possible IVF procedures in this ART era. Sperm, however, cannot be retrieved when suppressed by excessive androgen replacement. Our finding that one of the patients underwent unsuccessful testicular sperm aspiration at a local infertility clinic and was told to consider donor sperm usage for fertilization shows that medical personnel involved in fertility issue should have basic knowledge about transient reversible azoospermia induced by external androgen supplement therapy.
For most patients, androgen replacement therapy with testosterone is a safe and effective treatment for testosterone deficiency. Androgen replacement therapy may have negative effects on the prostate and the cardiovascular system. However, the fertility problems associated with excessive androgen replacement in young fertile men are rarely mentioned. In addition, due to competitive commercial market conditions and poor understanding of male reproductive physiology by primary physicians, the use of androgen replacement is rapidly increasing in Korea. As is true for all forms of medical therapy, before initiating androgen replacement therapy, one must understand and balance the possible therapeutic benefits against the potential risks.
In summary, misusage of external androgen replacement in infertile men with hypogondal symptoms can cause temporary spermatogenic dysfunction. Considering current infertility treatment practices and the wide usage of androgen replacement therapy, potential side effect of androgen replacement should be well recognized by related medical personnel.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare & Family affairs, Republic of Korea (A084923). The sponsor had no role in the design and conduct of the study, in the collection, analysis, or interpretation of the data, or in the preparation, review or approval of the manuscript.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2:366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 2.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien JH, Lazarou S, Deane L, Jarvi K, Zini A. Erectile dysfunction and andropause symptoms in infertile men. J Urol. 2005;174:1932–1934. doi: 10.1097/01.ju.0000177453.14334.a2. [DOI] [PubMed] [Google Scholar]
- 4.Monga M, Alexandrescu B, Katz SE, Stein M, Ganiats T. Impact of infertility on quality of life, marital adjustment, and sexual function. Urology. 2004;63:126–130. doi: 10.1016/j.urology.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Shabsigh R, Kaufman JM, Steidle C, Padma-Nathan H. Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. J Urol. 2004;172:658–663. doi: 10.1097/01.ju.0000132389.97804.d7. [DOI] [PubMed] [Google Scholar]
- 6.Harle L, Basaria S, Dobs AS. Nebido: a long-acting injectable testosterone for the treatment of male hypogonadism. Expert Opin Pharmacother. 2005;6:1751–1759. doi: 10.1517/14656566.6.10.1751. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- 8.Jan Z, Pfeifer M, Zorn B. Reversible testosterone-induced azoospermia in a 45-year-old man attending an infertility outpatient clinic. Andrologia. 2012;44(Suppl 1):823–825. doi: 10.1111/j.1439-0272.2011.01192.x. [DOI] [PubMed] [Google Scholar]
- 9.Lenzi A, Lombardo F, Salacone P, Gandini L, Jannini EA. Stress, sexual dysfunctions, and male infertility. J Endocrinol Invest. 2003;26(3 Suppl):72–76. [PubMed] [Google Scholar]
- 10.Seidman SN, Roose SP. The relationship between depression and erectile dysfunction. Curr Psychiatry Rep. 2000;2:201–205. doi: 10.1007/s11920-996-0008-0. [DOI] [PubMed] [Google Scholar]
- 11.Davidson JM, Camargo CA, Smith ER. Effects of androgen on sexual behavior in hypogonadal men. J Clin Endocrinol Metab. 1979;48:955–958. doi: 10.1210/jcem-48-6-955. [DOI] [PubMed] [Google Scholar]
- 12.Skakkebaek NE, Bancroft J, Davidson DW, Warner P. Androgen replacement with oral testosterone undecanoate in hypogonadal men: a double blind controlled study. Clin Endocrinol (Oxf) 1981;14:49–61. doi: 10.1111/j.1365-2265.1981.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto AM. Hormonal therapy of male hypogonadism. Endocrinol Metab Clin North Am. 1994;23:857–875. [PubMed] [Google Scholar]
- 14.Practice Committee of the American Society for Reproductive Medicine. Treatment of androgen deficiency in the aging male. Fertil Steril. 2004;82(Suppl 1):S46–S50. doi: 10.1016/j.fertnstert.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Wu FC. Hormonal approaches to male contraception: approaching reality. Mol Cell Endocrinol. 2006;250:2–7. doi: 10.1016/j.mce.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Gui YL, He CH, Amory JK, Bremner WJ, Zheng EX, Yang J, et al. Male hormonal contraception: suppression of spermatogenesis by injectable testosterone undecanoate alone or with levonorgestrel implants in chinese men. J Androl. 2004;25:720–727. doi: 10.1002/j.1939-4640.2004.tb02846.x. [DOI] [PubMed] [Google Scholar]