Abstract
Several clinical theories propose that borderline personality disorder (BPD) is characterized by a biologically based affective vulnerability to intense affective experiences and impaired modulation of affective states, which might manifest in high emotional intensity, hyperreactivity, and impaired recovery to baseline. However, few studies have tested these theories based on emotional and biological responses of BPD participants in response to psychosocial stressors. This study examined cortisol, alpha-amylase, and subjective emotional reactivity to social evaluative stress among women with BPD compared with two healthy female control groups: a trait-matched (TM) group scoring similarly to the BPD group on trait measures of negative affect and impulsivity, and a non-trait-matched (NTM) group. Results generally suggested high emotional intensity and high baseline psychobiological arousal among individuals with BPD, but not emotional hyperreactivity or impaired recovery specific to the stressor. Relative to both control groups, BPD participants had higher baseline and overall subjective negative affect, higher baseline cortisol levels, and attenuated stress-related cortisol reactivity. In addition, both the BPD and TM groups had attenuated alpha-amylase reactivity in comparison to the NTM group. The differences between BPD and TM groups on most of the dependent measures suggest that emotional dysregulation in BPD is not merely an extreme variant of normative personality traits. These results suggest that women with BPD demonstrate intense and chronic negative affectivity along with high resting psychobiological arousal and attenuated psychobiological reactivity specific to laboratory stressors.
Keywords: borderline personality disorder, emotional dysregulation, psychobiological, hypothalamicpituitary-adrenal axis, autonomic nervous system
Evidence suggests that emotional dysregulation is one of the most prominent, problematic, and enduring features of borderline personality disorder (BPD; McGlashan et al., 2005; Tragesser, Solhan, Schwartz-Mette, & Trull, 2007). A number of clinical theories propose that BPD is characterized by emotional intensity, rapid and heightened affective reactivity, and impaired modulation of affective states, particularly within social contexts (Clarkin & Posner, 2005; Linehan, 1993). Accordingly, studies suggest abnormalities in frontolimbic circuitry in those with BPD that are consistent with difficulties modulating emotional arousal (Wingenfeld, Spitzer, Rullkötter, & Löwe, 2010). These abnormalities could be mediated at least in part by dysregulation of the stress response system, which is comprised of the autonomic nervous system (ANS) and hypothalamic-pituitary-adrenal (HPA) axis. Upon onset of a stressful or emotionally evocative event, the sympathetic division of the ANS is first activated, resulting in the rapid release of catecholamines as part of the body’s “fight or flight” response. Minutes later, the HPA axis is activated by sympathetic nervous system input, culminating in release of cortisol. This system is acutely sensitive to social evaluative stress (Dickerson & Kemeny, 2004) and can become dysregulated through repeated and chronic activation, having wide-spread deleterious effects on general health and cognitive functioning (McEwen, 2004). Thus, the functioning of the stress response system can have important implications for understanding emotional dysregulation, health, and mortality among those with BPD.
Evidence to date suggests that patients with BPD may differ from healthy controls in stress response system functioning, but results from previous studies are ambiguous and often contradictory. One study found that a subgroup of patients with BPD with dissociative tendencies had higher peak cortisol levels in response to stress relative to healthy controls (Simeon, Knutelska, Smith, Baker, & Hollander, 2007). Another investigation suggested delayed HPA axis reactivity to stress and impaired poststress recovery in patients with BPD (Walter et al., 2008). In addition, ANS hyperarousal, as assessed by cardiac sympathetic index and respiratory sinus arrhythmia, has been demonstrated in those with BPD (Weinberg, Klonsky, & Hajcak, 2009). A more recent report showed evidence of elevated cortisol levels in the anticipation of a conflict discussion among young women with BPD (Lyons-Ruth, Choi-Kain, Pechtel, Bertha, & Gunderson, 2011). On the other hand, one study indicates lower resting cortisol and salivary alpha-amylase (sAA, a marker for ANS arousal) levels and attenuated cortisol and sAA reactivity (Nater et al., 2010). However, many previous studies are limited by small samples, use of invasive methods (e.g., venipuncture), lack of clinically referred patient samples, and lack of control for confounds such as time of day and comorbid Axis I symptoms that are known to influence stress responses.
The psychological substrates of emotional dysregulation in BPD are also not well-defined. Trait negative affect and impulsivity (NA/IMP) are normative personality traits that are often elevated in those with BPD (e.g., Widiger & Costa, 2002) and that could potentially increase vulnerability to heightened subjective and psychobiological stress reactivity. Such trait dimensions are likely to be important aspects of personality disorder assessment in DSM–5 (Skodol & Bender, 2009). However, the role of normative traits in BPD-related emotional dysregulation has not yet been examined, leaving uncertainty as to the clinical utility of these constructs for defining BPD. Recent evidence suggests that BPD symptoms and personality traits are overlapping but not redundant constructs, and that some core elements of BPD are not fully captured by normative traits (Hopwood & Zanarini, 2010). In addition, some research indicates that traits cannot explain psychobiological reactivity to novel stressors (e.g., van Eck, Berkhof, Nicolson, & Sulon, 1996). Hence, whereas trait NA/IMP may reflect temperamental aspects of BPD, they might not explain episodic and reactive responses to environmental events.
Because of the mixed results of previous studies, the biological and psychological concomitants of emotional arousal in BPD are still poorly understood. This study is designed to simultaneously test three theorized aspects (i.e., emotional intensity, hyperreactivity, and impaired recovery to baseline) of affective dysregulation in BPD. We assessed HPA axis, ANS, and negative affective reactivity in response to a psychosocial stressor among women with BPD compared with two healthy comparison groups: (a) trait-matched (TM) controls who scored similarly to BPD participants on trait measures of NA/IMP; and (b) non-trait-matched (NTM) controls who scored in the average range on these measures. To address several limitations of previous studies, we recruited a treatment-seeking BPD sample, used noninvasive salivary measures of neurobiological stress responses (i.e., cortisol and sAA), and controlled for several factors known to affect neurobiological responses to stress (e.g., time of day, dissociation, major depressive disorder [MDD], and posttraumatic stress disorder [PTSD]). We hypothesized that participants with BPD would show greater intensity (higher baseline and overall levels), greater reactivity (faster rate of change from baseline), and impaired recovery (slower rate of decline after stress) on each of the dependent measures in comparison to both control groups.
Method
Participants
Participants were 90 women between the ages of 18 and 50 (BPD n = 33; TM n = 27; NTM n = 30). BPD participants were recruited from an outpatient community mental health clinic. Comparison participants were identified through online screening of university students and community residents using subscales from the Revised NEO Personality Inventory (NEO-PI-R; Costa & McCrae, 1992). TM participants were selected based on T scores within 1 SD of the BPD sample on these measures, whereas NTM participants were selected who scored within 1 SD of the normative population means.1 Exclusion criteria for the patient group included diagnoses of psychotic disorders, Bipolar I disorder, delirium, dementia, and mental retardation. Exclusion criteria for the two control groups included current DSM–IV Axis I or II diagnoses (including probable Axis II diagnoses and probable or definite Personality Disorder Not Otherwise Specified [PD-NOS] diagnoses), suicidal or self-injurious behaviors, or meeting more than two DSM–IV criteria for BPD. Other exclusion criteria that applied to all participants included heart disease, pregnancy within the last 6 months, current lactation, and endocrinological diseases other than diabetes and thyroid disorders, because evidence suggests that these are common illnesses among psychiatric populations and their exclusion would limit generalizability of study results (Jones, Macias, Barreira, Fisher, Hargreaves, & Harding, 2004).
Sample demographics are summarized in Table 1. Groups did not differ significantly in race, ethnicity, employment status, or education. The BPD group was older, on average, and more likely to be divorced, than controls. Although not shown in Table 1, groups did not differ in the number of days since the beginning of their last menstrual cycle or in use of hormonal contraceptives. With regard to current Axis I diagnoses in the BPD group, 7 (21%) had mood disorders, 12 (36%) had anxiety disorders, 7 (21%) had substance-related disorders, and 6 (18%) had other disorders (e.g., somatoform or eating disorders). The majority of BPD participants (79%) were taking at least one psychotropic medication, including antidepressants (58%), anticonvulsants or mood stabilizers (30%), antipsychotics (33%), sedatives (30%), and stimulants (15%). Only two comparison participants (one TM and one NTM) were taking psychotropic medications, both of which were taking an antidepressant. In accordance with Granger, Hibel, Fortunato, and Kapelewski (2009) we calculated an index of the total likely influence of all medications on salivary biomarkers for a given participant, taking into account the various pathways by which medications can affect biomarkers. This value was explored as a potential covariate in each analysis.
Table 1.
Sample Characteristics
| BPD (n = 33) | TM (n = 27) | NTM (n = 30) | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | Test statistic (df) | |
| Race | |||||||
| White/Caucasian | 28 | 84.8 | 22 | 81.5 | 24 | 80.0 | χ2(2) = 0.27 |
| Ethnicity | |||||||
| Hispanic/Latina | 0 | 0 | 4 | 14.8 | 5 | 16.7 | χ2(2) = 5.84 |
| Marital status | |||||||
| Single | 20 | 60.6 | 22 | 81.5 | 26 | 86.7 | χ2(4) = 10.06* |
| Divorced | 8 | 24.2 | 1 | 3.7 | 1 | 3.3 | |
| Married | 5 | 15.2 | 4 | 14.8 | 3 | 10.0 | |
| Employed | 17 | 51.5 | 20 | 74.1 | 18 | 60.0 | χ2(2) = 3.20 |
| M | SD | M | SD | M | SD | ||
| Age | 30.42a | 7.64 | 23.74b | 7.51 | 22.70b | 7.59 | F(2, 87) = 9.64*** |
| Education (years) | 14.00 | 1.54 | 13.78 | 2.38 | 13.53 | 2.21 | F(2, 73.21) = 0.39 |
| NEO-PI-R scales | |||||||
| Angry hostility | 68.28a | 10.29 | 63.26a | 10.28 | 46.93b | 6.85 | F(2, 86) = 44.15*** |
| Anxiety | 64.94a | 7.07 | 57.93b | 9.57 | 47.83c | 6.90 | F(2, 86) = 36.93*** |
| Depression | 70.34a | 6.94 | 60.41b | 8.75 | 44.47c | 5.99 | F(2, 86) = 99.76*** |
| Impulsivity | 62.13a | 11.00 | 58.59a | 6.84 | 43.47b | 6.25 | F(2,71.75) = 43.80*** |
| SSPRS | 6.38a | 1.57 | 6.65a | 1.51 | 4.97b | 1.55 | F(2, 87) = 10.11*** |
| DSS | 1.24 | 1.30 | 1.01 | 1.37 | 0.68 | 0.93 | F(2, 86) = 1.69 |
Note. BPD = borderline personality disorder; TM = trait-matched group; NTM = non-trait-matched group; NEO-PI-R = Revised NEO Personality Inventory; SSPRS = Subjective Stress Perception Rating Scale; DSS = Dissociation Tension Scale. Degrees of freedom with decimal places denote Brown-Forsythe Robust Test of Equality of Means (correcting for inhomogeneity of variance). Row means with different subscripts are significantly different from each other at p < .05 or less using Bonferroni-corrected (or Tamhane’s T2 in the case of inhomogeneity of variance) post-hoc tests. Degrees of freedom differ for NEO-PI-R scales because of missing data.
p < .05.
p < .001.
Measures
NEO-PI-R facet scales
The Angry Hostility, Anxiety, Depression, and Impulsivity facet scales of the NEO-PI-R (Costa & McCrae, 1992) were administered to assess trait NA/IMP. Items were rated on a five-point scale (0 = strongly disagree, 4 = strongly agree). Each scale demonstrated high internal consistency in this study (α = .84 to .92).
Health Form
The Health Form was constructed by the authors and administered prior to the stress procedure to assess recent stressors, food and beverage intake, physical activity, substance use, recent dental hygiene activity, sleep-wake patterns, and menstrual cycle phase.
Positive and Negative Affect Schedule: Negative Affect subscale (PANAS-NA)
The PANAS-NA (Watson, Clark, & Tellegen, 1988) was administered three times (at baseline, immediately after the stressor, and 40 min later). Participants were asked to rate the extent to which they were experiencing each emotion at the present moment. Items were rated on a five-point scale (1 = very slightly, 5 = extremely). The PANAS-NA demonstrated high internal consistency across the three administrations (α = .81 to .87).
Subjective Stress Perception Rating Form (SSPRS)
The SSPRS was created by the authors as a manipulation check of the effectiveness of the stressor, and contained six items rated on a nine-point scale (1 = not at all, 9 = very much) to assess participants’ subjective perceptions of the stressor as difficult, stressful, uncontrollable, threatening, hostile, and evaluative. Interitem correlations and exploratory factor analysis suggested that items comprised one robust factor with high internal consistency (α = .82). Hence, we utilized the mean of these six items.
Dissociation Tension Scale (DSS)
The DSS (Stiglmayr et al., 2010) is a self-report measure of present-state dissociative experiences, and was administered immediately after the stress procedure. Participants rated the intensity with which they experienced each item during the course of the stress procedure using a Likert scale from 0 to 9. Scores were calculated based on the mean of all items. The DSS had excellent internal consistency (α = .92).
Structured Clinical Interview for DSM–IV (SCID-I)
The SCID-I (First, Gibbon, Spitzer, & Williams, 1997) is a well-validated and widely used semistructured clinical interview for diagnosing DSM–IV Axis I disorders in persons 18 years of age or older. Interrater reliability Kappas (κ) for SCID-I Axis I diagnoses ranged from .64 to 1.0 in this study.
International Personality Disorder Examination (IPDE)
The IPDE (Loranger, 1999) is a semistructured interview for diagnosing DSM–IV personality disorders. Interrater reliability Kappas ranged from .71 to 1.0 for IPDE diagnoses (κ = .88 for BPD diagnosis). Intraclass correlations were .94 for number of BPD criteria met and .98 for BPD dimensional scores.
Procedures
Based on a brief telephone screen, eligible participants were invited to the laboratory to complete questionnaires and diagnostic interviews. After complete description of the study to participants, written informed consent was obtained. All procedures were approved by the institution’s Office for Research Protections. Interviewers were advanced clinical psychology graduate students who were trained in diagnostic interviewing and were blind to participants’ recruitment source (i.e., clinical referral vs. community). Interviews were videotaped and 20% were randomly selected from each group and scored by independent raters in order to calculate interrater reliability Kappas (κ) for diagnoses (reported under Measures).
On a separate day, participants completed the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993), which involves public speaking and verbal arithmetic tasks in front of three “judges.” To control for factors known to influence salivary biomarkers, participants completed the TSST during the mid to late afternoon and during the follicular phase of the menstrual cycle. Participants were also asked to follow specific instructions on the day of the TSST: no alcohol for 24 hrs; no medications or drugs (except prescribed medications) for 6 hrs; no caffeine, tobacco, rigorous exercise, tooth brushing/flossing, or dental work for 2 hrs; no food or beverages (other than water) for 1 hr; no dairy or citrus foods or beverages for 30 min prior to the appointment; and remain awake for at least 4 hrs prior to their appointment. Compliance with instructions was assessed via the Health Form. Noncompliant participants were rescheduled to complete the study at another time.
Procedures for the TSST closely followed those outlined by Kirschbaum et al. (1993). Participants rinsed their mouths with water, completed the Health Form and the first PANAS-NA, provided the first saliva sample, and then rested quietly for 30 min before the TSST began. Participants were then introduced to the TSST in a separate room. They were given 5 min to prepare a speech explaining why they would be the best candidate for their ideal job and were told that their performance was to be videotaped and rated by the judges for coherence, poise, and expressiveness. After the speech, participants were asked to perform a 5-min serial subtraction task (i.e., counting aloud backward in increments of 13 starting at 1,022). They then sat quietly for 40 min and completed the SSPRS, DSS, and PANAS-NA. Participants were then fully debriefed and assessed for any signs of residual distress.
A total of eight saliva samples were collected from each participant via the passive drool method: (a) 10 min after arrival at the laboratory and after rinsing with water (time = 0 min); (b) 30 min later, after resting quietly; (c) immediately prior to starting the speech portion of the TSST; (d) immediately following completion of the TSST; and (e– h) each occurring 10 min after the last sample. Saliva samples were assayed for cortisol in duplicate using a commercially available enzyme immunoassay (Salimetrics, LLC). The assay uses 25 µl of saliva per determination, has a lower limit of detection of 0.003 µg/dl, standard curve range from 0.012 to 3.0 µg/dl, and average intra- and interassay coefficients of variation less than 5% and 10%, respectively. Samples were assayed for sAA using a commercially available kinetic reaction assay (Salimetrics, LLC), which employs a chromagenic substrate, 2-chloro-p-nitrophenol, linked to maltotriose. The enzymatic action of sAA on this substrate yields 2-chloro-p-nitrophenol, which can be measured spectrophotometrically at 405 nm using a standard laboratory plate reader. The amount of sAA activity present in the sample is directly proportional to the increase (over a 2-min period) in absorbance at 405 nm. The intraassay variation (CV) based on 30 replicate tests was less than 7.5%. The interassay variation based on 16 separate runs was less than 6%.
Statistical Analysis
Analyses were conducted using SPSS 19 (SPSS Inc., Chicago, IL). Data were tested for normality of distributions and homogeneity of variance with Kolmogorov–Smirnov and Levene’s tests. To examine group differences in demographic and clinical characteristics, univariate analyses of variance (ANOVAs) for continuous variables and χ2 analyses for categorical variables were conducted. Brown-Forsythe F values are reported (as reflected in degrees of freedom with decimal values) for data that violated the homogeneity of variance assumption. Bonferroni corrections were applied where required for multiple comparisons, except in the case of inhomogeneity of variance, in which case the Tamhane’s T2 test is reported.
Study hypotheses were tested using piecewise multilevel linear mixed (MLM) models (Llabre, Spitzer, Saab, & Schneiderman, 2001), which simultaneously estimated an intercept (i.e., first measure upon study entry) and three separate linear slopes for each biomarker (i.e., for cortisol and sAA): (a) prestress decline (resting), (b) stress-related increase (reactivity), and (c) poststress decline (recovery). Time was coded into three separate level-1 predictors for changes in cortisol and sAA, and two level-1 predictors (reactivity and recovery) for changes in negative affect (NA). Time was coded in minutes from the first measure, and divided by 10 so that one unit of time was equal to 10 min. The coding scheme is illustrated in Table 2 (see also Llabre et al., 2001 for further details on coding time in piecewise models). Because of the more rapid reactivity and recovery of sAA relative to cortisol, coding schemes differed between these measures. Random effects for intercept and slopes were included in all models, with the exception of slopes comprised of only two data points. Restricted maximum likelihood (REML) estimation was used to assess the significance of random effects, and an unstructured covariance matrix was specified to obtain robust SEs. Degrees of freedom were estimated using the Satterthwaite method. In all models, the intercept represents the predicted average baseline level (represented by the first measure upon study entry) of the dependent variable.
Table 2.
Coding Scheme for Time in Piecewise Linear Mixed Models
| Measurement/sample number | ||||||||
|---|---|---|---|---|---|---|---|---|
| Linear slopes | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Cortisol | ||||||||
| Resting | 0 | 3 | 4 | 4 | 4 | 4 | 4 | 4 |
| Reactivity | 0 | 0 | 0 | 1.4 | 2.4 | 2.4 | 2.4 | 2.4 |
| Recovery | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 3 |
| Alpha-amylase | ||||||||
| Resting | 0 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Reactivity | 0 | 0 | 1 | 2.4 | 2.4 | 2.4 | 2.4 | 2.4 |
| Recovery | 0 | 0 | 0 | 0 | 1 | 2 | 3 | 4 |
| Negative affect | ||||||||
| Reactivity | 0 | 5.4 | 5.4 | |||||
| Recovery | 0 | 0 | 4 | |||||
Note.. One unit of time is equal to 10 min. See Llabre et al. (2001) for further details on coding time in piecewise models.
In addition, analyses of covariance (ANCOVAs) were performed to test hypotheses regarding overall intensity of response. For cortisol and sAA, we calculated the area under the curve from ground (AUCG; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003), which gives an index of total biomarker output (i.e., elevation), independent from baseline levels or changes over time. To assess overall levels of NA, we calculated the average of all three measures of the PANAS-NA.
Results
Preliminary Analyses
Dependent variables were first examined for outliers (±3 SD from the group means). There were 5 participants (2 BPD, 3 comparisons) with extreme cortisol values, 5 participants (3 BPD, 2 comparisons) with extreme sAA values, and 3 participants (1 BPD, 2 comparisons) with extreme PANAS-NA values. In accordance with previous studies (e.g., Edwards et al., 2003; Eiden et al., 2009), outliers were winsorized according to Tukey’s (1977) method (i.e., replaced with values exactly 3 SD from the mean). Less than 1% of biomarker data points were missing for reasons such as sample viscosity or insufficient sample volumes. Because AUCs can still be calculated with missing values and mixed models can handle missing data, all participants were included in the analyses, including those with missing values. One BPD participant was missing the last PANAS-NA because of experimenter error; this value was replaced by the BPD group mean for the last PANAS-NA measure prior to calculation of the overall NA mean.
Group differences in NA/IMP, SSPRS, and DSS scales are presented in Table 1. Both the BPD and TM groups were significantly higher than the NTM group in each of the four trait NA/IMP scales, suggesting successful differentiation of the TM group from the NTM group. In addition, the TM group did not differ significantly from the BPD group in trait Angry Hostility or Impulsivity, indicating successful matching on these trait variables. The TM group was significantly lower than the BPD group in trait Anxiety and Depression, but the TM group was still significantly higher than the NTM group on these traits, suggesting successful differentiation from the NTM group on these variables. Mean SSPRS scores demonstrated that each group reported experiencing the TSST as at least moderately stressful and evaluative, and both the BPD and TM groups experienced the TSST as significantly more stressful than did the NTM group.
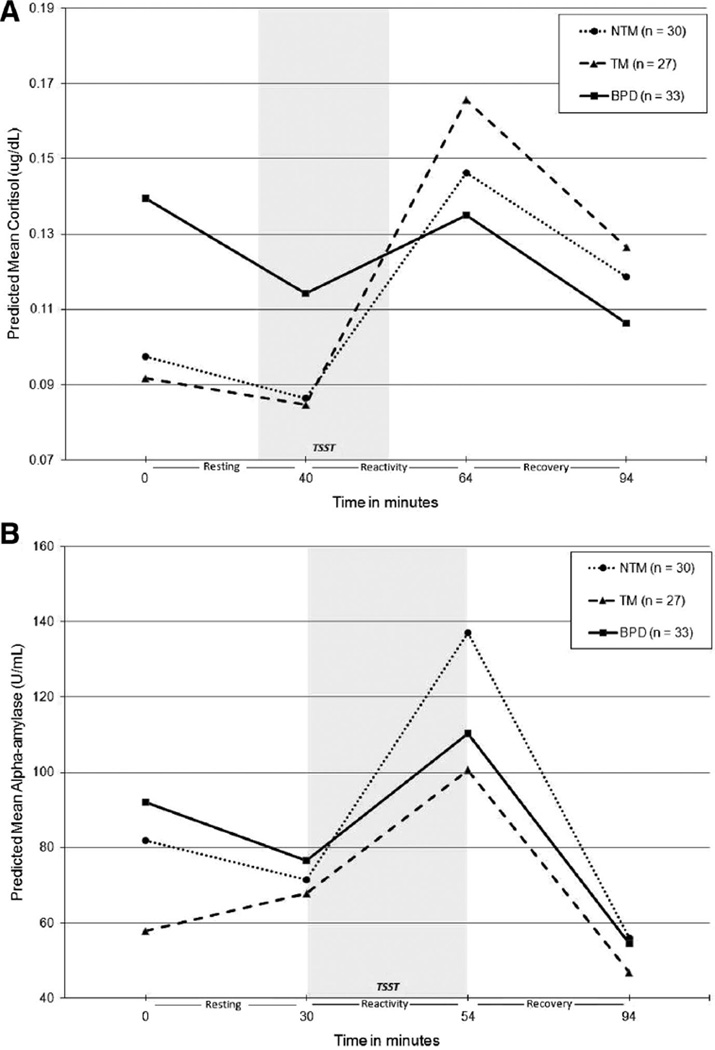
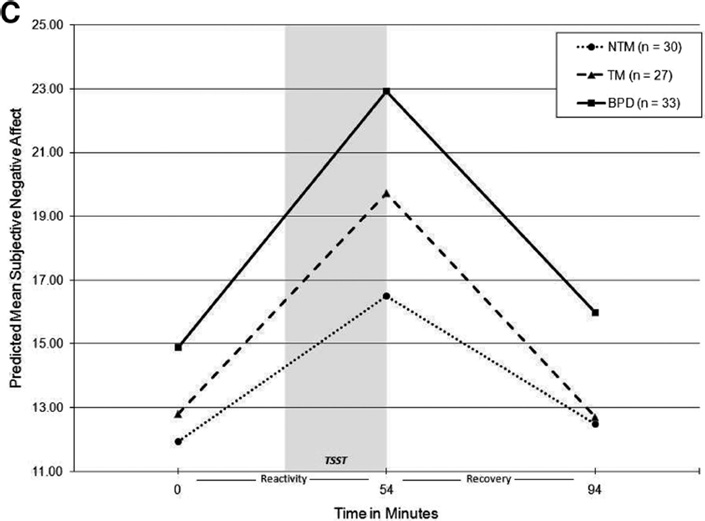
Raw cortisol and PANAS-NA values were log-transformed and sAA was square root-transformed prior to MLM analyses to correct for non-normality of the distributions. Age, time of day, medication influence codes, dissociation (DSS), and total current symptoms of MDD and PTSD (based on the SCID-I) were entered as covariates in the initial models. Covariates reaching at least marginal significance (p < .10) were retained in subsequent analyses. In all level-1 MLM models, all variance components for intercepts and slopes (random effects) were significant, indicating significant variability between individuals to warrant the inclusion of level-2 predictors. Table 3 displays final level 2 MLM model coefficients, demonstrating both the significance of within-group slopes (denoted by asterisks) and between-groups differences in intercepts and slopes (denoted by subscripts). Figure 1 illustrates model-predicted piecewise linear trajectories for cortisol, sAA, and NA in each group.
Table 3.
Fixed Effects Estimates for Final Piecewise Linear Mixed Models
| Parameter | BPD (n = 33) | TM (n = 27) | NTM (n = 30) |
F value (df) for Omnibus tests of group differences in parameters |
|---|---|---|---|---|
| Salivary cortisol (LOG) | ||||
| Intercept | −1.97a (0.13)*** | −2.39b (0.12)*** | −2.33b (0.11)*** | F(2, 85.08) = 3.13* |
| Resting slope | −0.05 (0.02)** | −0.02 (0.02) | −0.03 (0.02) | F(2, 86.33) = 0.91 |
| Reactivity slope | 0.07a (0.05) | 0.28b (0.06)*** | 0.22b (0.05)*** | F(2, 84.92) = 4.75** |
| Recovery slope | −0.08 (0.02)*** | −0.09 (0.03)*** | −0.07 (0.02)** | F(2, 86.09) = 0.24 |
| Salivary alpha-amylase (SQRT) | ||||
| Intercept | 9.59 (0.81)*** | 7.60 (0.73)*** | 9.05 (0.69)*** | F(2, 110.90) = 1.90 |
| Resting slope | −0.28a (0.13)* | 0.21b (0.14) | −0.20a (0.13) | F(2, 440.71) = 3.74* |
| Reactivity slope | 0.73a (0.20)*** | 0.75a (0.22)*** | 1.36b (0.21)*** | F(2, 118) = 2.84 |
| Recovery slope | −0.78 (0.13)*** | −0.80 (0.14)*** | −1.06 (0.13)*** | F(2, 87.14) = 1.39 |
| Subjective negative affect (LOG) | ||||
| Intercept | 2.70a (0.06)*** | 2.55b (0.06)*** | 2.48b (0.05)*** | F(2, 181.08) = 4.73** |
| Reactivity slope | 0.08 (0.01)*** | 0.08 (0.01)*** | 0.06 (0.01)*** | F(2, 171.26) = 0.88 |
| Recovery slope | −0.09 (0.01)*** | −0.11 (0.01)*** | −0.07 (0.01)*** | F(2, 171.58) = 1.80 |
Note. BPD = borderline personality disorder; TM = trait-matched group; NTM = non-trait-matched group; LOG = natural log-transformed; SQRT = square-root-transformed. SEs are in parentheses. Coefficients are in the scale of transformed variables. Model for cortisol was adjusted for age, time of day, and posttraumatic stress disorder (PTSD) symptoms; model for salivary alpha-amylase (sAA) was adjusted for major depressive disorder (MDD) and PTSD symptoms; model for negative affect was adjusted for age, dissociation, and PTSD. The significance of within-group model coefficients (i.e., intercept and changes over time within groups) are denoted by asterisks. Row means with different subscripts are significantly different from each other at p < .05.
p < .05.
p < .01.
p < .001.
Figure 1.
Model-predicted group trajectories for salivary cortisol (A), alpha-amylase (B), and subjective negative affect (C). BPD = borderline personality disorder; TM = trait-matched. NTM = non-trait-matched; TSST = Trier Social Stress Test. Model coefficients were back-transformed into original units of measurement for calculation of predicted mean values. Model for cortisol was adjusted for age, time of day, and current posttraumatic stress disorder (PTSD) symptoms; model for salivary alpha-amylase (sAA) was adjusted for current major depressive disorder (MDD) and PTSD symptoms; model for negative affect (NA) was adjusted for age, dissociation, and PTSD.
Primary Analyses
Cortisol
Cortisol models were adjusted for age, time of day, and current PTSD symptoms. The BPD group had significantly higher baseline cortisol relative to both comparison groups, with no significant difference between NTM and TM groups in baseline cortisol. As shown in Table 3, only the BPD group showed a significant decrease in cortisol during the resting phase prior to stress, although groups did not differ significantly in resting slopes.2 In addition, the BPD group showed a slower rate of stress-related cortisol increase relative to both comparison groups. In fact, only the BPD group failed to show significant stress-related cortisol increase, as evidenced by the nonsignificant reactivity slope. All groups demonstrated a significant decrease in cortisol during the recovery period, and groups did not differ in rates of cortisol recovery. Means (with SDs in parentheses) for total cortisol output (AUCG [in µg/dL]) in the NTM, TM, and BPD groups were 13.76 (7.39), 13.96 (7.76), and 12.96 (8.92), respectively. Groups did not differ in total cortisol output, F(2, 84) = 1.11, p = .34, η2 = .03.
Alpha-amylase
All sAA models were adjusted for MDD and PTSD symptoms. Groups did not differ in sAA intercept, suggesting no significant difference in baseline sAA levels upon study entry. However, as with resting cortisol, only the BPD group demonstrated a significant decrease in sAA during the resting phase prior to stress. The BPD and NTM groups showed a more rapid resting decline in sAA relative to the TM group prior to stress, and there was no significant difference in rate of decrease between BPD and NTM groups. Although the omnibus test of group differences in sAA reactivity slope did not quite reach significance, F(2, 118) = 2.84, p = .06, estimates of individual fixed effects revealed significantly slower rates of stress-related sAA reactivity in both the BPD and TM groups compared with the NTM group, and the BPD and TM groups did not differ in rates of stress-related sAA reactivity. All three groups demonstrated a significant decrease in sAA during the recovery phase, and groups did not differ in rates of sAA recovery. Means (and SDs) for total sAA output (AUCG [in U/mL]) in the NTM, TM, and BPD groups were 9,086.57 (5,249.05), 8,326.89 (6,367.78), and 9,798.98 (6,850.04), respectively. Groups did not differ in total sAA output, F(2, 85) = 0.27, p = .76, η2 = .01.
NA
NA models were adjusted for age, dissociation (DSS), and PTSD. The BPD group reported higher baseline NA compared with both NTM and TM groups, and there was no difference between NTM and TM groups in baseline NA. All groups demonstrated significant increases in NA in response to stress and significant decreases in NA during recovery, with no significant differences between groups in rates of stress-related NA reactivity or recovery. Means (and SDs) for average NA across all three administrations of the PANAS in the NTM, TM, and BPD groups were 15.54 (4.12), 17.38 (4.34), and 20.64 (5.65), respectively. Groups differed in average NA, F(2, 83) = 9.63, p < .001, η2 = .19, with the BPD group reporting higher overall elevation in NA relative to both control groups across the laboratory task (NTM vs. BPD, p = .001; TM vs. BPD, p = .009; NTM vs. TM, p = .50).
Discussion
This study sought to test clinical theories of affective dysregulation in BPD by examining subjective affective and psychobiological responses to a psychosocial stressor. BPD participants showed evidence of intense subjective negative affectivity at baseline that remained high with stress, as well as high resting HPA axis arousal, compared with both NTM and TM groups. However, specific to the stressor, the BPD group showed attenuated cortisol reactivity relative to both control groups. Our results for sAA were less clear, but suggested attenuated stress-related sAA reactivity in the BPD and TM groups relative to the NTM group. Groups did not differ in NA reactivity to stress, recovery to baseline on any measure, or total biomarker output levels.
Our results are partially consistent with one report of attenuated cortisol and sAA reactivity among those with BPD (Nater et al., 2010). However, we found that BPD participants had higher pre-stress resting cortisol levels compared with both control groups, which is congruent with studies suggesting high resting physiological arousal among individuals with BPD (e.g., Kuo & Linehan, 2009; Lyons-Ruth et al., 2011). This may reflect HPA axis hyper-arousal in anticipation of an ambiguous event; alternatively, this may suggest heightened arousal in response to daily life stress that is independent of the experimental situation. Although the BPD group’s cortisol did not differ significantly from controls by the start of the TSST, the residual elevation at baseline meant that BPD participants would have needed to reach a higher stress-related cortisol peak in order to show a similar rate of increase from baseline relative to controls. Thus, results suggest a different overall organization of HPA axis response in the BPD group, characterized by high baseline levels and a lack of response specific to the stressor. Meanwhile, both control groups start with low cortisol levels and then show a sharp increase with stress, followed by the expected decrease during poststress recovery. Combined with results from previous studies (Lyons-Ruth et al., 2011; Nater, 2010), this finding suggests a complex picture of HPA axis dysregulation in those with BPD, characterized by high resting arousal and dysregulated reactivity specific to environmental stress.
The high NA in the BPD group compared with both control groups is consistent with a number of studies suggesting intense NA but not hyperreactivity of NA in those with BPD (e.g., Herpertz et al., 1999; Jacob et al., 2008, 2009; Kuo & Linehan, 2009). These findings suggest that emotional dysregulation in those with BPD might be more appropriately characterized as intense subjective negative affectivity, rather than emotional hyperreactivity and impaired recovery as suggested by current theories. However, a recent study (Gratz, Rosenthal, Tull, Lejuez, & Gunderson, 2010) suggested that BPD participants show hyperreactivity and impaired recovery only with respect to shame in response to negative feedback. Although there is an evaluative component to the TSST, there is no negative feedback provided, and our measure of general negative affect does not allow for much differentiation between specific emotions. Future research in this area would benefit from finer-grained investigation of specific emotions and contextual precipitants of emotional responses in those with BPD.
The differences between the BPD and TM groups in subjective NA and cortisol levels suggests that emotional dysregulation in BPD must be understood as more than just extreme variation on these normative personality traits, which has implications for assessment of BPD in DSM-5, as well as for theories of emotional dysregulation in BPD. Our assessment procedures ensured that although the TM group showed elevated BPD-related personality traits, they were without clinically significant Axis I or II symptoms. Thus, individuals can be high on BPD-related personality traits, yet not show significant signs of psychopathology or aberrant patterns of HPA axis or subjective emotional responses to stress. On the other hand, the similarity between BPD and TM groups in sAA reactivity suggests that blunted autonomic responsiveness in those with BPD might potentially be related to affective traits that these groups share in common. These findings indicate that the role of emotional and impulsive traits in autonomic dysregulation should be further explored, perhaps with additional measures of ANS functioning.
The mixed results across previous studies of emotional responding in those with BPD might be attributable at least in part to differences between studies in biological systems assessed by distinct types of measures. Several studies suggest heightened psychophysiological reactivity to aversive stimuli in those with BPD (e.g., Ebner-Priemer et al., 2005; Hazlett et al., 2007; Weinberg et al., 2009), but salivary biomarker measures often differ from psychophysiological indices. Accordingly, previous findings suggest that no single measure in isolation can sufficiently capture the complexity and diversity of psychobiological stress responses (Bauer, Quas, & Boyce, 2002). Previous studies also differ in the type of environmental stimuli used to elicit responses. It is possible that the TSST, which involves interaction with strangers, may fail to tap into BPD-related emotional dysregulation, which often occurs in the context of intimate relationships. However, the BPD participants reported experiencing the procedure as highly stressful and personally relevant. Discrepancies between study results may also be attributable to the heterogeneity of BPD, which may lead to differences across studies between clinical samples in symptom profiles, medication use, or severity of impairment.
With regard to the assessment of emotional functioning in those with BPD, these results suggest that underlying biological stress responses among patients with BPD are not fully captured by subjective experiences of emotion and can be considered separate dimensions of emotional response. In addition, the high baseline and attenuated stress-related cortisol reactivity in the BPD group suggests that current clinical theories may not adequately capture the complexity of emotional dysregulation in BPD. Although many existing interventions for BPD focus on reducing emotional hyperreactivity, a lack of stress-related cortisol increase may be equally problematic. For instance, evidence suggests that children who have low cortisol reactivity to threat respond poorly to psychosocial interventions (van de Wiel, van Goozen, Matthys, Snoek, & van Engeland, 2004). Hence, disruptions in affective processing and arousal may be important mechanisms by which many individuals with BPD fail to improve with psychotherapy. Accordingly, increasing the flexibility of patients’ emotional responses (e.g., downregulating chronic emotional arousal and upregulating normative responsiveness to environmental input) could be a potentially important new treatment target, pending further investigation.
Strengths of this study include the use of a well-validated and naturalistic environmental stressor, repeated and careful measurement of noninvasive salivary biomarkers, a clinically referred and severely impaired BPD sample, and the inclusion of two healthy control groups, one of which was matched to the BPD group in traits relevant to BPD. Relatively few studies have noninvasively examined biological responses to social evaluative stressors among patients with BPD, and this is only the second study to examine sAA as a marker of ANS arousal in BPD participants. In addition, the BPD participants were clinically referred and representative of those seen in clinical practice in terms of severity, medication use, and comorbidity. It is noteworthy that all of the BPD participants met the criterion of affective instability; hence, the current sample is particularly relevant for examining emotional dysregulation in BPD. Moreover, the TM comparison group is an innovative aspect of the current study, which allowed for the investigation of extreme variation on normative personality traits as putative underlying mechanisms of emotional dysregulation in BPD.
Interpretation of these findings is limited by differences between groups in factors such as age, medications, and comorbid symptoms, which cannot be completely ruled out as potential confounds based solely on covariate analyses. Several BPD participants also met criteria for substance-related disorders, which could have influenced psychobiological response patterns. Experiences of adversity and trauma may also have influenced psychobiological responses in ways that are not necessarily reflected in symptoms of PTSD. Although the heterogeneity and complex comorbidity of the BPD sample enhances generalizability, these features obfuscate the specificity of these findings to BPD pathology. A recommendation for future studies is to examine clinical characteristics that predict distinct patterns of emotional responding within large samples with elevated BPD features. In addition, the TM and BPD groups differed in trait anxiety and depression, leaving uncertainty as to the role of these specific traits in explaining differences between the BPD and TM groups. The lack of clear differences between the BPD group and both comparison groups in sAA also limits our ability to draw firm conclusions about the respective roles of BPD and trait NA/IMP in patterns of autonomic responding. Furthermore, this study is limited by the lack of psychophysiological measures and by the lack of a control (i.e., nonstress) condition. Finally, these results cannot be generalized to men with BPD or to emotional responses in the context of intimate relationships.
Overall, our findings suggest heightened resting cortisol levels and intense subjective negative emotional arousal among those with BPD, as well as reduced psychobiological reactivity specific to a generalized psychosocial stressor. Our results provide some support for the high emotional intensity aspect, but not hyperreactivity and impaired recovery aspects, of current clinical theories of affective dysregulation in BPD. Findings also indicate that extreme variation on normative personality traits does not fully explain emotional dysregulation in BPD, providing further support for the continued use of traditional personality disorder criteria along with normative traits in DSM-5. These results highlight the importance of understanding the altered functioning of the stress response system as a component of emotional dysregulation in BPD.
Acknowledgments
This research was supported by grants to Kenneth N. Levy from the Pennsylvania State University Social Science Research Institute, International Psychoanalytic Association, and American Psychoanalytic Association, and by grants to Lori N. Scott from the National Institute of Mental Health (F31 MH081395, PI: Lori N. Scott), Pennsylvania State University College of Liberal Arts, and American Psychological Association. This article is based on data also used in the doctoral dissertation of the first author. We would like to thank Joseph E. Beeney, M.S., William D. Ellison, M.S., Ann B. Stonebraker, M.S., Christina M. Temes, Rachel H. Wasserman, Ph.D., and Samantha Bernecker for their assistance in conducting interview assessments. We would also like to thank Amber L. Walser, Psy.D., Rachel L. Tomko, Stevie N. Grassetti, M.A., Samantha Bernecker, and Laura Moser for their assistance in recruiting participants and technical assistance, and the members of the lab who contributed to the study by serving as judges in the Trier and coding data. In the interest of full disclosure, Douglas A. Granger is the founder of Salimetrics LLC (State College, PA), serves as the company’s chief scientific and strategy advisor, and this relationship is managed by the policies of the Johns Hopkins University committee on conflict of interest at the School of Medicine.
Footnotes
Drs. Lori N. Scott and Kenneth N. Levy report no competing interests.
Until a sizable BPD group was recruited in this study for matching control participants on NA/IMP dimensions, potential TM comparisons were initially selected based on Morey et al.’s (2002) reported descriptive statistics from a sample of patients with BPD on the same NA/IMP scales used in this study.
In Figure 1(A), the high baseline cortisol level in the BPD group appears to persist through the 30-min resting period. However, supplemental univariate ANCOVAs for log-transformed cortisol values at measures 2 and 3 demonstrated that groups did not differ significantly in cortisol levels at the end of the resting phase/beginning of stress reactivity phase.
Contributor Information
Lori N. Scott, Department of Psychology, Pennsylvania State University
Kenneth N. Levy, Department of Psychology, Pennsylvania State University
Douglas A. Granger, Center for Interdisciplinary Salivary Bioscience Research, Johns Hopkins University
References
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental and Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Clarkin JF, Posner M. Defining the mechanisms of borderline personality disorder. Psychopathology. 2005;28:56–63. doi: 10.1159/000084812. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Revised NEO PersonalityInventory (NEO–PI–R) and NEO Five-Factor Inventory (NEO–FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol reactivity: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Badeck S, Beckmann C, Wagner A, Feige B, Weiss I, Bohus M. Affective dysregulation and dissociative experience in female patients with borderline personality disorder: A startle response study. Journal of Psychiatric Research. 2005;39:85–92. doi: 10.1016/j.jpsychires.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Edwards S, Hucklebridge F, Clow A, Evans P. Components of the diurnal cortisol cycle in relation to upper respiratory symptoms and perceived stress. Psychosomatic Medicine. 2003;65:320–327. doi: 10.1097/01.psy.0000033123.70631.8e. [DOI] [PubMed] [Google Scholar]
- Eiden RD, Veira Y, Granger DA. Prenatal cocaine exposure and infant cortisol reactivity. Child Development. 2009;80:528–543. doi: 10.1111/j.1467-8624.2009.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured clinical interview for Axis I DSM-IV disorders (SCID-I) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1997. [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Gratz KL, Rosenthal MZ, Tull MT, Lejuez CW, Gunderson JG. An experimental investigation of emotional reactivity and delayed emotional recovery in borderline personality disorder: The role of shame. Comprehensive Psychiatry. 2010;51:275–285. doi: 10.1016/j.comppsych.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Speiser LJ, Goodman M, Roy M, Carrizal M, Wynn JK, New AS. Exaggerated affect-modulated startle during unpleasant stimuli in borderline personality disorder. Biological Psychiatry. 2007;62:250–255. doi: 10.1016/j.biopsych.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Kunert HJ, Schwenger UB, Sass H. Affective responsiveness in borderline personality disorder: A psychophysiological approach. American Journal of Psychiatry. 1999;156:1550–1556. doi: 10.1176/ajp.156.10.1550. [DOI] [PubMed] [Google Scholar]
- Hopwood CJ, Zanarini MC. Borderline personality traits and disorder: Predicting prospective patient functioning. Journal of Consulting and Clinical Psychology. 2010;78:585–589. doi: 10.1037/a0019003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob GA, Guenzler C, Zimmerman S, Scheel CN, Rüsch N, Rainer L, Lieb K. Time course of anger and other emotions in women with borderline personality disorder: A preliminary study. Journal of Behavior Therapy and Experimental Psychiatry. 2008;39:391–402. doi: 10.1016/j.jbtep.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Jacob GA, Kathrin H, Ower N, Pillmann M, Scheel CN, Rüsch N, Lieb K. Emotional reactions to standardized stimuli in women with borderline personality disorder: Stronger negative affect, but no differences in reactivity. Journal of Nervous and Mental Disease. 2009;197:808–815. doi: 10.1097/NMD.0b013e3181bea44d. [DOI] [PubMed] [Google Scholar]
- Jones DR, Macias C, Barreira PJ, Fisher WH, Hargreaves WA, Harding CM. Prevalence, severity, and co-occurrence of chronic physical health problems of persons with serious mental illness. Psychiatric Services. 2004;55:1250–1257. doi: 10.1176/appi.ps.55.11.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Social Stress Test: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kuo JR, Linehan MM. Disentangling emotion processes in borderline personality disorder: Physiological and self-reported assessment of biological vulnerability, baseline emotional intensity, and reactivity to emotionally evocative stimuli. Journal of Abnormal Psychology. 2009;118:531–544. doi: 10.1037/a0016392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York,, NY: Guilford; 1993. [Google Scholar]
- Llabre MM, Spitzer SB, Saab PG, Schneiderman N. Piecewise latent growth curve modeling of systolic blood pressure reactivity and recovery from the cold pressor test. Psychophysiology. 2001;38:951–960. doi: 10.1111/1469-8986.3860951. [DOI] [PubMed] [Google Scholar]
- Loranger AW. International Personality Disorder Examination (IPDE) manual. Odessa, FL: Psychological Assessment Resources, Inc.; 1999. [Google Scholar]
- Lyons-Ruth K, Choi-Kain L, Pechtel P, Bertha E, Gunderson J. Perceived parental protection and cortisol responses among young females with borderline personality disorder and controls. Psychiatry Research. 2011;189:426–432. doi: 10.1016/j.psychres.2011.07.038. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress. Annals of the New York Academy of Sciences. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Grilo CM, Sanislow E, Ralevski LC, Morey LC, Gunderson JG, Pagano M. Two-year prevalence and stability of individual DSM-IV criteria for schizotypal, borderline, avoidant, and obsessive-compulsive personality disorders: Toward a hybrid model of axis II disorders. American Journal of Psychiatry. 2005;162:883–889. doi: 10.1176/appi.ajp.162.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey LC, Gunderson JG, Quigley BD, Shea T, Skodol AE, McGlashan TH, Zanarini MC. The representation of borderline, avoidant, obsessive-compulsive, and schizotypal personality disorders by the five-factor model. Journal of Personality Disorders. 2002;16:215–234. doi: 10.1521/pedi.16.3.215.22541. [DOI] [PubMed] [Google Scholar]
- Nater UM, Bohus M, Abbruzzese E, Ditzen B, Gaab J, Kleindienst N, Ehlert U. Increased psychological and attenuated cortisol and alpha-amylase responses to acute psychosocial stress in female patients with borderline personality disorder. Psychoneuroendocrinology. 2010;35:1565–1572. doi: 10.1016/j.psyneuen.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Simeon D, Knutelska M, Smith L, Baker BR, Hollander E. A preliminary study of cortisol and norepinephrine reactivity to psychosocial stress in borderline personality disorder with high and low dissociation. Psychiatry Research. 2007;149:177–184. doi: 10.1016/j.psychres.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Bender DS. The future of personality disorders is DSM-V? American Journal of Psychiatry. 2009;166:388–391. doi: 10.1176/appi.ajp.2009.09010090. [DOI] [PubMed] [Google Scholar]
- Stiglmayr CE, Schimke P, Wagner T, Braakmann D, Schweiger U, Sipos V, Kienast T. Development and psychometric characteristics of the Dissociation Tension Scale. Journal of Personality Assessment. 2010;92:269–277. doi: 10.1080/00223891003670232. [DOI] [PubMed] [Google Scholar]
- Tragesser SL, Solhan M, Schwartz-Mette R, Trull TJ. The role of affective instability and impulsivity in predicting future BPD features. Journal of Personality Disorders. 2007;21:603–614. doi: 10.1521/pedi.2007.21.6.603. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory data analysis. Don Mills, Ontario: Addison-Wesley; 1997. [Google Scholar]
- van de Wiel NMH, Van Goozen SHM, Matthys W, Snoek H, Van Engeland H. Cortisol and treatment effect in children with disruptive behavior disorders: A preliminary study. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1011–1018. doi: 10.1097/01.chi.0000126976.56955.43. [DOI] [PubMed] [Google Scholar]
- van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosomatic Medicine. 1996;58:432–446. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Walter M, Bureau J, Holmes BM, Bertha EA, Hollander M, Wheelis J, Lyons-Ruth K. Cortisol response to interpersonal stress in young adults with borderline personality disorder: A pilot study. European Psychiatry. 2008;23:201–204. doi: 10.1016/j.curpsy.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klonsky ED, Hajcak G. Autonomic impairment in borderline personality disorder: A laboratory investigation. Brain and Cognition. 2009;71:279–286. doi: 10.1016/j.bandc.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Widiger TA, Costa PT. FFM personality disorder research. In: Costa PT Jr, Widiger TA, editors. Personality disorders and the Five-Factor Model of personality. 2nd ed. Washington, DC: American Psychological Association; 2002. pp. 59–87. [Google Scholar]
- Wingenfeld K, Spitzer C, Rullkötter N, Löwe B. Borderline personality disorder: Hypothalamus pituitary adrenal axis and findings from neuroimaging studies. Psychoneuroendocrinology. 2010;35:154–170. doi: 10.1016/j.psyneuen.2009.09.014. [DOI] [PubMed] [Google Scholar]