Abstract
Purpose
Nociceptin/orphanin FQ (N/OFQ) as an endogeneous hexadecapeptide is known to exert antinociceptive effects spinally. The aims of this study were to demonstrate the antinociceptive effects of i.t. N/OFQ and to investigate the possible interaction between N/OFQ and endogenous opioid systems using selective opioid receptor antagonists in rat formalin tests.
Materials and Methods
I.t. N/OFQ was injected in different doses (1-10 nmol) via a lumbar catheter prior to a 50 µL injection of 5% formalin into the right hindpaw of rats. Flinching responses were measured from 0-10 min (phase I, an initial acute state) and 11-60 min (phase II, a prolonged tonic state). To observe which opioid receptors are involved in the anti-nociceptive effect of i.t. N/OFQ in the rat-formalin tests, naltrindole (5-20 nmol), β-funaltrexamine (1-10 nmol), and norbinaltorphimine (10 nmol), selective δ-, µ- and κ-opioid receptor antagonists, respectively, were administered intrathecally 5 min after i.t. N/OFQ.
Results
I.t. N/OFQ attenuated the formalin-induced flinching responses in a dose-dependent manner in both phases I and II. I.t. administration of naltrindole and β-funaltrexamine dose-dependently reversed the N/OFQ-induced attenuation of flinching responses in both phases; however, norbinaltorphimine did not.
Conclusion
I.t. N/OFQ exerted an antinociceptive effect in both phases of the rat-formalin test through the nociceptin opioid peptide receptor. In addition, the results suggested that δ- and µ-opioid receptors, but not κ-opioid receptors, are involved in the antinociceptive effects of N/OFQ in the spinal cord of rats.
Keywords: Antinociception, formalin test, nociceptin/orphanin FQ, opioid receptor
INTRODUCTION
Nociceptin/orphanin FQ (N/OFQ) is a 17-amino-acid endogenous peptide, which is known to be an endogenous ligand for the nociceptin opioid peptide (NOP) receptor. N/OFQ and the NOP receptor exhibit sequence homology with dynorphin A and other members of the opioid receptor family, respectively.1 Despite the structural homology between the NOP receptor and other opioid receptors, N/OFQ displays low affinity for other opioid receptors. In addition, the NOP receptor is insensitive to naloxone, an opioid antagonist, as shown in in vitro and in vivo studies.2
The pharmacologic actions of N/OFQ have been reported to increase food intake,3 decrease heart rate and blood pressure,4 and produce diuresis.4 However, the pharmacologic actions of N/OFQ, especially with respect to pain in rodents, have not yet been clearly defined. The effect of N/OFQ has been shown to vary from hyperalgesia, allodynia and even analgesia, depending on the route of administration, dose of the drug, genetic differences of the species and characteristics of nociceptive stimuli.5-8 For example, N/OFQ at the supraspinal level, intracisternally or intracerebroventricularly, has been shown to exert pro-nociceptive and hyperalgesic effects in rodents.9,10 However, N/OFQ at the spinal level, intrathecally, has been shown to exert antinociceptive and analgesic effects in rodent pain models of hot plate11 or paw formalin injection,9,10,12,13 which have been used as models of noxious thermal stimulation or inflammatory pain, respectively.
As N/OFQ and its NOP receptor are co-localized with other opioid peptides and their respective receptors in dorsal root ganglia and the dorsal spinal cord,14 it is possible that an interaction between N/OFQ and other opioid peptides may be responsible for the antinociceptive effect of N/OFQ. Indeed, intrathecal injection of µ- or δ-antagonists blocks the antinociceptive effect of intrathecal N/OFQ upon noxious thermal stimulation.11 However, it is not clear at present which types of opioid receptors are involved in the antinociceptive effect of intrathecally administered N/OFQ in rat formalin tests. Thus, the aims of the present study were to demonstrate the antinociceptive effects of i.t. N/OFQ and to investigate the possible interactions between N/OFQ and endogenous opioid systems using selective opioid receptor antagonists in rat formalin tests.
MATERIALS AND METHODS
Animals
Male, Sprague-Dawley rats (Orient, Seoul, Korea) weighing 220-260 g (8-9 weeks of age) were used in this study. The animals were housed in individual cages with free access to food and water at a room temperature of 22±1℃ and a relative humidity of 50±5% under 12-h light-dark cycles (lights on at 8:00 AM). All experiments were conducted according to a protocol approved by the Institutional Animal Care and Use Committee of Ewha Womans University.
Chemicals
The following chemicals in this study were purchased from Sigma Chemical (Sigma-Aldrich, St. Louis, MO, USA): N/OFQ, [Nphe1]NC(1-13)-NH2, naltrindole hydrochloride, β-funaltrexamine hydrochloride and norbinaltorphimine. All drugs were dissolved in sterile 0.9% saline in a volume of 10 µL. Ten microliters of 0.9% saline was used as a control.
I. t. catheterization
An i.t. catheter was implanted according to the direct lumbar catheterization method.15,16 A 2-3 cm midline longitudinal incision was made between the two anterior iliac spines under Zoletil TM®, Bayer Korea, Seoul, Korea (Tiletamine : Zolazepam=1 : 1) and xylazine anesthesia (50 and 10 mg/kg, respectively, IM). A 20 cm polyethylene-10 tube (Becton Dickinson, Sparks, MD, USA) was implanted via the wire pull-through technique between the lumbar L5 and L6 vertebrae. A tail flick or paw retraction was used as confirmation of correct positioning of the wire. The guide wire was then withdrawn approximately 2 cm, and the catheter was pushed gently upward to reach the L4 lumbar enlargement. The catheter was tied in a loose knot and sutured on the back under the skin. The external end of the catheter was tunneled subcutaneously to exit at the occipital region for secure placement, flushed with sterile 0.9% saline, and sealed by heat melting. All the animals were housed individually and allowed to recover for 6-7 days prior to behavioral study. Animals exhibiting disturbances in general well-being, abnormal behavior, or neurological deficits and dysfunction were excluded. The external end of the tube was closed by fire flare. Placement of the i.t. catheter was confirmed after each experiment by injecting indigo blue and checking for distribution within the lumbar subarachnoid space. Only data obtained from rats with proper i.t. catheter placement were analyzed.
Formalin nociceptive assay
Randomly selected rats were habituated for 20 min to an individual Plexiglas observation chamber (55 cm long×30 cm wide×26 cm high) before testing. A mirror was placed behind the chamber, which provided a clear view of the rat's paw movement. Formalin (5%, 50 µL) was injected subcutaneously via a 29-gauge needle into the plantar surface of the right hindpaw while the rat was manually restrained. Immediately following the injection, rats were returned to the observation chamber and individuals who were not familiar with the experimental conditions recorded the number of flinches by the animal. Flinching was defined as a rapid and brief withdrawal or flexing of the injected paw. The ability of the test compound to inhibit formalin-induced flinches was measured as the number of flinching incidences in a 5-min period for 60 min. Flinching responses were determined for two characteristic time periods: 0-10 min (phase I, an initial acute state) and 11-60 min (phase II, a prolonged tonic state) after formalin administration.15,17 At the end of the experiments, rats were euthanized in a CO2 chamber and i.t. catheter placement was verified.
I.t. injection
Various doses of N/OFQ and opioid antagonists were administered 10 min and 5 min before the formalin test, respectively, as determined in pilot studies. All drugs were administered via the catheter with a 10-µL micro-injector syringe (Hamilton, Reno, NV, USA) with a 30-gauge needle in a total volume of 10 µL over 20 s, followed by a flush with 10 µL of 0.9% sterile saline. The dead space of the i.t. catheter was placed at 8.5±1.2 mL (n=10), flushed with sterile 0.9% saline, and sealed by heat melting after all drugs had been administered via i.t. injection. The drugs administered were as follows: 1) 1, 5, and 10 nmol of N/OFQ; 2) 10 nmol of [Nphe1]NC(1-13)-NH2 (the selective NOP antagonist); 3) 5, 10, and 20 nmol of naltrindole hydrochloride (NTI, the selective δ-opioid receptor antagonist); 4) 1, 5, and 10 nmol of β-funaltrexamine hydrochloride (FNX, the selective µ-opioid receptor antagonist); and 5) 10 nmol of norbinaltorphimine (nor-BNI, the selective κ-opioid receptor antagonist).
Data analysis
All data are presented as means±standard deviation (SD). The time-response data are presented as the mean number of flinches for each 5-min period. For the dose-response analysis, the cumulative number of formalin-induced flinching responses during phases I and II were calculated separately. The number of flinches was converted to a percentage of control according to the following formula:
Effective dose 50 (ED50) values and 95% confidence limits (CL95) values of N/OFQ were calculated for phase I and phase II, respectively, by least square (linear) regression with the portion of the dose-response curves that spanned 50% of response. The time course and total flinching response data were analyzed using a two-way analysis of variance (ANOVA), followed by Bonferroni test. Statistical significance was determined for each drug condition in phases I and II using a one-way ANOVA, followed by a Tukey test. A value of p<0.05 was considered statistically significant.
RESULTS
A total of 184 rats were used in these experiments with 17 rats excluded before the formalin test because of infection, self-cutting of the i.t. catheter or injection failure. Fifteen rats were also excluded because of improper i.t. catheter placement.
Effect of i.t. administration of NC on the formalin nociceptive assay: the role of the NOP receptor
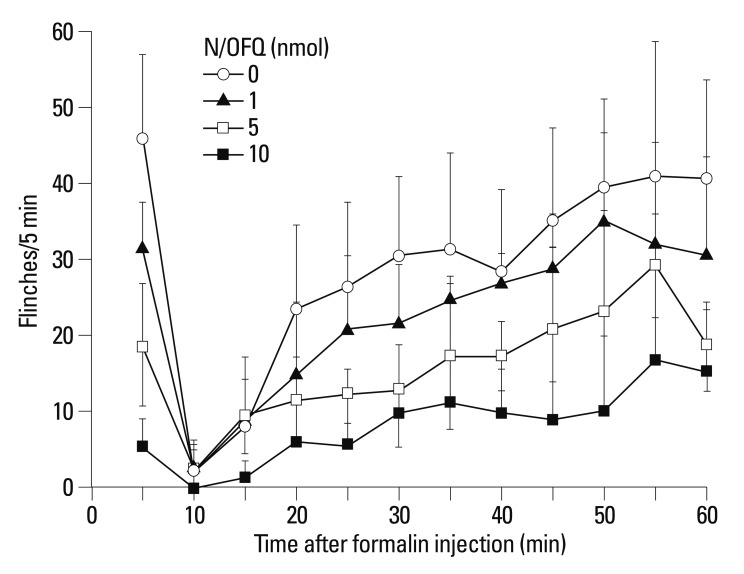
I.t. administration of N/OFQ dose-dependently attenuated formalin-induced flinching responses [F (3336)=98.92, p<0.05] (Fig. 1). ED50 values (CL95 values) of N/OFQ were 5.3 (4.2-6.4) nmol in the dose-response curves and 4.3 (3.2-5.4) nmol and 6.6 (4.8-8.3) nmol in phases I and II, respectively. The ED50 values of the dose response curves were calculated by the following least squares regression equations (percent control was set as the outcome and dose was set as the predictor):
Fig. 1.
Time-effect curves of intrathecal nociceptin/orphanin FQ (N/OFQ) for flinching responses in the formalin test. X-axis: time (min) after formalin injection. Y-axis: number of flinches for each 5-min session. Different doses of NC and saline (as a vehicle) were administered 10 min before formalin injection. I.t. administration of N/OFQ dose-dependently attenuated formalin-induced flinching responses (p<0.05). Each value represents mean±SD (n=8). Symbols represent different dosing conditions for each panel. ED50 values (Cl95 values) of N/OFQ were 5.3 (4.2-6.4) nmol in dose-response curves and 4.3 (3.2-5.4) nmol and 6.6 (4.8-8.3) nmol for phase I and phase II, respectively. SD, standard deviation.
ED50 Total=[Percent control (set as 50)-81.924]/(-5.994)
ED50 Phase I=[Percent control (set as 50)-78.618]/(-6.633)
ED50 Phase II=[Percent control (set as 50)-85.230]/(-5.356)
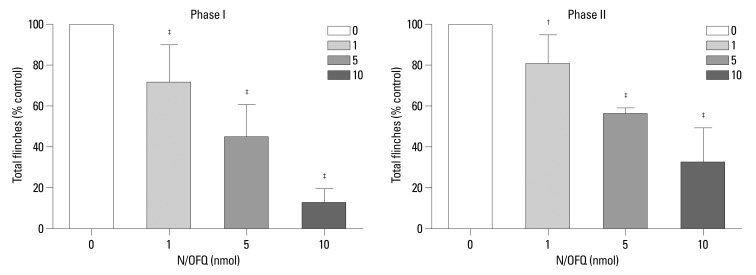
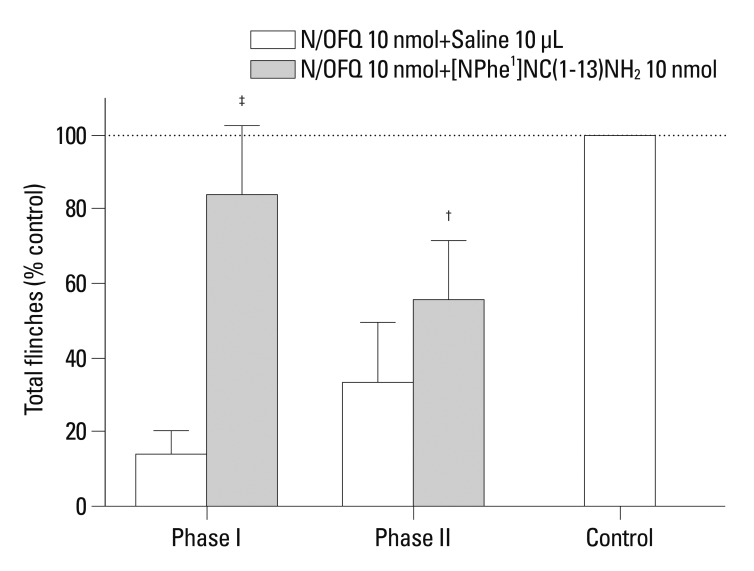
These results showed that N/OFQ dose-dependently inhibited the flinching responses in both phases of the formalin test (p<0.05) (Fig. 2). To investigate the receptor selectivity of N/OFQ, 10 nmol of [Nphe1]NC(1-13)-NH2, was administered 5 min after the i.t. administration of N/OFQ (10 nmol). Fig. 3 illustrates that [Nphe1]NC(1-13)-NH2 significantly antagonized the N/OFQ-induced attenuation of flinching responses in both phases of the formalin test (p<0.05).
Fig. 2.
The cumulative flinching scores of the rat formalin test after intrathecal administration of nociceptin/orphanin FQ (N/OFQ). X-axis: various doses of N/OFQ (1, 5, 10 nmol in 10 µL) and vehicle (0.9% sterile saline, 10 µL). The drugs and tested vehicle were administered 10 min before formalin injection. Y-axis: percentage of control for total flinches during phase I (1-10 min, top panel) and phase II (11-60 min, right panel) after formalin injection. Each values represents mean±SD (n=8). †p<0.01, ‡p<0.001 versus control. SD, standard deviation.
Fig. 3.
The cumulative flinching scores of rat formalin test after intrathecal administration of nociceptin/orphanin FQ (N/OFQ) and (Nphe1)NC(1-13)-NH2. X-axis: bars represent the different dose conditions of each phase. (Nphe1)NC(1-13)-NH2 or 10 mL of 0.9% sterile saline (control) was administered 5 min after i.t. injection of N/OFQ (10 nmol). Y-axis: percentage of control for total flinches during phase I (1-10 min, top panel) and phase II (11-60 min, bottom panel) after formalin injection. Each values represents mean±SD (n=8). †p<0.01, ‡p<0.001 versus control. SD, standard deviation.
Effect of i.t.-administered NTI on N/OFQ-induced inhibition on formalin flinching responses
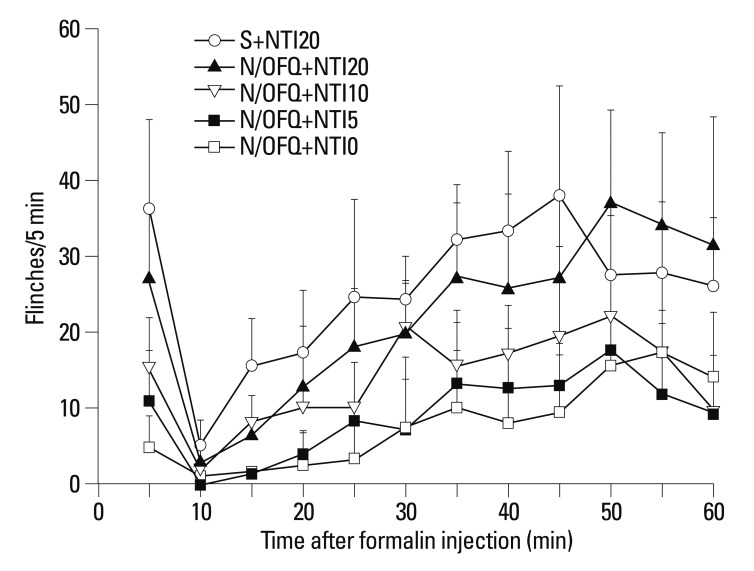
Rats first received N/OFQ (10 nmol, i.t.), followed 5 min later by 5, 10 or 20 nmol of NTI and saline vehicle (n=8 per group). The N/OFQ-induced attenuation of flinching responses was dose-dependently inhibited by NTI [F (4420)=95.63, p<0.05)] (Fig. 4). For the cumulative flinching responses, data are presented as the percentage of control. For the control, 0.9% sterile saline (10 µL, i.t.) instead of N/OFQ (10 nmol, i.t.) was administered, followed 5 min later by 20 nmol of NTI (n=8 per group). Rats treated with i.t. administration of N/OFQ followed by 20 nmol of NTI (N/OFQ+NTI20) showed statistical differences, compared to those in the vehicle pre-treated group (N/OFQ+NTI0) in phase I (‡p<0.001, top panel) (Fig. 5) Rats treated with i.t. administration of N/OFQ followed by 10 or 20 nmol of NTI (N/OFQ+NTI10, N/OFQ+NTI20) showed statistical differences, compared to those in the vehicle pre-treated group (N/OFQ+NTI0) in phase II (*p<0.05, ‡p<0.001 respectively, bottom panel) (Fig. 5).
Fig. 4.
Time-effect curves of intrathecal nociceptin/orphanin FQ (N/OFQ) and co-administered naltrindole (NTI) for flinching responses in the formalin test. X-axis: time (min) after formalin injection. Y-axis: number of flinches for each 5-min session. Each value represents mean±SD (n=8). Symbols represent the different dose conditions for each panel. Ten nmol of N/OFQ was administered intrathecally, followed 5 min later by 5, 10, or 20 nmol of NTI and vehicle (0.9% sterile saline), before formalin injection. I.t. co-administration of NTI dose-dependently reversed N/OFQ-induced flinching attenuations in the formalin test (p<0.05). SD, standard deviation.
Fig. 5.
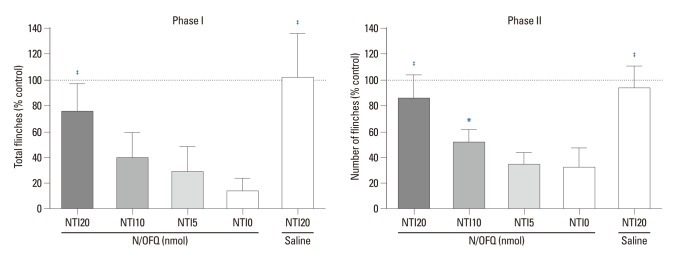
The cumulative flinching scores after intrathecal administration of nociceptin/orphanin FQ (N/OFQ) and naltrindole (NTI) in the formalin test. X-axis: doses of NTI (0, 5, 10, or 20 nmol). Ten nmol of N/OFQ was administered intrathecally, followed 5 min later with NTI. Y-axis: percentage of control for total flinches during phase I (1-10 min, top panel) and phase II (11-60 min, bottom panel) after formalin injection. Each value represents mean±SD (n=8). *p<0.05, ‡p<0.001 versus vehicle pre-treated group (N/OFQ+NTI0). SD, standard deviation.
Effect of i.t. administration of FNX on N/OFQ-induced inhibition in formalin flinching responses
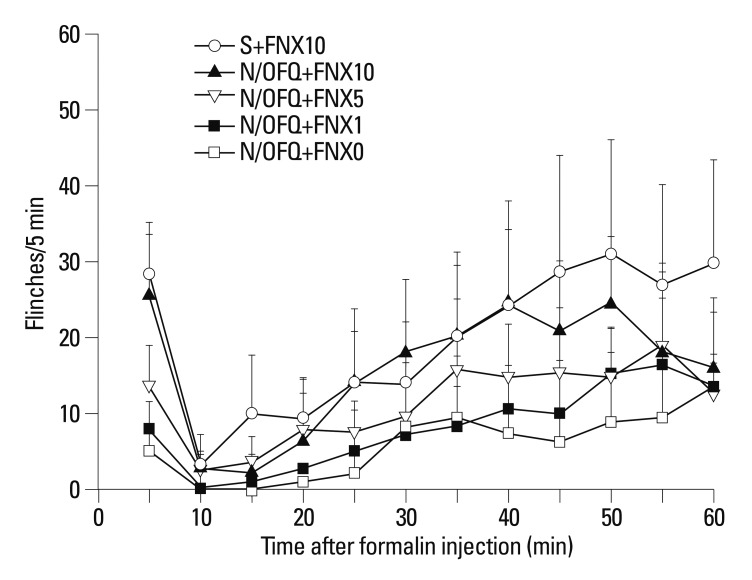
Rats first received N/OFQ (10 nmol, i.t.), followed 5 min later by 1, 5, or 10 nmol of FNX and saline vehicle (n=8 per group). The N/OFQ-induced attenuation of flinching responses was dose-dependently inhibited by FNX [F (4420)=56.27, p<0.05) (Fig. 6). For the control, 0.9% sterile saline (10 µL, i.t.) instead of N/OFQ (10 nmol, i.t.) was administered, followed 5 min later by 10 nmol of FNX (n=8 per group). Rats treated with 10 nmol of N/OFQ followed by 5 or 10 nmol of FNX (N/OFQ+FNX5 or N/OFQ+FNX10) showed statistical differences compared to those in the vehicle pre-treated group (N/OFQ+FNX0) in phase I (†p< 0.01, ‡p<0.001 respectively, top panel) (Fig. 7). Rats treated with 10 nmol of N/OFQ followed by 10 nmol of FNX (N/OFQ+FNX10) showed statistical differences compared to those in the vehicle pre-treated group (N/OFQ+FNX0) in phase II (‡p<0.001, bottom panel) (Fig. 7).
Fig. 6.
Time-effect curves of intrathecal nociceptin/orphanin FQ (N/OFQ) and co-administered β-funaltrexamine (FNX) for flinching responses in the formalin test. X-axis: time (min) after formalin injection. Y-axis: number of flinches for each 5-min session. Each value represents mean±SD (n=8). Symbols represent the different dose conditions for each panel. Ten nmol of N/OFQ was administered intrathecally, followed 5 min later by 1, 5, or 10 nmol of FNX and vehicle (0.9% sterile saline), before the formalin injection. I.t. co-administration of FNX dose-dependently reversed N/OFQ-induced flinching attenuations in the formalin test (p<0.05). SD, standard deviation.
Fig. 7.
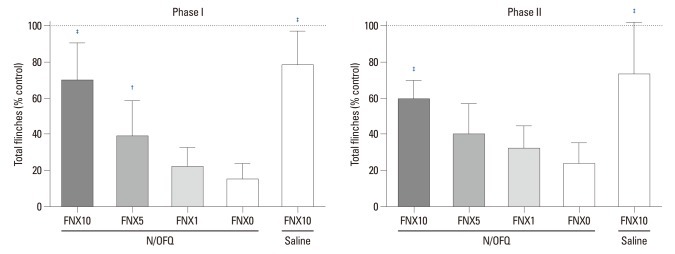
The cumulative flinching scores of the rat formalin test after intrathecal administration of nociceptin/orphanin FQ (N/OFQ) and β-funaltrexamine (FNX). X-axis: doses of FNX (0, 1, 5, or 10 nmol). Ten nmol of N/OFQ was administered intrathecally, followed 5 min later with FNX. Y-axis: percentage of control for total flinches during phase I (1-10 min, top panel) and phase II (11-60 min, bottom panel) after formalin injection. Each value represents mean±SD (n=8). †p<0.01, ‡p<0.001 versus vehicle pre-treated group (N/OFQ+FNX0). SD, standard deviation.
Effect of i.t. administration of nor-BNI on N/OFQ-induced inhibition in formalin flinching responses
Rats first received N/OFQ (10 nmol, i.t.), followed 5 min later by 10 or 20 nmol of nor-BNI and a saline vehicle (n=8 per group). The results showed that 10 nmol and 20 nmol (data not shown) of nor-BNI failed to antagonize N/OFQ-induced attenuation of flinching responses in both phases of the formalin test (p<0.05). The group that received 10 nmol of N/OFQ followed by 10 nmol of nor-BNI (N/OFQ+nor-BNI10) showed no statistical differences compared to the vehicle pre-treated group (N/OFQ+nor-BNI0) in either phase (p>0.05) (Fig. 8).
Fig. 8.
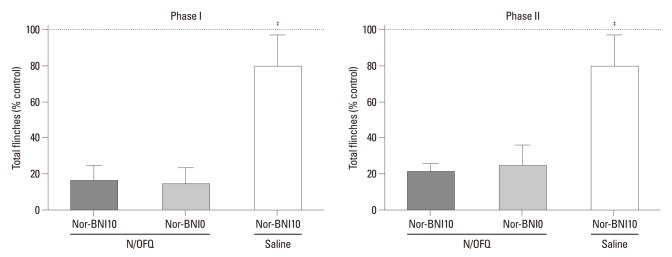
The cumulative flinching scores of rat formalin test after intrathecal-administered nociceptin/orphanin FQ (N/OFQ) and norbinaltorphimine (nor-BNI). X-axis: doses of nor-BNI (0 or 10 nmol). Ten nmol of N/OFQ was administered intrathecally, followed 5 min later with nor-BNI. Y-axis: percentage of control for total flinches during phase I (1-10 min, top panel) and phase II (11-60 min, bottom panel) after formalin injection. Each value represents mean±SD (n=8). ‡p<0.001 versus vehicle pre-treated group (N/OFQ+nor-BNI0). SD, standard deviation.
Moreover, the effects of the three opioid antagonists alone were compared. The means±SD of flinching scores in phase I were 42.1±14.2, 32.8±7.5 and 41.4±11.3 for S+NTI20, S+FNX10 and S+nor-BNI10, respectively. The means±SD of flinching scores in phase II were 271±51.2, 213.7±83.9 and 290.3±39.3 for S+NTI20, S+FNX10 and S+nor-BNI10, respectively. There were no statistical differences between groups for both phases I and II (p>0.05).
DISCUSSION
In the present study, i.t. administration of N/OFQ (1-10 nmol) dose-dependently attenuated formalin-induced flinching responses. The selected doses of N/OFQ for the antinociceptive effect in the present study (1, 5, 10 nmol) were similar to doses of N/OFQ used in previous studies.18,19 The observed antinociceptive effect of i.t. N/OFQ in the rat formalin tests was consistent with the effect noted in previous studies.9,10,12,13 This same antinociceptive effect of i.t. N/OFQ was also observed in other pain models such as tail flick, hot plate, and paw-pressure tests in rats,11,20 although there is some controversy regarding the hot plate test.2,13 This antinociceptive effect is not due to motor dysfunction or other impairments, as demonstrated by the fact that similar doses of N/OFQ do not affect the righting reflex13 or inclined plane test.11 These results suggest that N/OFQ has antinociceptive effects at the spinal level in tonic inflammatory models such as the formalin test. Interestingly, evidence shows that N/OFQ is more efficacious than morphine in the particular types of pain associated with nerve injury21 or in morphine tolerant animals.22 The antinociceptive effect of N/OFQ in the spinal cord seems to be mediated via NOP receptors, since affinity of N/OFQ for NOP receptors was 500-fold higher than that for the µ- and κ-opioid receptors and 5000-fold higher than that for the δ-opioid receptor.23 Moreover, the antinociceptive effect of i.t. N/OFQ was significantly attenuated by [Nphe1]NC(1-13)-NH2, a selective NOP antagonist,24 indicating that an NOP receptor mediates N/OFQ-induced antinociception in both phases of the rat formalin test. Similar reversal of the anti-nociceptive effect of N/OFQ with [Nphe1]NC(1-13)-NH2 was observed in the pain model of thermal and mechanical stimulation.11,25
In the present study, i.t. administration of NTI (5-20 nmol) and FNX (1-10 nmol), selective δ- and µ-opioid antagonists, respectively, dose-dependently inhibited the N/OFQ-induced attenuation of flinching responses in phases I and II in the formalin test. The effective doses of i.t. NTI for antagonizing δ-opioid receptors have been reported as 10 nmol,25,26 11.3 nmol,27 and sometimes, 24 nmol28 in various studies. The effective doses of i.t. FNX for antagonizing µ-opioid receptors have been reported as 10 pmol29 and 10 nmol11,26 in various studies. We observed that i.t. doses of selective δ- and µ-opioid antagonists for inhibition of N/OFQ-induced anti-nociception were similar to those of opioid-induced anti-nociception. The effective doses of i.t. nor-BNI for antagonizing κ-opioid receptors have been reported as 10 nmol26 and 13.3 nmol27 in various studies. However, nor-BNI (10 nmol) failed to antagonize N/OFQ-induced anti-nociception in the present study. Although nor-BNI has non-specific effects at high doses,30 10 nmol of nor-BNI is reported to be relatively κ-selective.11 This indicates that in the spinal dorsal horn, δ- and µ-opioid receptors, but not the κ-opioid receptor, are involved in the anti-nociceptive effects of N/OFQ. The lack of an antagonistic effect by κ-opioid may reflect the very low density of κ-opioid receptors in the lumbosacral spinal cord (2-3%) compared to µ- and δ-opioid receptors.31 Thus, the present study suggests a possible interaction between N/OFQ and δ- and µ-opioid receptors in processing inflammatory pain in the spinal cord.
NOP receptors are distributed throughout the central and peripheral nervous system and overlap with other opioid receptors in the spinal cord.14 NOP receptor mRNAs are expressed throughout the whole length of the spinal cord of rats. NOP receptor mRNAs are especially abundant in the lamina I and II (substantia gelatinosa) as well as lamina X (central communicating regions).32,33 This distribution of NOP receptors is similar to that of opioid receptors.34 The lamina I and II of the spinal cord are the major locations for the first synapses between primary afferent sensory neurons and secondary afferent sensory neurons. N/OFQ and opioid peptides may modulate pain processing and attenuate the intensity of pain signals in these locations. The opioid antagonists may interact with NOP receptors by an indirect way during pain processing. N/OFQ nerve terminals are also found in the dorsal horn layer, where enkephalin-positive neurons are enriched.35 This anatomical proximity probably contributes to interactions between N/OFQ and opioids in pain modulation.
The involvement of opioid receptors in the antinociceptive effect of N/OFQ in the formalin test is controversial. Previous studies demonstrated that the effects of i.t. N/OFQ were not reversed by nonselective opioid antagonists such as naloxone.8,13,36 In addition, opioid antagonists failed to antagonize the effects of i.t. N/OFQ in inflammatory or hyperalgesic pain models after neuropathic injury induced either mechanically or chemically.37 Moreover, the analgesic effect of i.t. N/OFQ could not be attenuated by subcutaneous naltrexone, but only by J-113397, an NOP selective antagonist, in primates.8 Therefore, these discrepancies in results seem to depend on experimental design, animal species, and route of administration of opioid antagonists. Others reported that differences in the type of animal pain model could be another factor of this discrepancy.11,36 It was interesting that the antinociceptive effect of i.t. N/OFQ was inhibited by administration of i.t. opioid antagonists in the rat formalin test, a model of inflammatory pain. In contrast to our investigation, naloxone could not inhibit the effect of i.t. N/OFQ in a hyperalgegic37 and neuropathic36 pain model as well as an inflammatory13 pain model. Additionally, a relatively small dose of naloxone was more effective than a higher dose in inhibiting the antihyperalgesic effect of i.t. N/OFQ.37
The mechanism of interaction between N/OFQ and opioids in the present study is not clear. As N/OFQ has a low affinity for µ-opioid receptors, it is not possible to activate opioid receptors directly. In the dorsal root ganglion, N/OFQ and opioids both produce analgesia by inhibiting the presynaptic release of neurotransmitters such as substance P, which is related to calcium influx through N-type voltage-gated calcium channels.7 Thus, one possible mechanism is that N/OFQ may reduce substance P release through opioid receptor activation at the presynaptic terminal. However, N/OFQ antagonizes the inhibition of Ca2+ currents induced by µ-opioid receptors,38 similar to those induced by the κ-opioid receptors,39 at the cellular level. Moreover, N/OFQ-induced substance P release is not blocked by µ-, δ- or κ-opioid receptor antagonists in the isolated renal pelvis.40 Alternatively, N/OFQ may increase the release of endogenous opioid peptides via activation of NOP receptor in the spinal cord dorsal horn,11 although this needs further study. Even though our data do not support the complete understanding of the exact mechanisms between N/OFQ and opioid systems, our results may partially elucidate their actions. Opioids are the most powerful anti-nociceptives in clinical settings. Therefore, our notions that N/OFQ-induced anti-nociception was inhibited by opioid antagonists in rat formalin tests may contribute to increasing our understanding of pain modulation treatment in the near future.
Taken together, our study demonstrated that N/OFQ dose-dependently attenuates formalin-induced pain in rats and that opioid antagonists (δ and µ, but not κ) inhibit the N/OFQ-induced antinociceptive effects in formalin-induced pain. Such evidence supports the notion that i.t. N/OFQ plays an important role in modulating pain processing at the spinal cord level. The mechanism and molecular basis of N/OFQ-induced alleviation of inflammatory and neuropathic pain in the spinal cord requires further investigation.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 2.Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 3.Pomonis JD, Billington CJ, Levine AS. Orphanin FQ, agonist of orphan opioid receptor ORL1, stimulates feeding in rats. Neuroreport. 1996;8:369–371. doi: 10.1097/00001756-199612200-00072. [DOI] [PubMed] [Google Scholar]
- 4.Kapusta DR, Sezen SF, Chang JK, Lippton H, Kenigs VA. Diuretic and antinatriuretic responses produced by the endogenous opioid-like peptide, nociceptin (orphanin FQ) Life Sci. 1997;60:PL15–PL21. doi: 10.1016/s0024-3205(96)00593-0. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Geller EB, Adler MW. Nociceptin/orphanin FQ blocks the antinociception induced by mu, kappa and delta opioid agonists on the cold water tail-flick test. Eur J Pharmacol. 2007;557:32–36. doi: 10.1016/j.ejphar.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hara N, Minami T, Okuda-Ashitaka E, Sugimoto T, Sakai M, Onaka M, et al. Characterization of nociceptin hyperalgesia and allodynia in conscious mice. Br J Pharmacol. 1997;121:401–408. doi: 10.1038/sj.bjp.0701146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue M, Shimohira I, Yoshida A, Zimmer A, Takeshima H, Sakurada T, et al. Dose-related opposite modulation by nociceptin/orphanin FQ of substance P nociception in the nociceptors and spinal cord. J Pharmacol Exp Ther. 1999;291:308–313. [PubMed] [Google Scholar]
- 8.Ko MC, Wei H, Woods JH, Kennedy RT. Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: behavioral and mass spectrometric studies. J Pharmacol Exp Ther. 2006;318:1257–1264. doi: 10.1124/jpet.106.106120. [DOI] [PubMed] [Google Scholar]
- 9.Rizzi A, Nazzaro C, Marzola GG, Zucchini S, Trapella C, Guerrini R, et al. Endogenous nociceptin/orphanin FQ signalling produces opposite spinal antinociceptive and supraspinal pronociceptive effects in the mouse formalin test: pharmacological and genetic evidences. Pain. 2006;124:100–108. doi: 10.1016/j.pain.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Wang JL, Zhu CB, Cao XD, Wu GC. Distinct effect of intracerebroventricular and intrathecal injections of nociceptin/orphanin FQ in the rat formalin test. Regul Pept. 1999;79:159–163. doi: 10.1016/s0167-0115(98)00161-x. [DOI] [PubMed] [Google Scholar]
- 11.Yu LC, Lu JT, Huang YH, Meuser T, Pietruck C, Gabriel A, et al. Involvement of endogenous opioid systems in nociceptin-induced spinal antinociception in rats. Brain Res. 2002;945:88–96. doi: 10.1016/s0006-8993(02)02743-9. [DOI] [PubMed] [Google Scholar]
- 12.Ahmadi S, Kotalla C, Gühring H, Takeshima H, Pahl A, Zeilhofer HU. Modulation of synaptic transmission by nociceptin/orphanin FQ and nocistatin in the spinal cord dorsal horn of mutant mice lacking the nociceptin/orphanin FQ receptor. Mol Pharmacol. 2001;59:612–618. doi: 10.1124/mol.59.3.612. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Nozaki-Taguchi N, Kimura S. Analgesic effect of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, in the rat formalin test. Neuroscience. 1997;81:249–254. doi: 10.1016/s0306-4522(97)00166-8. [DOI] [PubMed] [Google Scholar]
- 14.Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999;406:503–547. [PubMed] [Google Scholar]
- 15.Kang S, Kim CH, Lee H, Kim DY, Han JI, Chung RK, et al. Antinociceptive synergy between the cannabinoid receptor agonist WIN 55,212-2 and bupivacaine in the rat formalin test. Anesth Analg. 2007;104:719–725. doi: 10.1213/01.ane.0000255291.38637.26. [DOI] [PubMed] [Google Scholar]
- 16.Zhao C, Tall JM, Meyer RA, Raja SN. Antiallodynic effects of systemic and intrathecal morphine in the spared nerve injury model of neuropathic pain in rats. Anesthesiology. 2004;100:905–911. doi: 10.1097/00000542-200404000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T, Shono K, Tanabe S. Buprenorphine activates mu and opioid receptor like-1 receptors simultaneously, but the analgesic effect is mainly mediated by mu receptor activation in the rat formalin test. J Pharmacol Exp Ther. 2006;318:206–213. doi: 10.1124/jpet.105.100859. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Grass S, Hao J, Xu IS, Wiesenfeld-Hallin Z. Nociceptin/orphanin FQ in spinal nociceptive mechanisms under normal and pathological conditions. Peptides. 2000;21:1031–1036. doi: 10.1016/s0196-9781(00)00234-5. [DOI] [PubMed] [Google Scholar]
- 19.Tian JH, Xu W, Fang Y, Mogil JS, Grisel JE, Grandy DK, et al. Bidirectional modulatory effect of orphanin FQ on morphine-induced analgesia: antagonism in brain and potentiation in spinal cord of the rat. Br J Pharmacol. 1997;120:676–680. doi: 10.1038/sj.bjp.0700942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jhamandas KH, Sutak M, Henderson G. Antinociceptive and morphine modulatory actions of spinal orphanin FQ. Can J Physiol Pharmacol. 1998;76:314–324. [PubMed] [Google Scholar]
- 21.Chu X, Xu N, Li P, Wang JQ. The nociceptin receptor-mediated inhibition of the rat rostral ventrolateral medulla neurons in vitro. Eur J Pharmacol. 1999;364:49–53. doi: 10.1016/s0014-2999(98)00816-4. [DOI] [PubMed] [Google Scholar]
- 22.Spagnolo B, Calo G, Polgar WE, Jiang F, Olsen CM, Berzetei-Gurske I, et al. Activities of mixed NOP and mu-opioid receptor ligands. Br J Pharmacol. 2008;153:609–619. doi: 10.1038/sj.bjp.0707598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson G, McKnight AT. The orphan opioid receptor and its endogenous ligand--nociceptin/orphanin FQ. Trends Pharmacol Sci. 1997;18:293–300. [PubMed] [Google Scholar]
- 24.Calo' G, Guerrini R, Bigoni R, Rizzi A, Marzola G, Okawa H, et al. Characterization of [Nphe(1)]nociceptin(1-13)NH(2), a new selective nociceptin receptor antagonist. Br J Pharmacol. 2000;129:1183–1193. doi: 10.1038/sj.bjp.0703169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu JT, Huang YH, Palmer PP, Xie GX, Gabriel A, Grond S, et al. Blockade effects of (Nphe1)Nociceptin(1-13)-NH(2) on anti-nociception induced by intrathecal administration of nociceptin in rats. Regul Pept. 2001;101:81–85. doi: 10.1016/s0167-0115(01)00263-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhang YP, Yu LC, Lundeberg T. An interaction of opioids and galanin in dorsal horn of the spinal cord in mononeuropathic rats. Regul Pept. 2000;86:89–94. doi: 10.1016/s0167-0115(99)00091-9. [DOI] [PubMed] [Google Scholar]
- 27.Wu HE, Hung KC, Mizoguchi H, Nagase H, Tseng LF. Roles of endogenous opioid peptides in modulation of nocifensive response to formalin. J Pharmacol Exp Ther. 2002;300:647–654. doi: 10.1124/jpet.300.2.647. [DOI] [PubMed] [Google Scholar]
- 28.Ochi T, Ohkubo Y, Mutoh S. Blockade of the antinociceptive effect of spinally administered kyotorphin by naltrindole in mice. Neurosci Lett. 2002;322:95–98. doi: 10.1016/s0304-3940(02)00109-x. [DOI] [PubMed] [Google Scholar]
- 29.Goodchild CS, Nadeson R, Cohen E. Supraspinal and spinal cord opioid receptors are responsible for antinociception following intrathecal morphine injections. Eur J Anaesthesiol. 2004;21:179–185. doi: 10.1017/s0265021504003035. [DOI] [PubMed] [Google Scholar]
- 30.Guirimand F, Strimbu-Gozariu M, Willer JC, Le Bars D. Effects of mu, delta and kappa opioid antagonists on the depression of a C-fiber reflex by intrathecal morphine and DAGO in the rat. J Pharmacol Exp Ther. 1994;269:1007–1020. [PubMed] [Google Scholar]
- 31.Stevens CW, Kajander KC, Bennett GJ, Seybold VS. Bilateral and differential changes in spinal mu, delta and kappa opioid binding in rats with a painful, unilateral neuropathy. Pain. 1991;46:315–326. doi: 10.1016/0304-3959(91)90114-D. [DOI] [PubMed] [Google Scholar]
- 32.Darland T, Grandy DK. The orphanin FQ system: an emerging target for the management of pain? Br J Anaesth. 1998;81:29–37. doi: 10.1093/bja/81.1.29. [DOI] [PubMed] [Google Scholar]
- 33.Meunier JC. Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor. Eur J Pharmacol. 1997;340:1–15. doi: 10.1016/s0014-2999(97)01411-8. [DOI] [PubMed] [Google Scholar]
- 34.Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 35.Riedl M, Shuster S, Vulchanova L, Wang J, Loh HH, Elde R. Orphanin FQ/nociceptin-immunoreactive nerve fibers parallel those containing endogenous opioids in rat spinal cord. Neuroreport. 1996;7:1369–1372. doi: 10.1097/00001756-199605310-00007. [DOI] [PubMed] [Google Scholar]
- 36.Sotgiu ML, Bellomi P, Biella GE. Efficacy of nociceptin inhibition on WDR neuron activity is enhanced in mononeuropathic rats. Brain Res. 2004;998:251–254. doi: 10.1016/j.brainres.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto T, Nozaki-Taguchi N. Effects of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, and N-methyl-D-aspartate receptor antagonists on the thermal hyperalgesia induced by partial sciatic nerve injury in the rat. Anesthesiology. 1997;87:1145–1152. doi: 10.1097/00000542-199711000-00019. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Wang X, Zhang D, Xu G, Dong H, Yu Y, et al. Orphanin FQ antagonizes the inhibition of Ca(2+) currents induced by mu-opioid receptors. J Mol Neurosci. 2005;25:21–27. doi: 10.1385/JMN:25:1:021. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Sun QL, Wan Y, Yao L, Yu YX, Han JS. OFQ reverses the kappa-opioid receptor-mediated depression of calcium current in rat dorsal root ganglion neurons. Neuroreport. 1998;9:2095–2098. doi: 10.1097/00001756-199806220-00034. [DOI] [PubMed] [Google Scholar]
- 40.Giuliani S, Maggi CA. Inhibition of tachykinin release from peripheral endings of sensory nerves by nociceptin, a novel opioid peptide. Br J Pharmacol. 1996;118:1567–1569. doi: 10.1111/j.1476-5381.1996.tb15576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]