Abstract
Purpose
Transcatheter aortic valve implantation (TAVI) has become an attractive therapeutic strategy for severe aortic stenosis (AS) in elderly patients due to its minimally-invasive nature. Therefore, early results of its clinical outcomes in elderly Korean patients were evaluated.
Materials and Methods
We compared early clinical outcomes of TAVI, surgical aortic valve replacement (SAVR), and optimal medical therapy (OMT) in patients aged ≥80 years with symptomatic severe AS. Treatment groups were allocated as follows: TAVI (n=10), SAVR (n=14), and OMT (n=42).
Results
Baseline clinical characteristics including predicted operative mortality were similar among the three groups. However, patients with New York Heart Association functional class III or IV symptoms and smaller aortic valve area were treated with TAVI or SAVR rather than OMT. In-hospital combined safety endpoints (all-cause mortality, major stroke, peri-procedural myocardial infarction, life-threatening bleeding, major vascular complication, and acute kidney injury) after TAVI or SAVR were significantly lower in the TAVI group than in the SAVR group (10.0% vs. 71.4%, respectively, p=0.005), along with an acceptable rate of symptom improvement and device success. During the follow-up period, the TAVI group showed the lowest rate of 3-month major adverse cardiovascular and cerebrovascular events, a composite of all-cause mortality, myocardial infarction, major stroke, and re-hospitalization (TAVI 0.0% vs. SAVR 50.0% vs. OMT 42.9%, p=0.017).
Conclusion
Treatment with TAVI was associated with lower event rates compared to SAVR or OMT. Therefore, TAVI may be considered as the first therapeutic strategy in selected patients aged ≥80 years with symptomatic severe AS.
Keywords: Aortic stenosis, transcatheter aortic valve implantation, treatment outcome
INTRODUCTION
Aortic stenosis (AS) is a progressive disease with a poor prognosis if treated medically after symptom onset.1-4 Although surgical aortic valve replacement (SAVR) has been shown to improve symptoms and survival,5-7 patients with advanced age are at increased risk of surgical complications or death.8-10 With aging of the population, the number of very old patients with symptomatic severe AS has increased, and a significant portion of them are not candidates for SAVR because of advanced age and its associated comorbidities.11 Therefore, a less invasive treatment strategy is desired in such patients.
Transcatheter aortic valve implantation (TAVI) is a new procedure, in which a bioprosthetic valve is inserted through a catheter and implanted within the diseased native aortic valve. TAVI is a minimally-invasive procedure that avoids median sternotomy and the need for cardiopulmonary bypass support. After several clinical studies, including the pivotal United States placement of aortic transcatheter valve trial,12,13 TAVI is now considered as a safe and effective treatment modality in elderly patients with symptomatic severe AS.11,14-20
Therefore, this study was performed to compare 3-month clinical outcomes of TAVI, SAVR, and optimal medical therapy (OMT) in patients aged ≥80 years with symptomatic severe AS.
MATERIALS AND METHODS
We enrolled 66 consecutive patients aged ≥80 years who were newly diagnosed with symptomatic severe AS at our institute. Transthoracic echocardiography was used to diagnose AS according to the guidelines of the American Society of Echocardiography,21 using a digital ultrasound scanner. In the apical 5-chamber view, peak and mean transvalvular pressure gradient across the aortic valve were calculated using the Bernoulli equation. The effective aortic valve area was calculated using a continuity equation.22 All 66 patients exhibited New York Heart Association (NYHA) functional class ≥I symptoms, and their echocardiographic findings were consistent with severe AS (aortic valve area <1 cm2 with or without transvalvular mean pressure gradient ≥40 mm Hg).1 The decision for TAVI or SAVR was made for each patient according to the severity of clinical symptoms, combined comorbid disease, physical performance status and echocardiographic parameters. Patients were selected for TAVI after they were considered as inoperable or high-risk patients, assessed by two independent cardiovascular surgeons. The final treatment groups included 10 patients in the TAVI group, 14 in the SAVR group, and 42 in the OMT group.
All 10 TAVI procedures were performed using the AccuTrak CoreValve System (Medtronic Inc., Minneapolis, MN, USA) under general anesthesia. The prosthetic aortic valve was inserted in a retrograde fashion using the femoral artery (n=7), subclavian artery (n=1), or ascending aorta (n=2). Balloon valvuloplasty was performed prior to prosthetic valve deployment. All patients had transvenous temporary cardiac pacing during the procedure. Rapid ventricular pacing at 150 to 200 beats/min was performed to reduce cardiac motion and transvalvular flow during balloon dilation. Positioning and deployment of the prosthetic valve was performed under fluoroscopic guidance. Size 26 mm and 29 mm prosthetic valves were used. The 26 mm valve was selected if the aortic annulus size was 20 to 23 mm (n=6), and the 29 mm valve was selected for an aortic annulus size of 24 to 27 mm (n=4). Pre-procedural echocardiography and multi-slice computed tomography were used to measure the size of the aortic annulus. Immediately after deployment of the prosthetic valve, transesophageal echocardiography was performed to confirm good motion of the prosthetic valve and identify any paravalvular leakage.
We compared in-hospital and 3-month clinical outcomes of TAVI, SAVR, and OMT using medical, echocardiographic and angiographic data. Major adverse cardiovascular and cerebrovascular events (MACCE) during 3-month follow-up were defined as a composite of all-cause mortality, myocardial infarction, major stroke (modified Rankin score ≥2), and re-hospitalization due to severe AS or complications of TAVI or SAVR. Definition of each endpoint was in accordance with the Valve Academic Research Consortium guidelines.23 Other clinical events including transient ischemic attack,23 major bleeding, new-onset permanent atrial fibrillation, and complete atrioventricular block were also analyzed. Life-threatening bleeding23 or bleeding which required blood transfusion24 was considered major bleeding. In addition, procedural burden (procedure time and length of intensive care unit stay), combined safety endpoints (all-cause mortality, major stroke, peri-procedural myocardial infarction, life-threatening bleeding, major vascular complication, and acute kidney injury) according to the Valve Academic Research Consortium guidelines,23 and procedural efficacy (improvement of clinical symptoms and echocardiographic parameters) during hospitalization were compared between the TAVI and SAVR groups. Improvement of clinical symptoms was defined as at least one class improvement in NYHA functional class.
All statistical analyses were performed using Statistical Package for Social Sciences (SPSS) statistical software package, version 17.0 (SPSS, Chicago, IL, USA). Continuous variables were expressed as a median (interquartile range) and compared using Mann-Whitney test or Kruskal-Wallis test. Categorical variables were described as a number (%) and analyzed using chi-square test or Fisher's exact test, as appropriate. In case of results with a p-value <0.05 from the 3-group comparison, three additional pairwise comparisons were performed between each two groups. p-values <0.05 were considered statistically significant.
RESULTS
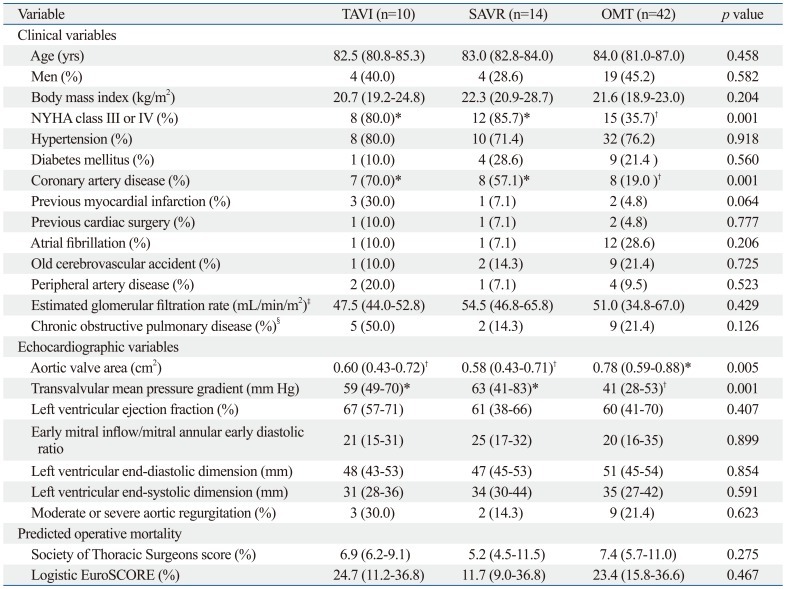
The baseline clinical and echocardiographic characteristics of the three groups are presented in Table 1. More patients in the TAVI and SAVR groups exhibited NYHA class III or IV symptoms compared to those who were treated with OMT (80.0%, 85.7% and 35.7%, p=0.001, respectively). The incidence of pre-existing coronary artery disease was also significantly higher in the TAVI and SAVR groups compared to the OMT group (70.0%, 57.1% and 19.0%, p=0.001, respectively). Smaller aortic valve area and higher mean transvalvular pressure gradient were observed in the TAVI and SAVR groups than the OMT group.
Table 1.
Baseline Clinical and Echocardiographic Characteristics
TAVI, transcatheter aortic valve implantation; SAVR, surgical aortic valve replacement; OMT, optimal medical therapy; NYHA, New York Heart Association; Logistic EuroSCORE, logistic European System for Cardiac Operative Risk Evaluation.
*,†In case of results with a p-value <0.05 from the 3-group comparison, three additional pairwise comparisons were performed between each two groups. Groups with a p-value <0.05 from the pairwise comparisons were labeled with *,†representing the groups with the higher and lower rates, respectively; i.e. The comparison between *(higher) vs. †(lower) was significant.
‡Calculated by the modification of diet in renal disease formula.
§Long-term use of bronchodilators or steroids for chronic lung disease, or forced expiratory volume in 1 second <75% of predicted.
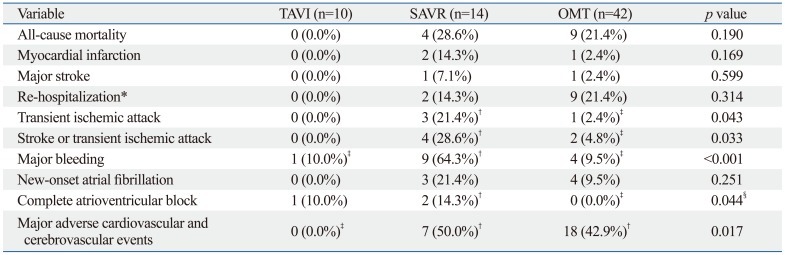
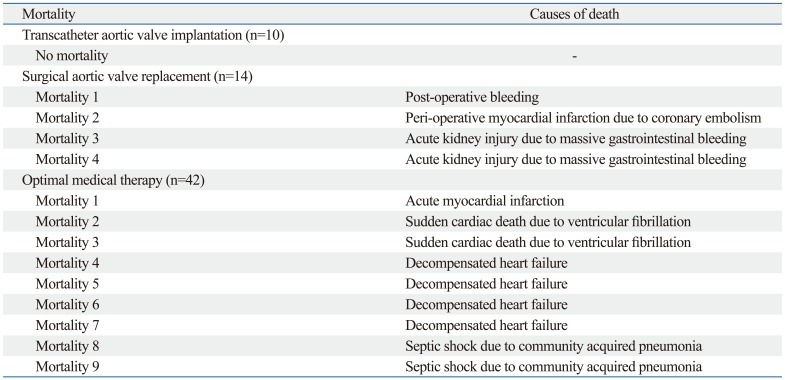
Table 2 shows the 3-month clinical outcomes among the three groups. Although there was no statistical difference in all-cause mortality among the three groups, no death occurred in the TAVI group, whereas the SAVR group had four (28.6%) cases of all-cause mortality and the OMT group had nine (21.4%). The rate of 3-month MACCE was significantly lowest in the TAVI group (0.0% in TAVI vs. 50.0% in SAVR vs. 42.9% in OMT, p=0.017). The causes of death among the three groups are listed in Table 3.
Table 2.
Clinical Outcome at 3 Months
TAVI, transcatheter aortic valve implantation; SAVR, surgical aortic valve replacement; OMT, optimal medical therapy.
Major adverse cardiovascular and cerebrovascular events, a composite of all-cause mortality, myocardial infarction, major stroke and re-hospitalization.
*Due to aortic stenosis or complications of TAVI or SAVR.
†,‡In case of results with a p-value <0.05 from the 3-group comparison, three additional pairwise comparisons were performed between each two groups. Groups with a p-value <0.05 from the pairwise comparisons were labeled with †,‡representing the groups with the higher and lower rates, respectively; i.e. The comparison between †(higher) vs. ‡(lower) was significant.
§For complete atrioventricular block, the comparison between SAVR vs. OMT was only marginally significant with p=0.059.
Table 3.
Detailed Causes of Death at 3 Months
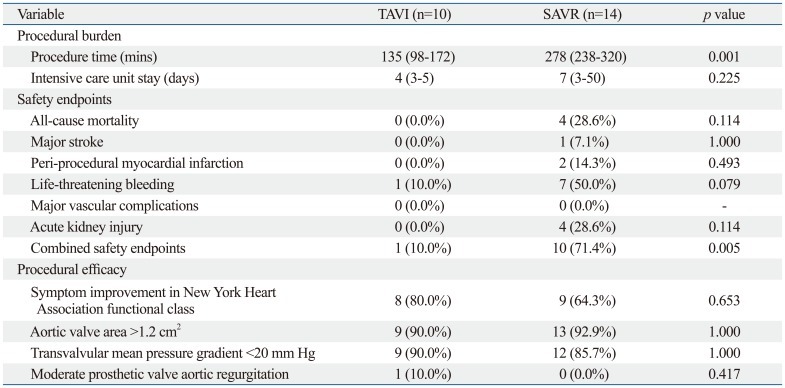
Additionally, procedural burden, safety, and efficacy during hospitalization were compared between the TAVI and SAVR groups (Table 4). Compared with the SAVR group, the TAVI group showed a lower rate of combined safety endpoints (71.4% vs. 10.0%, p=0.005, respectively).
Table 4.
Procedural Burden, Safety, and Efficacy of TAVI and SAVR during Hospitalization
TAVI, transcatheter aortic valve implantation; SAVR, surgical aortic valve replacement.
DISCUSSION
The main finding of the present study is that the TAVI group showed the lowest rate of 3-month MACCE when compared to the SAVR and OMT groups in symptomatic patients aged ≥80 years with severe AS.
The prognosis in patients with symptomatic severe AS is poor if treated medically.1-4 After symptom onset, the 1-year mortality rate in patients with severe AS and without SAVR is reportedly around 30%.2,4 Moreover, one could easily expect a higher mortality rate in the patients of the present study, aged ≥80 years, than those of previous studies,2,4 even though there are no available published data regarding the prognosis of medically treated patients in the selected populations. This was also confirmed in the present study with a reported 3-month mortality rate of 21.4% in the OMT group.
SAVR is the treatment of choice for patients with symptomatic severe AS.1 However, the risk of surgical mortality increases significantly with age.8-10 Although early mortality rate of combined valve and coronary bypass surgery was low in some reports,25 in-hospital mortality of SAVR with concomitant coronary bypass surgery has been reported as high as 24% in patients aged ≥80 years.9 In the present study, the in-hospital mortality rate of SAVR was 28.6%. Because of these high surgical mortality rates, surgeons sometimes hesitate to perform SAVR in patients aged ≥80 years, or patients themselves are reluctant to undergo SAVR in real-world clinical practice. Therefore, SAVR is occasionally reserved only for patients with very small aortic valve area or high transvalvular pressure gradient whose clinical symptoms are not stabilized by OMT alone. In a previous study by Bouma, et al.,26 the actual rate of SAVR in patients aged ≥80 years was only 23.8%. In addition, the surgery rate in patients with NYHA class I/II dyspnea or transvalvular peak pressure gradient <75 mm Hg was as low as 20%. Similarly, in the present study, the rate of SAVR was 21.2% (14 of 66 patients), and patients with more severe symptom of dyspnea, smaller aortic valve area, and greater transvalvular mean pressure gradient inevitably underwent SAVR.
Facing this dilemma between the need for surgery and the high surgical mortality rate in patients aged ≥80 years, TAVI with minimally-invasive nature could be an attractive alternative treatment modality. With proven safety and efficacy,11-20 more patients aged ≥80 years who would have been candidates for SAVR are now undergoing TAVI. Like the SAVR group, patients with more severe symptom of dyspnea, higher incidence of pre-existing coronary artery disease, smaller aortic valve area, and greater transvalvular mean pressure gradient underwent TAVI in this study.
The other main finding of the present study is that TAVI had a lower rate of in-hospital combined safety endpoints than SAVR. This was mainly due to a lower rate of in-hospital all-cause mortality and life-threatening bleeding in TAVI than in SAVR. Previous publications report that a 30-day mortality rate after TAVI ranges from 3.2% to 15.2%.12-20,27,28 Although the mean age was over 80 years in these previous studies, some of the patients were under 80 years. In the present study, in-hospital all-cause mortality rate of TAVI was 0.0%, even though all patients were over the age of 80. The excellent mortality outcomes of TAVI may be due to meticulous patient selection, pre-procedural planning, or post-procedural care in strict compliance with recommendations from TAVI experts and accumulated data. In addition, they could be due to an evolving device profile, skillful operators, or the minimally-invasive nature of TAVI itself. Only one case (10.0%) of major bleeding (bloody pericardial effusion necessitating pericardial window formation) occurred in the TAVI group, whereas nine cases (64.3%) of major bleeding (four cases of post-operative bleeding and five cases of gastrointestinal bleeding) occurred in the SAVR group. This difference may be due to the invasive nature of SAVR or anticoagulation after SAVR. Because TAVI is a catheter-based procedure, it entails a high possibility of major vascular complication,29,30 cerebrovascular accident,31,32 or significant prosthetic valve aortic regurgitation.33 However, no major vascular complication or stroke occurred after TAVI. Only one case of prosthetic valve aortic regurgitation of moderate grade occurred without significant symptoms. These low rates of complications were able to be achieved by a careful 'pre-closure' technique,34 catheter manipulation, meticulous evaluation of peripheral vascular structure using computed tomography, and pre-procedural accurate measurement of annular size using computed tomography and echocardiogram. Another common concern with TAVI is a high rate of permanent pacemaker insertion due to atrioventricular conduction block.35,36 We were able to avoid this complication by accurately choosing the size and implantation depth of the prosthetic valve. As a result, complete atrioventricular conduction block occurred in only 1 patient (10.0%) after TAVI. However, 2 patients (14.3%) in the SAVR group had complete atrioventricular conduction block after surgery and needed permanent pacemaker insertion.
The main limitation of the present study was the small sample size and short follow-up duration. Because TAVI is a relatively new procedure, long-term follow-up of a large patient population is required for more accurate comparison with SAVR and OMT. However, our results could be helpful in choosing the best therapeutic strategy for patients aged ≥80 years with symptomatic severe AS.
In conclusion, TAVI demonstrated the best 3-month clinical outcomes when compared to SAVR and OMT. Therefore, TAVI might be considered as the first therapeutic strategy in patients aged ≥80 years with symptomatic severe AS.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (nos. A085012 and A102064), a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (no. A085136), and the Cardiovascular Research Center, Seoul, Korea.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 2.Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg. 2006;82:2111–2115. doi: 10.1016/j.athoracsur.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 3.Iung B, Cachier A, Baron G, Messika-Zeitoun D, Delahaye F, Tornos P, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26:2714–2720. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- 4.Chizner MA, Pearle DL, deLeon AC., Jr The natural history of aortic stenosis in adults. Am Heart J. 1980;99:419–424. doi: 10.1016/0002-8703(80)90375-0. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. Ann Thorac Surg. 2009;88(1 Suppl):S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 6.Shahian DM, O'Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 3--valve plus coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(1 Suppl):S43–S62. doi: 10.1016/j.athoracsur.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 7.Lund O. Preoperative risk evaluation and stratification of long-term survival after valve replacement for aortic stenosis. Reasons for earlier operative intervention. Circulation. 1990;82:124–139. doi: 10.1161/01.cir.82.1.124. [DOI] [PubMed] [Google Scholar]
- 8.Bose AK, Aitchison JD, Dark JH. Aortic valve replacement in octogenarians. J Cardiothorac Surg. 2007;2:33. doi: 10.1186/1749-8090-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolh P, Kerzmann A, Honore C, Comte L, Limet R. Aortic valve surgery in octogenarians: predictive factors for operative and long-term results. Eur J Cardiothorac Surg. 2007;31:600–606. doi: 10.1016/j.ejcts.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Leontyev S, Walther T, Borger MA, Lehmann S, Funkat AK, Rastan A, et al. Aortic valve replacement in octogenarians: utility of risk stratification with EuroSCORE. Ann Thorac Surg. 2009;87:1440–1445. doi: 10.1016/j.athoracsur.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 11.Faxon DP. Transcatheter aortic valve implantation: coming of age. Circulation. 2011;124:e439–e440. doi: 10.1161/CIRCULATIONAHA.111.065243. [DOI] [PubMed] [Google Scholar]
- 12.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 13.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 14.Ewe SH, Ajmone Marsan N, Pepi M, Delgado V, Tamborini G, Muratori M, et al. Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am Heart J. 2010;160:1113–1120. doi: 10.1016/j.ahj.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Gotzmann M, Bojara W, Lindstaedt M, Ewers A, Bösche L, Germing A, et al. One-year results of transcatheter aortic valve implantation in severe symptomatic aortic valve stenosis. Am J Cardiol. 2011;107:1687–1692. doi: 10.1016/j.amjcard.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 16.Eltchaninoff H, Prat A, Gilard M, Leguerrier A, Blanchard D, Fournial G, et al. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J. 2011;32:191–197. doi: 10.1093/eurheartj/ehq261. [DOI] [PubMed] [Google Scholar]
- 17.Zahn R, Gerckens U, Grube E, Linke A, Sievert H, Eggebrecht H, et al. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J. 2011;32:198–204. doi: 10.1093/eurheartj/ehq339. [DOI] [PubMed] [Google Scholar]
- 18.Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–1090. doi: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Buellesfeld L, Gerckens U, Schuler G, Bonan R, Kovac J, Serruys PW, et al. 2-year follow-up of patients undergoing transcatheter aortic valve implantation using a self-expanding valve prosthesis. J Am Coll Cardiol. 2011;57:1650–1657. doi: 10.1016/j.jacc.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 20.Moat NE, Ludman P, de Belder MA, Bridgewater B, Cunningham AD, Young CP, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol. 2011;58:2130–2138. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 21.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Otto CM. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol. 2006;47:2141–2151. doi: 10.1016/j.jacc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, et al. Standardized endpoint definitions for Transcatheter Aortic Valve Implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–269. doi: 10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 24.The GUSTO investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Chang BC, Yoo KJ. Surgical management of coexisting coronary artery and valvular heart disease. Yonsei Med J. 2010;51:326–331. doi: 10.3349/ymj.2010.51.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouma BJ, van Den Brink RB, van Der Meulen JH, Verheul HA, Cheriex EC, Hamer HP, et al. To operate or not on elderly patients with aortic stenosis: the decision and its consequences. Heart. 1999;82:143–148. doi: 10.1136/hrt.82.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lange R, Bleiziffer S, Mazzitelli D, Elhmidi Y, Opitz A, Krane M, et al. Improvements in transcatheter aortic valve implantation outcomes in lower surgical risk patients: a glimpse into the future. J Am Coll Cardiol. 2012;59:280–287. doi: 10.1016/j.jacc.2011.10.868. [DOI] [PubMed] [Google Scholar]
- 28.Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. [DOI] [PubMed] [Google Scholar]
- 29.Van Mieghem NM, Nuis RJ, Piazza N, Apostolos T, Ligthart J, Schultz C, et al. Vascular complications with transcatheter aortic valve implantation using the 18 Fr Medtronic CoreValve System: the Rotterdam experience. EuroIntervention. 2010;5:673–679. doi: 10.4244/eijv5i6a111. [DOI] [PubMed] [Google Scholar]
- 30.Tchetche D, Dumonteil N, Sauguet A, Descoutures F, Luz A, Garcia O, et al. Thirty-day outcome and vascular complications after transarterial aortic valve implantation using both Edwards Sapien and Medtronic CoreValve bioprostheses in a mixed population. EuroIntervention. 2010;5:659–665. doi: 10.4244/eijv5i6a109. [DOI] [PubMed] [Google Scholar]
- 31.Rodés-Cabau J, Dumont E, Boone RH, Larose E, Bagur R, Gurvitch R, et al. Cerebral embolism following transcatheter aortic valve implantation: comparison of transfemoral and transapical approaches. J Am Coll Cardiol. 2011;57:18–28. doi: 10.1016/j.jacc.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 32.Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al-Rashid F, Weber M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation. 2010;121:870–878. doi: 10.1161/CIRCULATIONAHA.109.855866. [DOI] [PubMed] [Google Scholar]
- 33.Détaint D, Lepage L, Himbert D, Brochet E, Messika-Zeitoun D, Iung B, et al. Determinants of significant paravalvular regurgitation after transcatheter aortic valve: implantation impact of device and annulus discongruence. JACC Cardiovasc Interv. 2009;2:821–827. doi: 10.1016/j.jcin.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Kahlert P, Eggebrecht H, Erbel R, Sack S. A modified "preclosure" technique after percutaneous aortic valve replacement. Catheter Cardiovasc Interv. 2008;72:877–884. doi: 10.1002/ccd.21711. [DOI] [PubMed] [Google Scholar]
- 35.Guetta V, Goldenberg G, Segev A, Dvir D, Kornowski R, Finckelstein A, et al. Predictors and course of high-degree atrioventricular block after transcatheter aortic valve implantation using the CoreValve Revalving System. Am J Cardiol. 2011;108:1600–1605. doi: 10.1016/j.amjcard.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Khawaja MZ, Rajani R, Cook A, Khavandi A, Moynagh A, Chowdhary S, et al. Permanent pacemaker insertion after CoreValve transcatheter aortic valve implantation: incidence and contributing factors (the UK CoreValve Collaborative) Circulation. 2011;123:951–960. doi: 10.1161/CIRCULATIONAHA.109.927152. [DOI] [PubMed] [Google Scholar]