Abstract
Few studies have examined stroke risk in T1DM. Stroke incidence, predictors, and survival were thus explored. Pittsburgh EDC Study participants (n=658) with childhood-onset T1DM were followed biennially for 18 years. Baseline (1986–1988) mean age and diabetes duration were 28 and 19 years, respectively. Stroke incidence/type was determined via survey or physician interview and, when possible, confirmed with medical/autopsy records. During follow-up, 31 (4.7%) strokes occurred (21 ischemic, 8 hemorrhagic, 2 unclassified), mean age=40.2 years (range 23–60). In multivariable Cox modelling, diabetes duration, SBP, non-HDLc, WBC, and pulse significantly predicted ischemic stroke. Adding overt nephropathy (HR=4.4, 95% CI, 1.5–12.4) to the model replaced SBP. Survival after stroke was 80.6%, 45.2%, and 9.6% at 1, 5, and 10 years, respectively, significantly worse after hemorrhagic stroke (p=0.03). These risk factors merit careful evaluation and management to prevent stroke in T1DM, which occurs at least 20 years earlier than in the general population.
Introduction
Type 1 diabetes mellitus (T1DM) increases the risk for many long-term complications, especially renal and cardiovascular disease. Although many T1DM studies have included stroke as part of a composite cardiovascular endpoint, few have specifically focused on cerebrovascular disease in T1DM.1–6
Two recent studies have reported that T1DM does in fact increase the risk of stroke compared to nondiabetic persons, especially before 50 years of age.5–7 Mortality from stroke is increased 3- to 4-fold in T1DM compared to the general population.3 Prospective studies examining predictors of stroke in T1DM have shown that, univariately, conventional cardiovascular risk factors (i.e., hypertension and dyslipidemia), as well as proteinuria, increase stroke risk in this population.1,2,4
Using the Pittsburgh EDC cohort, our objectives were to: 1) determine the incidence of stroke in a large T1DM population; examine univariate and multivariable associations 2) between baseline variables and incident stroke, and 3) between time-varying variables and incident stroke; and 4) estimate median survival after incident stroke.
Participants and Methods
Study population
Complete methodology for patient selection, examination, and laboratory testing in the Pittsburgh EDC Study has been described in detail.8,9 Briefly, the EDC Study began in 1986–1988 consisting of 658 individuals diagnosed 1950–1980 with childhood-onset T1DM (age<17 years) and seen at Children’s Hospital of Pittsburgh within 1 year of diagnosis. Mean age diabetes duration were 28 and 19 years, respectively, at baseline (1986–1988). Participants have been followed biennially by survey and/or examination. Research protocols were approved by the University of Pittsburgh Institutional Review Board, and all participants provided written informed consent.
Outcome variable
Incident stroke (as well as hospitalization for any reason) was assessed biennially via survey. Medical records were obtained, when possible, to verify stroke occurrence and type and were reviewed by a physician (TJO). Stroke was defined as a neurological deficit of acute onset lasting ≥24 hours without other evident cause.
Predictor variables
Blood pressure was measured with a random-zero sphygmomanometer after a 5-min rest according to the Hypertension Detection and Follow-up Program protocol.10 Hypertension was defined as a blood pressure ≥140/90 mmHg or antihypertensive medication use. Height and weight were measured to calculate BMI. An ever smoker was defined as one who had smoked ≥100 lifetime cigarettes.
Fasting blood samples were taken to measure glycosylated hemoglobin (HbA1), lipids, lipoproteins, and serum creatinine. Original HbA1 values were converted to DCCT-aligned HbA1c for all analyses. Total cholesterol and triglycerides were measured enzymatically, and HDL cholesterol was determined with a heparin and manganese chloride precipitation technique.11 Non-HDL cholesterol was the difference between total and HDL cholesterol levels.
White blood cell counts were obtained using a Coulter Counter (model S-plus IV, Beckman-Coulter, Fullerton, CA) and fibrinogen using a biuret colorimetric procedure and a clotting method. Serum and urinary albumin were measured by immunonephelometry,12 and creatinine was assayed using an Ectachem 400 Analyzer (Eastman Kodak, Rochester, NY). Renal damage status was based on urinary albumin excretion rates (AER) from two of three timed urine collections as follows: microalbuminuria, AER=20–200 μg/min; overt nephropathy, AER>200 μg/min or, in the absence of urine, a serum creatinine level >2 mg/dl, renal failure or renal transplantation. Glomerular filtration rate (GFR) was estimated using the Cockcroft-Gault equation.13
CAD was defined as CAD death, fatal or nonfatal myocardial infarction confirmed either by hospital records or Q-waves on electrocardiogram (Minnesota code 1.1, 1.2), angiographic stenosis ≥50% confirmed by hospital records, revascularization, EDC-diagnosed angina, or ischemic ECG changes (Minnesota code 1.3, 4.1–4.3, 5.1–5.3, 7.1). Lower extremity arterial disease (LEAD) included claudication (based on the Rose questionnaire), amputation, or ankle brachial index <0.8.14
Proliferative retinopathy (PR) was assessed using stereoscopic fundus photographs of fields 1, 2, and 4 with a Zeiss camera, read by the Fundus Photography Reading Center, University of Wisconsin-Madison and classified based on the modified Arlie House system.15 PR was defined as receiving laser phototherapy for proliferative diabetic retinopathy for participants refusing fundus photographs or unable to attend an EDC clinic visit (n=43).
Mortality data
Vital status was determined as of 1 January 2010 via participant contact and searches in both the Social Security Death Index and the National Death Index. Each death was confirmed by death certificate. In addition, attempts were made to obtain the following, if appropriate: pertinent medical records, autopsy/coroner’s reports; and interview with next-of-kin regarding the circumstances surrounding the death. Each decedent’s underlying cause of death, and all contributing causes, was determined by a Mortality Classification Committee of at least 2 physician epidemiologists using all available data based on standardized procedures.16
Statistical analysis
Baseline traditional cardiovascular and diabetes-specific risk factors were analyzed univariately using the Student’s t test (or Mann-Whitney U for non-parametric comparisons) and the χ2 test (or Fisher’s exact test) by incident stroke status. Due to the small number of events, in addition to comparing ischemic stroke and no stroke, all incident strokes (ischemic and hemorrhagic) were combined for multivariable analysis. Significant univariate variables were made available to multivariable Cox regression models. The proportional hazards assumption was visually assessed, then verified by testing time-varying interaction terms.
Since many variables were significant univariately, exploratory analysis was performed by grouping variables into 3 groups: 1) demographic, 2) complications, and 3) clinical, with diabetes duration included in all exploratory models. Independent variables were analyzed both at baseline and as time-varying updated means. In the second method, updated means of each continuous variable were calculated as the overall mean of said variable for each participant at every cycle (i.e., the average of all data up to and including the present cycle), until either an event occurred or follow-up ended. Variables found to be significant in exploratory multivariable analysis were combined into Cox regression models with backward elimination. Survival plots by stroke status were based on Kaplan-Meier life-tables. Statistical significance was defined as p<0.05. All hazard ratios are reported per increase of 1 SD for continuous variables. SPSS 18.0 (IBM, Chicago, IL) was used for survival analyses and SAS 9.2 (SAS Institute, Cary, NC) for all other analyses.
Results
Four (0.6%) persons suffered a stroke prior to study entry and were excluded. In addition, one individual was lost to follow-up after the initial visit and thus excluded. During a mean (±SD) follow-up of 15.4 (±4.9 years), 31 participants (4.7%) suffered an incident stroke, six of which were fatal. This represents an unadjusted incidence rate of 3.1 (95% CI, 2.0–4.1) strokes/1000 person-years. The mean age at stroke onset was 40.2 years (range 23–60). Ten (32%) had evidence of coronary artery disease prior to the stroke, while 3 (10%) had a prior TIA. Stroke type was determined for 29 of the 31 incident strokes: 21 ischemic, 8 hemorrhagic, and 2 could not be classified.
Baseline predictors of stroke
Table 1 presents baseline data based on incident stroke status and type. Persons with incident stroke were significantly older and had a longer diabetes duration at baseline compared to no stroke, but no difference existed by sex or race. Baseline systolic blood pressure (SBP) was significantly higher for those with ischemic stroke, whereas diastolic blood pressure was significantly higher for those with hemorrhagic stroke. Compared to individuals without stroke, non-HDL cholesterol (non-HDLc), triglycerides, albumin excretion rate (AER), fibrinogen, and white blood cell count were elevated, and estimated glomerular filtration rate (eGFR) was decreased, in those with ischemic stroke. Compared to those without stroke, pulse, non-HDLc, triglycerides, and AER were elevated, but daily insulin dose and hematocrit were decreased, in those with hemorrhagic stroke. HbA1c was only univariately significant for hemorrhagic stroke. Of note, individuals with incident stroke (both types) were more likely to have a history of renal damage (microalbuminuria (MA) or overt nephropathy (ON)), but only those with ischemic stroke were more likely to have smoked.
Table 1.
Baseline characteristics (mean (±SD), % (n), or median (interquartile range)) of Pittsburgh EDC participants by incident stroke status (n=651)†
| No stroke | Incident ischemic stroke | Incident hemorrhagic stroke | |
|---|---|---|---|
| N | 623 | 21 | 8 |
| Age (yrs) | 27.3 (±7.8) | 31.6 (±8.7)* | 34.6 (±6.6)** |
| Diabetes duration (yrs) | 19.0 (±7.4) | 24.5 (±7.3)*** | 25.8 (±5.4)** |
| Female (%) | 49.3 (307) | 52.4 (11) | 50.0 (4) |
| African-American (%) | 2.2 (14) | 0.0 (0) | 12.5 (1) |
| HbA1c (%) | 8.7 (±1.5) | 9.1 (±1.8) | 10.0 (±1.7)* |
| Daily insulin dose (units/kg) | 0.79 (±0.24) | 0.89 (±0.42) | 0.58 (±0.12)* |
| Systolic BP (mmHg) | 113.2 (±15.5) | 123.6 (±14.1)** | 122.5 (±15.8) |
| Diastolic BP (mmHg) | 72.6 (±10.9) | 75.7 (±13.2) | 82.6 (±12.8)* |
| BP medication use (%) | 4.7 (28) | 15.0 (3) | 25.0 (2)* |
| Hypertension (%) | 15.0 (93) | 28.6 (6) | 37.5 (3) |
| Pulse (beats/min) | 78.3 (±9.9) | 75.1 (±9.4) | 89.3 (±9.9)** |
| Body mass index (kg/m2) | 23.5 (±3.2) | 24.4 (±3.5) | 22.0 (±2.4) |
| Waist-hip ratio | 0.82 (±0.07) | 0.85 (±0.09) | 0.85 (±0.11) |
| HDL cholesterol (mg/dL) | 53.9 (±12.4) | 54.4 (±11.8) | 50.4 (±11.5) |
| Non-HDL cholesterol (mg/dL) | 135.4 (±41.7) | 171.1 (±53.1)*** | 173.9 (±52.5)** |
| Triglycerides (mg/dL) | 81.0 (60.0–120.0) | 121.0 (79.0–186.0)** | 201.0 (89.8–266.8)** |
| Estimated GFR (ml min−1 1.73 m−2) | 113.5 (±43.6) | 90.1 (±36.9)* | 76.3 (±51.7)* |
| Albumin excretion rate (μg/min) | 15.2 (7.3–124.4) | 358.5 (30.7–1274)*** | 246.3 (91.1–531.2)* |
| Serum Albumin (g/dL) | 4.63 (±0.65) | 4.24 (±0.65)** | 4.45 (±0.51) |
| Serum Creatinine (mg/dL) | 0.90 (0.70–1.10) | 1.00 (0.80–1.10) | 1.10 (0.58–4.08) |
| Fibrinogen (mg/dL) | 270.0 (225–330) | 320.0 (278–375)** | 340.0 (248–420) |
| White blood cell count (× 109/L) | 6.55 (±1.88) | 8.31 (±2.43)*** | 5.61 (±1.43) |
| Hematocrit (%) | 44.3 (±4.7) | 43.7 (±5.8) | 40.6 (±6.9)* |
| Prevalent CAD (%) | 7.2 (45) | 14.3 (3) | 25.0 (2) |
| Prevalent LEAD (%) | 11.2 (70) | 23.8 (5) | 25.0 (2) |
| Prevalent Microalbuminuria (%) | 45.6 (284) | 90.5 (19)*** | 87.5 (7)* |
| Prevalent Overt Nephropathy (%) | 23.6 (147) | 66.7 (14)*** | 62.5 (5)* |
| Prevalent Proliferative Retinopathy (%) | 29.8 (182) | 52.4 (11)* | 50.0 (4) |
| Ever smoker (%) | 37.1 (225) | 66.7 (14)** | 37.5 (3) |
Excludes 4 prevalent cases and 2 unclassified strokes and 1 lost-to-follow-up Incident stroke vs. no stroke:
p≤0.05;
p≤0.01;
p≤0.001
Abbreviations: BP, blood pressure; CAD, coronary artery disease; GFR, glomerular filtration rate; HDL, high density lipoprotein; LEAD, lower extremity arterial disease; Non-HDL, non-high density lipoprotein
Multivariable analyses of baseline predictors consistently showed diabetes duration and non-HDLc as significant predictors of any stroke, and their effects did not materially change in the four models (Table 2A). The effects of SBP and HBA1c were only significant in Model 1, which also included a measure of renal function (eGFR). The effect of renal damage (ON) was highly predictive (HR=4.3), even after accounting for diabetes duration and non-HDLc, and replaced both SBP and HbA1c (Table 2A, Model 2). Substituting subclinical renal damage (MA) for clinical ON was also predictive of stroke (HR=5.1, 1.5–18.1), as was the continuous measure, AER (HR=1.8, 1.2–2.6 per SD increase).
Table 2.
Cox regression models for baseline predictors of (A) any stroke and (B) ischemic stroke in type 1 diabetes.
| (A) Incident stroke | |||
|---|---|---|---|
| SD | Model 1 | Model 2 | |
| Diabetes duration | 7.508 | 1.87 (1.21–2.87)** | 2.01 (1.33–3.03)*** |
| SBP | 15.984 | 1.39 (1.03–1.86)* | Not selected |
| Non-HDLc | 43.117 | 1.58 (1.17–2.13)** | 1.50 (1.11–2.02)** |
| HbA1c | 1.532 | 1.46 (1.02–2.09)* | Not selected |
| eGFR | 44.274 | 0.58 (0.36–0.91)* | Not selected |
| Prevalent ON | – | 4.33 (1.85–10.1)*** | |
| AIC (no covariates)a | 325.2 (366.1) | 324.1 (366.1) | |
| (B) Incident ischemic stroke only | |||
| SD | Model 3 | Model 4 | |
| Diabetes duration | 7.508 | 1.63 (1.00–2.67)* | 1.66 (1.02–2.70)* |
| SBP | 15.984 | 1.56 (1.05–2.31)* | Not selected |
| Non-HDLc | 43.117 | 1.66 (1.15–2.39)** | 1.50 (1.03–2.19)* |
| WBC | 1.917 | 1.64 (1.14–2.36)** | 1.58 (1.08–2.31)* |
| Pulse | 9.970 | 0.55 (0.34–0.88)* | 0.53 (0.32–0.88)* |
| Prevalent ON | – | 4.37 (1.54–12.4)** | |
| AIC (no covariates)a | 213.8 (239.2) | 209.6 (239.2) | |
Values shown are hazard ratios (95% CI), which are per 1 SD increase.
Model 1 allowed for diabetes duration, systolic blood pressure (SBP), non-HDL cholesterol (non-HDLc), HbA1c, glomerular filtration rate (eGFR), ever smoker, and prevalent lower extremity arterial disease.
Model 2 allowed for Model 1 variables + prevalent overt nephropathy (ON).
Model 3 allowed for diabetes duration, SBP, non-HDLc, white blood cell count (WBC), pulse, eGFR, and ever smoker.
Model 4 allowed for Model 3 variables + prevalent ON.
p≤0.05;
p≤0.01;
p≤0.001
AIC, Akaike’s Information Criterion, for models 1 and 2, n=642 with 31 events, and for models 3 and 4, n=628 with 21 events
Consistent baseline predictors of ischemic stroke included diabetes duration, SBP, non-HDLc, WBC, and pulse (Table 2B). Unlike the overall stroke models in Table 2A, when ON was included (Table 2B, Model 4), these other baseline predictors remained significant, except for SBP.
Despite the small numbers, multivariable analysis revealed that the strongest baseline predictors of hemorrhagic stroke were diabetes duration (HR=3.5, 95% CI, 1.6–7.6), HbA1c (HR=2.5, 1.4–4.5), and DBP (HR=2.0, 1.2–3.5).
Time-varying updated-means
Models using time-varying updated-means for all continuous variables were quite similar to the baseline models above. Predictors of incident stroke included longer diabetes duration (HR=1.57), higher SBP (HR=1.38), higher non-HDLc (HR=1.67), higher HbA1c (HR=1.26), and lower eGFR (HR=0.50) (Supplemental Table 1, Model 1). When prevalent ON was added to the model, it was strongly predictive of stroke (HR=4.54) and replaced both SBP and non-HDLc. Both higher HbA1c (HR=1.26) and lower eGFR (HR=0.53) remained in the fully-adjusted model (Suppl. Table 1, Model 2). The major predictors of ischemic stroke using time-varying updated-means were longer diabetes duration, ever smoker, higher SBP, and higher non-HDLc; however, neither diabetes duration nor ever smoker reached statistical significance (p=0.08 for both, Suppl. Table 1, Model 3). When prevalent ON was added, only longer diabetes duration (HR=1.70) and prevalent ON (HR=8.22) remained predictive in the final model (Suppl. Table 1, Model 4).
Survival after stroke
In this cohort, six (19.4%) incident strokes were fatal. The only significant baseline predictor of fatal incident stroke was HbA1c (HR=2.3, 1.1–4.9); sex, diabetes duration, renal damage, hypertension, and dyslipidemia were not significant (data not shown). In participants with a non-fatal incident stroke (n=25), 40.0% suffered at least one additional stroke over a median follow-up time of 3.9 years (range 0.6–14.9). As of 1 January 2010, 27 (87.1%) individuals with an incident stroke had died. Overall median survival time after incident stroke was 3.8 years; 5.1 and 1.0 years for ischemic and hemorrhagic stroke, respectively. The underlying cause of death was as follows: 9 (33.3%) died of a stroke, 9 (33.3%) died of a myocardial infarction or CAD death, 8 (29.6%) died of another diabetes-related cause (e.g., diabetic ketoacidosis, end-stage renal disease, sepsis), and 1 (3.7%) died of multiple sclerosis. Of note, two individuals with a fatal hemorrhagic stroke had a prior ischemic stroke.
Survival after incident stroke is shown in Figure 1. Overall, 1-year survival after stroke was 80.6%; 5-year survival was 45.2%; and 10-year survival was 9.6%. Stratification by stroke type, despite limited numbers (hemorrhagic stroke, n=8), showed significant differences in survival, with 1-year survival after ischemic stroke at 95.2% vs. 50.0% for hemorrhagic stroke (p=0.03). A similar pattern persisted for 5-year survival (52.4% vs. 25.0%, respectively).
Figure 1.
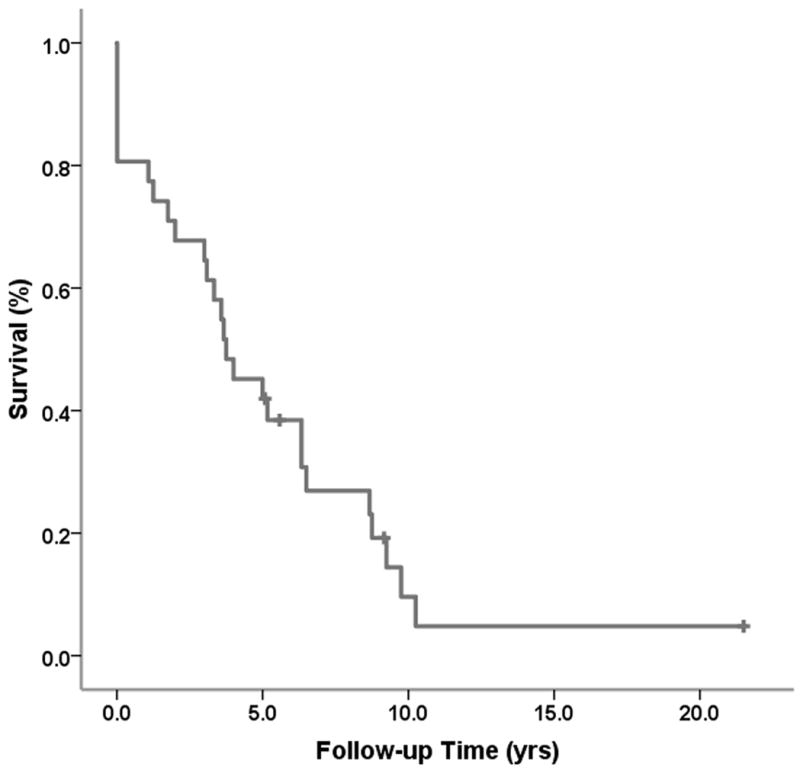
Survival (Kaplan-Meier) after incident stroke in type 1 diabetes participants of the Pittsburgh EDC Study.
Discussion
We report that stroke incidence in a large childhood-onset T1DM population is 3.0/1,000 person-years during follow-up (mean 15.4 years), with no differences seen by sex. More than two-thirds of the incident strokes were ischemic, consistent with other studies,2–4 and were usually not preceded by either CAD (32%) or TIA (10%), similar to the pattern seen in the general population. Baseline predictors of ischemic stroke included diabetes duration, SBP, pulse, non-HDLc, WBC, and renal measures; whereas, for hemorrhagic stroke, diabetes duration, DBP, and HbA1c were significant. Renal damage, both at baseline and during follow-up, was the strongest predictor of overall and ischemic stroke in T1DM. Survival after stroke was low, especially for hemorrhagic stroke, consistent with findings that hemorrhagic strokes are often more severe.17
Very few reports exist on stroke in T1DM for comparison. The largest study to date, the Diabetes UK Study, of >23,000 individuals with insulin-treated diabetes (diagnosed prior to age 30) only examined fatal strokes.3 The authors found overall stroke standardized mortality ratios of 3.1 for males and 4.4 for females. However, only 19% of our cohort had a fatal incident stroke, and >66% died of a cause other than stroke, so only capturing fatal strokes potentially misses a large segment of affected individuals.
The risk for any stroke in the U.K. General Practitioners Research Database Study was increased 3.7-fold for males and 4.8-fold for females with T1DM compared to matched controls.6 In the Nurses’ Health Study, the relative risk for hemorrhagic and ischemic stroke was 4.5 and 7.9, respectively, compared to non-diabetic women, over 24 years of follow-up.5 Our incidence rate (4.7%) for any stroke in the EDC Study is approximately four times higher than that seen in the 20–59 year-old general population in NHANES 2005–2008 (1.2%).
Few data exist on predictors of stroke in T1DM. In 2001, Fuller et al. combined data from multiple countries to look at risk factors for stroke (fatal and non-fatal) in T1DM.2 They found significant univariate associations between stroke and baseline SBP, ON, and ECG abnormalities, although the latter was only significant in men. However, a number of risk factors, including diabetes duration, plasma glucose, serum cholesterol, and smoking history, were not predictive. In contrast, diabetes duration was one of the strongest predictors in the present study. A more recent report from the Fremantle Diabetes Study found that the strongest univariate predictor of ischemic stroke in T1DM was serum HDL-cholesterol, with diabetes duration and history of blood pressure medication use also showing predictive value; however, only five incident ischemic strokes occurred in their cohort.4 Both of these studies only explored univariate predictors of stroke.
Not surprisingly, we found different predictors for ischemic vs. hemorrhagic stroke. Diabetes duration, which, in childhood-onset diabetes serves as a proxy for age, strongly predicted both types of stroke. Blood pressure was also a significant predictor for both, SBP for ischemic stroke and DBP for hemorrhagic stroke. Blood pressure has consistently been shown to be a risk factor for stroke both in T1DM2,18 and in the general population.19,20 It should be noted that SBP became non-significant with the addition of overt nephropathy to multivariable models of ischemic and overall stroke. The interplay between hypertension and derangements in the RAAS secondary to CKD are well known in both diabetes and the population at large,21,22 and early and consistent use of RAAS inhibitors (ACE inhibitors and angiotensin II receptor blockers) have been shown to delay and/or prevent CKD in diabetes.23,24 Similarly, increased non-HDL cholesterol levels predicted stroke in our population, and the timely addition of statin therapy for T1DM patients with dyslipidemia has shown clear antithrombotic benefit which translates into reduced rates of stroke.25,26 WBC, as a marker of inflammation, was also predictive of ischemic stroke, as has been reported in the general population.27–29 Baseline HbA1c was only predictive of hemorrhagic strokes, and not ischemic strokes, in our T1DM cohort. These results are not surprising, given the pathophysiology of each stroke type. Hemorrhagic stroke results from weakened or damaged blood vessels, similar to microvascular renal and retinal disease seen in diabetes patients, both of which are strongly associated with chronic hyperglycemia.30 However, similar to the ischemic stroke findings reported herein, we have shown previously that baseline HbA1c is not as predictive of CAD (i.e., ischemic heart disease) as other insulin resistance-related factors.31
When strokes occur in T1DM, they appear to have more detrimental effects on overall health and survival than seen in the general population. In fact, our median survival after incident stroke of 3.8 years is nearly half the median survival of 7 years after incident stroke in individuals aged 65–74 in the general population.19
Our EDC cohort has several strengths for evaluating the predictors of incident stroke and survival after stroke, including its prospective design with biennial examinations prior to stroke, its long-term follow-up to ascertain mortality, and its detailed classification of cause of death, including death certificates, autopsy and hospital records and review by an expert committee, using a standardized protocol to determine the primary cause of death and to rank contributory causes.16 Ascertainment of relevant demographic and clinical variables has allowed us to incorporate these into our analysis to minimize confounding. The longitudinal nature of the data also allows the assessment of the effect of progression from normoalbuminuria to renal disease on mortality. Renal disease classifications were based on multiple samples (and confirmed by persistence or progression at the next examination) using identical protocols throughout the study period. Our renal disease data are quite consistent with those published from the FinnDiane study, thus strengthening the overall validity, particularly given the different socioeconomic and healthcare backgrounds from which both cohorts derive.32,33
However, this study has its limitations. Our cohort is hospital-based, which may limit the generalizability of these results; however, a comparison of the EDC study population with the Allegheny County T1DM registry found our EDC cohort to be epidemiologically representative of the local T1DM population.34 Also, relatively few (n=31) strokes occurred during 18 years of follow-up. While we feel this figure is accurate, we are limited in our analyses to simple univariate and multivariable Cox modelling. As MRIs were not available to detect subclinical stroke, these data only capture clinical strokes, thus presenting only part of the stroke picture. In addition, we were unable to gather sufficient clinical data (especially hospital records from strokes occurring in the 1980s and 1990s) to adequately explore predictors of survival after stroke. Finally, the EDC population consists of participants with long-standing diabetes. Thus, these findings partially reflect outdated diabetes management and care, and may not be generalizable to individuals recently diagnosed with T1DM, whose experience may more closely resemble that of the DCCT intensive therapy cohort, as recently described.35
As with the general population, stroke in T1DM is largely ischemic and usually not preceded by either a CAD or TIA event. The strongest predictors of stroke in T1DM were diabetes duration and modifiable risk factors, including increased blood pressure (ischemic and hemorrhagic), dyslipidemia (ischemic), poor glycemic control (hemorrhagic), and overt nephropathy (ischemic). Thus, careful diabetes management could minimize or prevent this major complication, which occurs at least 20 years earlier than in the general population.
Supplementary Material
Acknowledgments
We are indebted to the EDC Study participants for their efforts in helping understand the natural history of type 1 diabetes. Preliminary data were presented at the 68th Annual American Diabetes Association Scientific Sessions in San Francisco, CA in June 2008. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK034818 and F30-DK082137 for A.M.S.).
Abbreviations
- AER
albumin excretion rate
- BMI
body mass index
- CAD
coronary artery disease
- CI
confidence interval
- CKD
chronic kidney disease
- DBP
diastolic blood pressure
- DCCT
Diabetes Complications and Control Trial
- EDC
Epidemiology of Diabetes Complications
- GFR
glomerular filtration rate
- HDLc
high-density lipoprotein cholesterol
- HR
hazard ratio
- LEAD
lower extremity arterial disease
- ON
overt nephropathy
- PR
proliferative retinopathy
- RAAs
renin-angiotensin-aldosterone system
- SBP
systolic blood pressure
- T1DM
type 1 diabetes mellitus
- TIA
transient ischemic attack
- WBC
white blood cells
Footnotes
The authors have no relevant conflicts of interest to disclose.
References
- 1.Deckert T, Poulsen JE, Larsen M. Prognosis of diabetics with diabetes onset before the age of thirty-one. I. Survival, causes of death, and complications. Diabetologia. 1978;14:363–70. doi: 10.1007/BF01228130. [DOI] [PubMed] [Google Scholar]
- 2.Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44 (Suppl 2):S54–64. doi: 10.1007/pl00002940. [DOI] [PubMed] [Google Scholar]
- 3.Laing SP, Swerdlow AJ, Carpenter LM, et al. Mortality from cerebrovascular disease in a cohort of 23 000 patients with insulin-treated diabetes. Stroke. 2003;34:418–21. doi: 10.1161/01.str.0000053843.03997.35. [DOI] [PubMed] [Google Scholar]
- 4.Davis TM, Bruce DG, Davis WA. Predictors of first stroke in Type 1 diabetes: The Fremantle Diabetes Study. Diabet Med. 2005;22:551–3. doi: 10.1111/j.1464-5491.2005.01462.x. [DOI] [PubMed] [Google Scholar]
- 5.Janghorbani M, Hu FB, Willett WC, et al. Prospective study of type 1 and type 2 diabetes and risk of stroke subtypes: the Nurses’ Health Study. Diabetes Care. 2007;30:1730–5. doi: 10.2337/dc06-2363. [DOI] [PubMed] [Google Scholar]
- 6.Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K: a cohort study using the general practice research database. Diabetes Care. 2006;29:798–804. doi: 10.2337/diacare.29.04.06.dc05-1433. [DOI] [PubMed] [Google Scholar]
- 7.Sundquist K, Li X. Type 1 diabetes as a risk factor for stroke in men and women aged 15–49: a nationwide study from Sweden. Diabet Med. 2006;23:1261–7. doi: 10.1111/j.1464-5491.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- 8.Orchard TJ, Dorman JS, Maser RE, et al. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116–24. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 9.Orchard TJ, Dorman JS, Maser RE, et al. Factors associated with avoidance of severe complications after 25 yr of IDDM. Pittsburgh Epidemiology of Diabetes Complications Study I. Diabetes Care. 1990;13:741–7. doi: 10.2337/diacare.13.7.741. [DOI] [PubMed] [Google Scholar]
- 10.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev Med. 1976;5:207–15. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 11.Warnick GR, Albers JJ. Heparin--Mn2+ quantitation of high-density-lipoprotein cholesterol: an ultrafiltration procedure for lipemic samples. Clin Chem. 1978;24:900–4. [PubMed] [Google Scholar]
- 12.Ellis D, Buffone GJ. New approach to evaluation of proteinuric states. Clin Chem. 1977;23:666–70. [PubMed] [Google Scholar]
- 13.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 14.Olson JC, Erbey JR, Forrest KY, Williams K, Becker DJ, Orchard TJ. Glycemia (or, in women, estimated glucose disposal rate) predict lower extremity arterial disease events in type 1 diabetes. Metabolism. 2002;51:248–54. doi: 10.1053/meta.2002.30021. [DOI] [PubMed] [Google Scholar]
- 15.Early Treatment of Diabetic Retinopathy Study Coordinating Center: Manual of Operations. Baltimore, MD: University of Maryland School of Medicine; 1980. [Google Scholar]
- 16.International evaluation of cause-specific mortality and IDDM. Diabetes Epidemiology Research International Mortality Study Group. Diabetes Care. 1991;14:55–60. doi: 10.2337/diacare.14.1.55. [DOI] [PubMed] [Google Scholar]
- 17.Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP. Hemorrhagic and Ischemic Strokes Compared. Stroke Severity, Mortality, and Risk Factors. Stroke. 2009 doi: 10.1161/STROKEAHA.108.540112. [DOI] [PubMed] [Google Scholar]
- 18.Morrish NJ, Stevens LK, Fuller JH, Jarrett RJ, Keen H. Risk factors for macrovascular disease in diabetes mellitus: the London follow-up to the WHO Multinational Study of Vascular Disease in Diabetics. Diabetologia. 1991;34:590–4. doi: 10.1007/BF00400279. [DOI] [PubMed] [Google Scholar]
- 19.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sturgeon JD, Folsom AR, Longstreth WT, Jr, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke. 2007;38:2718–25. doi: 10.1161/STROKEAHA.107.487090. [DOI] [PubMed] [Google Scholar]
- 21.Abuissa H, O’Keefe J., Jr The role of renin-angiotensin-aldosterone system-based therapy in diabetes prevention and cardiovascular and renal protection. Diabetes Obes Metab. 2008;10:1157–66. doi: 10.1111/j.1463-1326.2008.00898.x. [DOI] [PubMed] [Google Scholar]
- 22.Fogo AB. Mechanisms of progression of chronic kidney disease. Pediatr Nephrol. 2007;22:2011–22. doi: 10.1007/s00467-007-0524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis Risk in Communities Study. N Engl J Med. 2000;342:905–12. doi: 10.1056/NEJM200003303421301. [DOI] [PubMed] [Google Scholar]
- 24.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tehrani S, Mobarrez F, Antovic A, et al. Atorvastatin has antithrombotic effects in patients with type 1 diabetes and dyslipidemia. Thromb Res. 2010;126:e225–31. doi: 10.1016/j.thromres.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–25. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 27.Folsom AR, Rosamond WD, Shahar E, et al. Prospective study of markers of hemostatic function with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Circulation. 1999;100:736–42. doi: 10.1161/01.cir.100.7.736. [DOI] [PubMed] [Google Scholar]
- 28.Gillum RF, Ingram DD, Makuc DM. White blood cell count and stroke incidence and death. The NHANES I epidemiologic follow-up study. Am J Epidemiol. 1994;139:894–902. doi: 10.1093/oxfordjournals.aje.a117095. [DOI] [PubMed] [Google Scholar]
- 29.Margolis KL, Manson JE, Greenland P, et al. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women’s Health Initiative Observational Study. Arch Intern Med. 2005;165:500–8. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- 30.Brown WV. Microvascular complications of diabetes mellitus: renal protection accompanies cardiovascular protection. Am J Cardiol. 2008;102:10L–3L. doi: 10.1016/j.amjcard.2008.09.068. [DOI] [PubMed] [Google Scholar]
- 31.Orchard TJ, Olson JC, Erbey JR, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003;26:1374–9. doi: 10.2337/diacare.26.5.1374. [DOI] [PubMed] [Google Scholar]
- 32.Groop PH, Thomas MC, Moran JL, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58:1651–8. doi: 10.2337/db08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53:2312–9. doi: 10.1007/s00125-010-1860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagener DK, Sacks JM, LaPorte RE, Macgregor JM. The Pittsburgh study of insulin-dependent diabetes mellitus. Risk for diabetes among relatives of IDDM. Diabetes. 1982;31:136–44. doi: 10.2337/diab.31.2.136. [DOI] [PubMed] [Google Scholar]
- 35.Nathan DM, Zinman B, Cleary PA, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005) Arch Intern Med. 2009;169:1307–16. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


