Abstract
We investigated the correlation between metabolic response by 18F-FDG PET and objective response, glucose transporter type 4 (GLUT4) expression, and KIT/PDGFRA mutation status in patients with gastrointestinal stromal tumor undergoing neoadjuvant imatinib mesylate therapy.
Methods
18F-FDG PET was performed at baseline, 1–7 d, and 4 or 8 wk after imatinib mesylate initiation. Best objective response was defined by version 1.0 of the Response Evaluation Criteria in Solid Tumors (RECIST). Mutational analysis and tumor GLUT4 expression by immunohistochemistry were done on tissue obtained at baseline or surgery.
Results
18F-FDG PET showed high baseline tumor glycolytic activity (mean SUVmax, 14.2; range, 1.3–53.2), decreasing after 1 wk of imatinib mesylate (mean, 5.5; range, −0.5–47.7, P < 0.001, n = 44), and again before surgery (mean, 3.0; range, −0.5–36.1, P < 0.001, n = 40). At week 1, there were 3 patients with complete metabolic response (CMR), 33 with partial metabolic response (PMR), 6 with stable metabolic disease (SMD), and 2 with progressive metabolic disease (PMD). Before surgery, there were 3 with CMR, 33 with PMR, 4 with SMD, and none with PMD. The best response according to RECIST was 2 with partial response, 36 with stable disease, and 1 with progressive disease (n = 39). Of the patients with a posttreatment decrease in GLUT4 expression, 1 had CMR, 15 had PMR, 2 had SMD, and 1 had PMD at week 1, whereas before surgery 1 patient had CMR, 16 had PMR, 2 had SMD, and none had PMD. Among 27 patients with KIT exon 11 mutations, 1 had CMR, 22 had PMR, 3 had SMD, and 1 had PMD at week 1, whereas 1 had CMR, 22 had PMR, 2 had SMD, and 2 were unknown before surgery; among 4 patients with a wild-type genotype, 2 had PMR and 2 SMD at week 1, whereas 1 had CMR, 2 had PMR, and 1 had SMD before surgery.
Conclusion
After imatinib mesylate initiation, metabolic response by 18F-FDG PET was documented earlier (1–7 d) and was of much greater magnitude (36/44) than that documented by RECIST (2/39). Immunohistochemistry data suggest that GLUT4 may play a role in 18F-FDG uptake in gastrointestinal stromal tumor, GLUT4 levels decrease after imatinib mesylate therapy in most patients with PMR, and the biologic action of imatinib mesylate interacts with glycolysis and GLUT4 expression. A greater than 85% metabolic response was observed as early as days 1–7 in patients with exon 11 mutations.
Keywords: GIST, FDG-PET, GLUT4, genotype, therapeutic response
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal malignancy found in the alimentary tract (1). GIST cells show ultrastructural features and express cell markers typical of the normal interstitial cells of Cajal (2). The pathogenesis of GIST is related to the expression of the KIT receptor tyrosine kinase (CD117) in approximately 95% of patients, and gain-of-function activating mutations within the KIT gene are a key etiologic mechanism in approximately 80% of patients (3). Activating mutations in the PDGFRA gene are seen in about 8% of patients (4,5), and mutations in the serine-threonine kinase BRAF have also been identified in a small number of GISTs (6–8). No mutations are detectable in KIT, PDGFRA, or BRAF in approximately 10%–12% of patients, although uncontrolled KIT kinase activation has been noted even in the absence of mutation (4,9). Significant progress over the last decade in the understanding of aberrant signal transduction pathways in GIST has led to new paradigms regarding drug design and drug development and a significant breakthrough in the concept and application of molecular-targeted therapy.
Surgical resection is the initial therapy for patients with primary GIST considered to have resectable disease and no metastases. Metastatic GIST is both chemoresistant and insensitive to irradiation and had a dismal outcome before 2000 (10). Recent studies, however, have shown promising results and greatly improved survival when patients with GIST were treated with imatinib mesylate (STI571, Gleevec, Glivec; Novartis Pharmaceuticals), a selective small molecule that inhibits tumor growth by competitive interaction at the adenosine triphosphate (ATP)–binding site of the KIT receptor (11,12).
Multiple imaging modalities are available to evaluate patients with GIST, including CT, MRI, and PET with 18F-FDG, now integrated with CT (13). Although CT is more readily available, we and others have demonstrated that metabolic response by 18F-FDG PET can be observed within hours after initiation of imatinib mesylate and precedes significant changes in tumor size by weeks, months, or even years (11,14–18). This trial was designed before the publication by Choi et al., who proposed new CT response criteria based on changes in size and density (19), and these criteria were not used in this trial. There also remain controversies about anatomy-based response evaluation criteria in patients with GIST (20).
The objectives of this phase II trial by the American College of Radiology Imaging Network (ACRIN) and Radiation Therapy Oncology Group (RTOG) were first, to determine the outcome and toxicity of imatinib mesylate given as a neoadjuvant agent before a planned surgical resection; second, to assess metabolic response by 18F-FDG PET as the change in the maximum standardized uptake value (SUVmax) during week 1 of therapy and before surgery and correlate metabolic response with anatomic response assessed by version 1.0 of the Response Evaluation Criteria in Solid Tumors (RECIST) (21); third, to determine whether an early decline in SUVmax is an early predictor of response; fourth, to correlate glucose transporter type 4 (GLUT4) expression with SUVmax before and after imatinib mesylate; and fifth, to compare KIT/PDGFRA mutation status with metabolic response by 18F-FDG PET. The early clinical results of this prospective phase II multi-institutional trial were recently published (22). This report addresses the last 4 remaining objectives listed above.
Materials And Methods
Patients
ACRIN 6665/RTOG 0132 was open to accrual from February 2002 through June 2006. Eligible patients were required to have resectable primary or metastatic/recurrent GIST and documented tumor CD117 positivity. After institutional review board approval at each of the 18 participating sites, 63 patients gave written informed consent and were enrolled; 57 patients were considered to be eligible (Supplemental Fig.1; supplemental materials are available online only at http://jnm.snmjournals.org). Patients were treated with imatinib mesylate (600 mg daily, by mouth) for 8–12 wk until the day of surgery. Tumor tissue samples were obtained before imatinib mesylate treatment and at the time of surgical resection. Imatinib mesylate was stopped the day before surgery and then resumed postoperatively as adjuvant therapy and continued until disease progression or for 2 y.
Imaging, Image Analyses, and Response Assessments
Imaging
18F-FDG PET was performed using a standard acquisition protocol (described in the supplemental data) before initiation of drug therapy, during week 1 of therapy, and at week 4 in patients with progressive disease or at week 8 in patients with stable or responding disease before surgical resection.
Unless medically contraindicated, contrast-enhanced CT and MRI were performed in accordance with RECIST (version 1.0) specifications before treatment, after 4 wk of therapy, and before surgery. CT was used at initial staging in all patients, and MRI was used instead of CT after induction in 2 patients, but details on the MRI protocols used are not available.
Image Analysis
The 18F-FDG PET, CT, and MRI scans were submitted electronically to ACRIN, stored in an electronic database, and used for both visual interpretation and semiquantitative analysis of the 18F-FDG PET data. All analyses were performed at the PET core laboratory of the Dana–Farber Cancer Institute sponsored by ACRIN. All scans were interpreted independently by 2 readers without knowledge of the clinical history or pathology results. Target lesions on PET were chosen by the PET core laboratory independently of the CT, MRI, or clinical findings recorded at the participating sites. Target lesions on CT were chosen by the individual sites on the basis of the RECIST criteria independently of the PET findings. The study design did not require matching of target lesions between the 2 imaging modalities.
The pretreatment and posttreatment 18F-FDG PET scans were visually compared to determine the change in tumor 18F-FDG uptake using a 5-point ordinal scale, as well as semiquantitative analysis of the relative changes in background-subtracted SUVmax (supplemental data).
Conventional cross-sectional images were interpreted independently at participating sites by radiologists who measured the change in size of lesions by the RECIST criteria without knowledge of the clinical history, PET results, or biopsy results.
Semiquantitative analysis of background-subtracted SUVmax was correlated with size changes on conventional cross-sectional imaging and glucose transporter expression.
Response Assessment
Anatomic response to neoadjuvant imatinib mesylate therapy by CT or MRI was evaluated in accordance with RECIST (21). The criteria of Choi et al. (19) were not published when this trial was designed and were not used.
Metabolic response by 18F-FDG PET was determined in accordance with the criteria of the European Organization for Research and Treatment of Cancer (EORTC), with increases or decreases of more than 25% in SUVmax defining progressive metabolic disease (PMD) and partial metabolic response (PMR), respectively, and new lesions defining PMD (23). The percentage decline in SUVmax relative to baseline during week 1 and just before surgery (at week 4 in patients with progressive disease or week 8 in patients with stable or responding disease) was measured. The results of the week 1 scans were also analyzed to determine whether they were predictive of the response just before surgery. The 18F-FDG PET results were also compared with the pathologic results of glucose transporter expression at baseline and after treatment, as well as with the mutational status.
Tumor Tissue Analysis
Tumor samples were collected from core-needle biopsies at baseline before imatinib mesylate therapy and from the surgical specimen obtained at the time of surgery after neoadjuvant therapy. Immunohistochemical analysis of CD117 was performed to confirm GIST diagnosis and cellularity of samples. Immunohistochemical analysis of GLUT4 and KIT and PDGFRA DNA mutational status (supplemental data) were evaluated as correlative endpoints.
Statistical Analysis
The change in background-subtracted SUVmax before and after treatment was evaluated. Formal comparisons of SUVmax at baseline to week 1 and to the presurgery time point were made using a Wilcoxon signed-rank test. A secondary analysis using mixed models was also performed. Regression modeling was planned in order to assess the performance of change in SUVmax as a predictor of anatomic response, as defined by RECIST. However, because the observed response rate was high and the patient sample size was limited, we report only descriptive analyses using waterfall plots. Data on GLUT4 expression and KIT/PDGFR mutation status were summarized numerically and graphically. Similarly, Cox regression modeling was planned to examine the ability of SUVmax change to predict overall and progression-free survival; however, the observed mortality and disease progression rates were too low. Computations for these analyses were performed using SPlus graphics and subroutines from the SAS software (SAS9; SAS Institute Inc.)
Results
Patients
The median age of all 63 patients enrolled was 59 y (range, 24–84 y), and there were 34 men (54%). Among the 44 patients who had all imaging studies performed at 2 or all 3 time points (baseline, 1 wk, and 4 or 8 wk), the median age was 55 y (range, 36–78 y), and there was an equal male and female distribution. Twenty-nine patients (66%) had primary GIST, and 15 (34%) had recurrent GIST. Tumors were located most often in the stomach (n = 20, 45%); disease was also located in the small intestine (n = 5, 11%), large bowel (n = 1, 2%), and other sites (n = 18, 41%). There was metastatic disease in 4 patients (9%) and none in the remaining 40 (91%); all were considered to have resectable tumors.
Imaging, Image Analysis, and Response Assessment
Imaging
Forty-four patients underwent 18F-FDG PET at baseline and week 1, and 40 patients at week 4 or 8, for a total of 128 scans. The mean fasting period was 10.4 h (range, 4– 20 h). Mean glucose level was 99.1 mg/dL (range, 61–192 mg/dL). Emission scans were initiated 64.7 min on average (range, 44–118 min) after injection of 18F-FDG (mean, 610 MBq; range, 159–925 MBq). The 18F-FDG PET images were acquired in 2-dimensional mode for most scans (n = 109) and in 3-dimensional mode for the remainder; for patients imaged on dedicated PET scanners the mean transmission scan time was 4 min (range, 1–7 min) per bed position, and the mean emission scan time was 5.7 min (range, 2–10 min) per bed position for interleaved scans. Most 18F-FDG PET scans were reconstructed with an iterative algorithm. Image quality was considered adequate for 106 scans, was considered suboptimal in 21, and was not reported in 1.
Image Analysis and Response Assessment
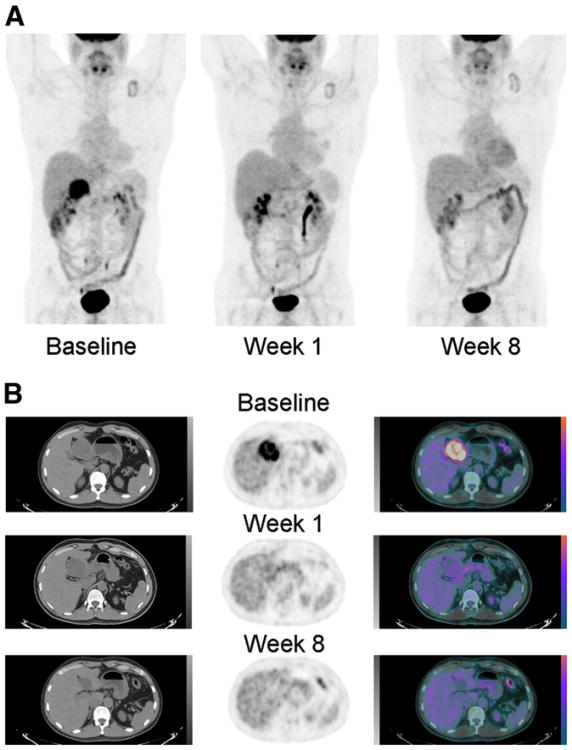
Most GIST tumors were intensely 18F-FDG–avid before treatment with imatinib mesylate, as is shown in Figure 1, and demonstrated a marked decrease in 18F-FDG uptake within 1 wk after initiation of treatment. All tumors that showed this pattern remained 18F-FDG–negative at the time of surgery.
Figure 1.
(A) 18F-FDG PET before therapy, 1 and 8 wk after imatinib mesylate initiation. Intense 18F-FDG uptake in seen at baseline within primary perigastric GIST, followed by complete resolution at 1 and 8 wk after therapy. Mild uptake seen in the left supraclavicular region is related to inflammatory changes around the site of the pacemaker. The remainder of 18F-FDG biodistribution is physiologic. (B) Axial CT, PET, and fused PET/CT slices through the right-upper-quadrant mass before and 1 and 8 wk after imatinib mesylate initiation. Intense 18F-FDG uptake is seen within the primary perigastric GIST at baseline, followed by marked decrease at 1 wk and complete resolution at 8 wk after therapy.
The range of SUVmax within the tumors at each time point is shown in Table 1. High tumor glycolytic activity was seen at baseline (mean SUVmax, 14.2; range, 1.3–53.2), significantly decreasing during the first week of imatinib mesylate therapy (mean, 5.5; range, −0.5–47.7, P < 0.001, n = 44) and again before surgery (mean, 3.0; range, −0.5–36.1, P < 0.001, n = 40). The percentage changes in SUVmax at week 1 and before surgery relative to baseline (P < 0.001), and of SUVmax at week 1 versus before surgery, are shown in Table 1.
Table 1. Distribution of Background-Corrected SUVmax, Percentage Change in SUVmax, and GLUT4 Fold Decrease.
| Measurement | Timing | Category | Number of participants | Number missing | Minimum | Median | Mean | Maximum | P |
|---|---|---|---|---|---|---|---|---|---|
| Background-corrected SUVmax | |||||||||
| Baseline | 44 | 0 | 1.3 | 10.1 | 14.2 | 53.2 | |||
| Week 1 | 44 | 0 | −0.5 | 2.7 | 5.5 | 47.7 | <0.0001* | ||
| Presurgery | 40 | 4 | −0.5 | 1.4 | 3.0 | 36.1 | <0.0001* | ||
| Change in SUVmax from baseline (%) | |||||||||
| Week 1 | 44 | 0 | −100.0 | −72.0 | −59.4 | 81.4 | <0.0001† | ||
| Presurgery | 40 | 4 | −100.0 | −89.6 | −76.4 | 13.4 | <0.0001† | ||
| GLUT4 fold decrease | |||||||||
| Week 1 | CMR | 1 | 2 | 4.3 | 4.3 | 4.3 | 4.3 | ||
| PMR | 17 | 16 | 1.0 | 2.3 | 9.1 | 100.0 | |||
| SMD | 3 | 3 | 1.0 | 2.0 | 2.5 | 4.5 | |||
| PMD | 1 | 1 | 1.7 | 1.7 | 1.7 | 1.7 | |||
| Presurgery | CMR | 1 | 2 | 3.0 | 3.0 | 3.0 | 3.0 | ||
| PMR | 19 | 14 | 1.0 | 2.1 | 8.4 | 100.0 | |||
| SMD | 2 | 2 | 1.7 | 3.1 | 3.1 | 4.5 | |||
| PMD | 0 | 0 |
Wilcoxon signed-rank test comparing week 1 (presurgery) absolute SUVmax against baseline.
Wilcoxon signed-rank test comparing week 1 (presurgery) percentage change in SUVmax from baseline.
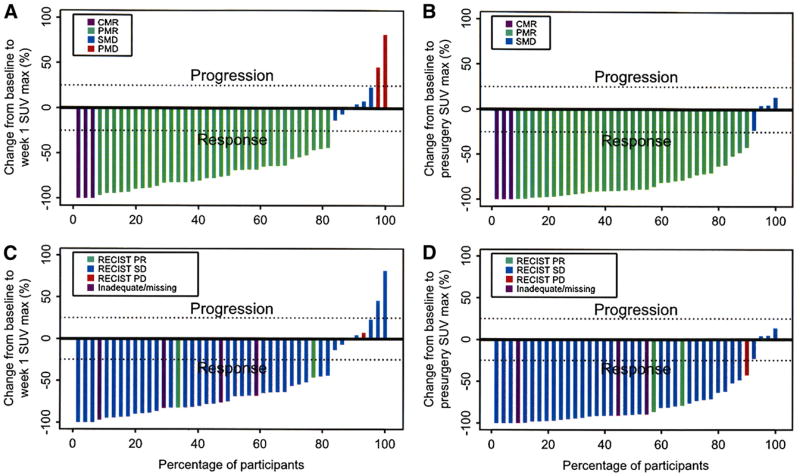
During week 1, on the basis of the criteria of the EORTC, 3 patients had a complete metabolic response (CMR), 33 had PMR, 6 had stable metabolic disease (SMD), and 2 had PMD (Fig. 2A). Before surgery, the distribution of metabolic responses was 3 CMR, 33 PMR, 4 SMD, and no PMD (Fig. 2B). We did not observe new lesions at either time point, and all tumors that showed reduction or resolution of 18F-FDG uptake at week 1 continued to show further reduction or remained negative at the time of surgery. All tumors within each patient responded similarly to imatinib therapy in this population of patients who were naïve to any tyrosine kinase inhibitor therapy.
Figure 2.
Percentage change in SUVmax from baseline to week 1 (A) and before surgery (B). CMR = complete metabolic response; PMR = partial metabolic response; SMD 5 stable metabolic disease; PMD = progressive metabolic disease. Percentage change in SUVmax from baseline to week 1 (C) and before surgery (D) by RECIST best response. PR = partial response; SD = stable disease; PD = progressive disease.
For 39 patients, the RECIST best response was 2 patients with partial response, 36 with stable disease, and 1 with progressive disease. Figures 2C and 2D present the RECIST overall best response superimposed onto the metabolic response data during week 1 and before surgery.
Outcome Analysis
The early clinical results of this prospective trial of preoperative imatinib in GIST were reported previously by Eisenberg et al. (22). Recently, 5-y clinical data were analyzed, and this analysis will be the subject of another article. There were no reported relapses, disease progression, or deaths during the imaging duration of this trial.
Tumor Tissue Analysis
GLUT4 expression was available in 22 of the 44 eligible and evaluable patients in the study cohort who had matching pairs of pre–imatinib mesylate (biopsy) and post–imatinib mesylate (resection) tissue samples that were of sufficient quality. These 22 patients included both exon 11 KIT mutations and wild-type GIST tissue samples (i.e., KIT/PDGRA-negative mutants) and were therefore representative of the larger group.
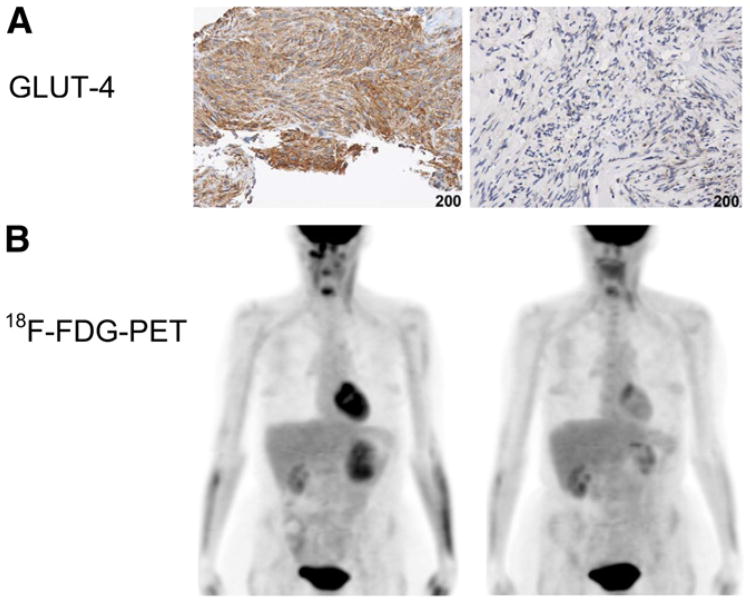
GLUT4 expression decreased from pretreatment to posttreatment specimens in 19 patients and remained unchanged in 3 patients. Table 1 summarizes changes in GLUT4 expression for each level of metabolic response according to the European Organization for Research and Treatment of Cancer. Of the patients with any decrease in GLUT4 expression, 1 patient had CMR, 15 had PMR, 2 had SMD, and 1 had PMD at week 1, whereas 1 patient had CMR, 16 had PMR, 2 had SMD, and none had PMD before surgery. Using a Fisher exact test of association on the categorization, the reduction in GLUT4 showed no association with the reduction in tumor 18F-FDG uptake. Figure 3 shows GLUT4 immunohistochemical and 18F-FDG PET studies performed on a patient with a primary gastric GIST before and 8 wk after imatinib mesylate therapy. The GLUT4 immunohistochemical study performed on a core biopsy sample at baseline shows intense brown staining that is no longer apparent on the week 8 surgical specimen obtained after resection of the left-upper-quadrant mass. 18F-FDG PET images of the same patient before imatinib mesylate therapy show intense 18F-FDG uptake in the gastric mass, which had resolved at week 8, before surgery. GLUT1 was also evaluated; however, its expression was absent in the initial cases tested (Supplemental Fig. 2) and was subsequently not evaluated in all samples.
Figure 3.
GLUT4 immunohistochemical (A) and 18F-FDG PET (B) studies of patient with primary gastric GIST before and 8 wk after imatinib mesylate therapy. GLUT4 intense brown staining at baseline is no longer apparent on week 8 surgical specimen. 18F-FDG PET images show intense 18F-FDG uptake in the gastric mass before treatment and resolution at week 8.
Genotype information was available for 31 (70%) of the 44 eligible and evaluable patients in the study cohort. Primary KIT exon 11 mutations were seen in 27 of 31 patients, PDGFRA mutations were detected in none of the samples (2 were unknown before surgery), and 4 cases lack mutations in either gene. The remaining 13 patients had insufficient samples for the mutational analysis or no samples. Among 27 patients with KIT exon 11 mutations, 1 had CMR, 22 PMR, 3 SMD, and 1 PMD at week 1; among 4 patients with the wild-type genotype, 2 had PMR and 2 SMD at week 1. In the presurgery study, among 25 patients with an exon 11 mutation, 1 had CMR, 22 PMR, and 2 SMD, versus 1 with CMR, 2 with PMR, and 1 with SMD among the 4 patients with the wild-type genotype.
Discussion
The early clinical results of this prospective phase II multiinstitutional ACRIN/RTOG trial have shown that the use of preoperative imatinib mesylate was feasible, with minimal drug-related toxicity and surgical morbidity (22). The preoperative use of imatinib mesylate has also been shown to improve resectability and reduce surgical morbidity in patients with unresectable or locally advanced GISTs (24).
The first 2 objectives of this report were to focus on the evaluation of 18F-FDG PET as a noninvasive functional imaging tool to assess metabolism of the tumor before and during imatinib mesylate administration, and to compare the tumor metabolic response using the EORTC criteria (23) to the traditional response evaluation by RECIST (21). A retrospective analysis of the CT scans to assess the methods proposed by Choi et al. (19) is not possible at this time.
The results demonstrate that the baseline glycolytic activity in these tumors before imatinib mesylate was high, as reflected by a mean absolute SUVmax of 14.2, supporting earlier observations that 18F-FDG PET is a sensitive means for staging patients with GIST (11,25). After therapy, a significant decrease in tumor metabolic activity was seen at all tumor sites in 82% of patients (36/44) as early as 1 wk after initiation of imatinib mesylate, including 3 patients with CMR. Before surgery, 90% of patients (36/40) had achieved CMR or PMR, and 4 patients had SMD. This rapid shutdown of glycolytic activity is consistent with our prior observation in the pivotal trial of imatinib mesylate in metastatic GIST (11,14) and later confirmed by others (15,26). The use of 18F-FDG PET soon after treatment can therefore help identify patients with primary resistance to the drug, given that up to 14% of primary GISTs may show initial resistance to imatinib mesylate therapy (11,27).
Conversely, therapeutic response evaluation using conventional criteria that are based solely on changes in tumor size was not predictive of response (15–18,28–30). Only 2 of 39 patients achieved partial response as best response by RECIST in this neoadjuvant trial. 18F-FDG PET is, therefore, the imaging modality of choice for the evaluation of therapeutic response to imatinib mesylate in patients with GIST who are naïve to tyrosine kinase inhibitor therapy (25,31).
Decreased tumor density is often observed in patients treated with tyrosine kinase inhibitors even in the absence of a decrease in tumor size. Proposals to refine anatomy-based response criteria using either no growth in tumor size (18,27) or a combination of tumor density and size criteria such as those proposed by Choi et al. have been made (19,30), but these refined criteria have been tested so far only in a GIST population that was naïve to the drug, and they are not yet universally accepted. The extrapolation and generalization of these criteria to any GIST population, including patients who have been exposed to prior targeted therapy, remains controversial (20).
A recent comparison of the RECIST and Choi criteria in patients with renal cell carcinoma treated with targeted therapy has shown that the better predictor of response was actually a simple 10% reduction in the longest unidimensional tumor diameter (32). Given the practical utility of a no-growth assessment or single linear measurement over the complexity of the methodology involved with the RECIST or Choi criteria, there is clearly an urgent need to validate which anatomic criteria are indeed the best predictors of response in GIST and other tumors treated with targeted therapies.
The abnormal use of glucose by tumor cells has been known since the last century (33) and is most likely related to oncogenic transformation, upregulation of the AKT pathway, and upregulation of glucose transporters. Glucose uptake in GIST cells has been shown to be mediated by gain-of-function mutations in KIT leading to constitutive activation of the KIT/PI3K/ AKT pathway. Results from in vitro experiments have demonstrated that imatinib mesylate directly leads to decreased glucose transport into GIST cells in an AKT-dependent manner (34). These in vitro data are quite complementary to the observations made on 18F-FDG PET clinically. GIST tumors show intense 18F-FDG uptake and high SUVmax consistent with an increased rate of glucose uptake and glucose metabolism relative to normal tissue. Conversely, after imatinib mesylate therapy, a marked decrease in 18F-FDG uptake is seen shortly after initiation of treatment, most likely related to decreased glucose (and 18F-FDG) transport into the tumor. We sought to study GLUT expression in the patients enrolled in this neoadjuvant trial and found that all patients expressed detectable levels of GLUT4 before imatinib mesylate therapy and that GLUT4 expression decreased in 19 of 22 patients for whom tumor samples were available at the time of surgery. Of the patients with any decrease in GLUT4 expression, 16 of 19 also showed metabolic response by 18F-FDG PET at week 1, and 17 of 19 had metabolic response before surgery. This decrease in GLUT4 expression and resulting decrease in 18F-FDG uptake may be reflective of the translocation via endocytosis of the plasma membrane–bound GLUT4 to the cytosol as described by Tarn et al. in GIST cells in culture treated with imatinib mesylate therapy (34). It is interesting to note that GLUT1, the other prominent glucose transporter that is responsible for the low level of basal glucose uptake required to sustain respiration in all cells, was not abundantly expressed in this patient population and in most cases was undetectable (Supplemental Fig. 2). This observation is consistent with a recent case report that found weak GLUT1 but intense GLUT4 membrane staining in an esophageal GIST (27).
A close relationship has also been reported between the genomic pattern of these tumors and response to tyrosine kinase inhibitors. The results of the gene expression profiling performed on tumor samples obtained from the patient population enrolled in this ACRIN/RTOG trial were reported separately (35). Of this population, 31 patients who underwent 18F-FDG PET had enough evaluable tissue to perform molecular characterization of their tumors as well. KIT exon 11 mutations were seen in 27 of 31 patients, whereas the remaining 4 patients tested had the wild-type genotype, that is, lacking KIT or PDGFRA mutations. Primary and secondary kinase genotypes do correlate with the biologic and clinical response to tyrosine kinase inhibitors, and KIT exon 11 mutant GISTs are more sensitive and responsive to imatinib mesylate therapy than KIT exon 9 mutant or wild-type GISTs (36–38). We observed a marked metabolic response by 18F-FDG PET in more than 85% (23/27) of patients with exon 11 mutations at both time points. The KIT/PDGFRA mutation–negative patients showed response in 50% (2/4) as early as 1 wk after initiation of treatment and in 75% (3/4) at 8 wk.
Conclusion
Intense 18F-FDG uptake is seen in most GIST tumors before therapy. After initiation of imatinib mesylate therapy, metabolic response is documented earlier (1–7 d) and is of much greater magnitude (36/44) than the anatomic response documented by RECIST (2/39), indicating that 18F-FDG PET is the imaging modality of choice in the evaluation of patients with GIST treated with imatinib mesylate and should be part of the routine management of patients with GIST.
A greater than 85% metabolic response was observed as early as days 1–7 in patients with exon 11 mutations.
The immunohistochemical data suggest that GLUT4 may play a role in 18F-FDG uptake in GIST, that GLUT4 levels decrease after imatinib mesylate therapy in most patients with metabolic response, and that the biologic action of imatinib mesylate directly interacts with glycolysis and GLUT4 expression, resulting in decreased 18F-FDG uptake.
Our results suggest that the clinical activity of imatinib mesylate is at least in part associated with modulation of the primary target and alteration of glycolysis, including a decrease in GLUT4 expression, as well as KIT exon 11 mutational status.
Supplementary Material
Acknowledgments
We thank all the patients who generously volunteered to participate in this study, the study team staffs at the participating institutions, and ACRIN. We acknowledge the contributions of Ramsey Badawi, and we recognize the pioneering work of George Demetri and his team in bringing novel therapies to patients with GISTs. This project was funded in part by the Department of Health and Human Services and the National Cancer Institute through grants U01 CA079778, U01 CA080098, and R01 CA106588. The contents of this publication do not necessarily reflect the views or policies of the Department of Health and Human Services, nor is endorsement by the U.S. government implied. This study was presented in part at the 2008 annual meeting of the American College of Radiology Imaging Network and at the 2009 annual meeting of the American Society of Clinical Oncology. No other potential conflict of interest relevant to this article was reported.
References
- 1.Miettinen M. Gastrointestinal stromal tumors: definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 2.Hirota S, Isozaki K, Nishida T, Kitamura Y. Effects of loss-of-function and gain-of-function mutations of c-kit on the gastrointestinal tract. J Gastroenterol. 2000;35(suppl 12):75–79. [PubMed] [Google Scholar]
- 3.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813–3825. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 4.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 5.Hirota S, Ohashi A, Nishida T, et al. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology. 2003;125:660–667. doi: 10.1016/s0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- 6.Agaram NP, Wong GC, Guo T, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008;47:853–859. doi: 10.1002/gcc.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belinsky MG, Skorobogatko YV, Rink L, et al. High density DNA array analysis reveals distinct genomic profiles in a subset of gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2009;48:886–896. doi: 10.1002/gcc.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinho O, Gouveia A, Viana-Pereira M, et al. Low frequency of MAP kinase pathway alterations in KIT and PDGFRA wild-type GISTs. Histopathology. 2009;55:53–62. doi: 10.1111/j.1365-2559.2009.03323.x. [DOI] [PubMed] [Google Scholar]
- 9.Rubin BP, Singer S, Tsao C, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118–8121. [PubMed] [Google Scholar]
- 10.DeMatteo RPLJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 12.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 13.Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors: report of the GIST Consensus Conference of 20–21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566–578. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 14.Van den Abbeele AD for the GIST Collaborative PET Study Group. F18-FDG-PET provides early evidence of biological response to STI571 in patients with malignant gastrointestinal stromal tumors (GIST) [abstract] Proc Am Soc Clin Oncol. 2001;(20):362a. [Google Scholar]
- 15.Stroobants S, Goeminne J, Seegers M, et al. 18FDG-positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec) Eur J Cancer. 2003;39:2012–2020. doi: 10.1016/s0959-8049(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 16.Van den Abbeele ADBR, Cliche JP, Spangler T, et al. FDG-PET as a surrogate marker for the response to Gleevec (imatinib mesylate) in patients with advanced gastrointestinal stromal tumors (GIST) [abstract] J Nucl Med. 2003;44(suppl):24P–25P. [Google Scholar]
- 17.Antoch G, Kanja J, Bauer S, et al. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004;45:357–365. [PubMed] [Google Scholar]
- 18.Holdsworth CH, Badawi RD, Manola JB, et al. CT and PET: early prognostic indicators of response to imatinib mesylate in patients with gastrointestinal stromal tumor. AJR. 2007;189:W324–W330. doi: 10.2214/AJR.07.2496. [DOI] [PubMed] [Google Scholar]
- 19.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 20.Dudeck O, Zeile M, Reichardt P, Pink D. Comparison of RECIST and Choi criteria for computed tomographic response evaluation in patients with advanced gastrointestinal stromal tumor treated with sunitinib. Ann Oncol. 2011;22:1828–1833. doi: 10.1093/annonc/mdq696. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42–47. doi: 10.1002/jso.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 24.Fiore M, Palassini E, Fumagalli E, et al. Preoperative imatinib mesylate for unresectable or locally advanced primary gastrointestinal stromal tumors (GIST) Eur J Surg Oncol. 2009;35:739–745. doi: 10.1016/j.ejso.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Basu S, Mohandas KM, Peshwe H, Asopa R, Vyawahare M. FDG-PET and PET/CT in the clinical management of gastrointestinal stromal tumor. Nucl Med Commun. 2008;29:1026–1039. doi: 10.1097/MNM.0b013e328313bbe7. [DOI] [PubMed] [Google Scholar]
- 26.Heinicke T, Wardelmann E, Sauerbruch T, Tschampa HJ, Glasmacher A, Palmedo H. Very early detection of response to imatinib mesylate therapy of gastrointestinal stromal tumours using 18fluoro-deoxyglucose-positron emission tomography. Anticancer Res. 2005;25:4591–4594. [PubMed] [Google Scholar]
- 27.Le Cesne A, Blay JY, Judson I, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group trial. J Clin Oncol. 2005;23:576–584. doi: 10.1200/JCO.2005.01.180. [DOI] [PubMed] [Google Scholar]
- 28.Van den Abbeele AD, Badawi RD, Cliche JP, et al. 18F-FDG-PET predicts response to imatinib mesylate (Gleevec™) in patients with advanced gastrointestinal stromal tumors (GIST) [abstract] Proc Am Soc Clin Oncol. 2002;21:403a. [Google Scholar]
- 29.Gayed I, Vu T, Iyer R, et al. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004;45:17–21. [PubMed] [Google Scholar]
- 30.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 31.Van den Abbeele AD. The lessons of GIST: PET and PET/CT—a new paradigm for imaging. Oncologist. 2008;13(suppl 2):8–13. doi: 10.1634/theoncologist.13-S2-8. [DOI] [PubMed] [Google Scholar]
- 32.Krajewski KM, Guo M, Van den Abbeele AD, et al. Comparison of four early posttherapy imaging changes (EPTIC; RECIST 1.0, tumor shrinkage, computed tomography tumor density, Choi criteria) in assessing outcome to vascular endothelial growth factor–targeted therapy in patients with advanced renal cell carcinoma. Eur Urol. 2011;59:856–862. doi: 10.1016/j.eururo.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 33.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 34.Tarn C, Skorobogatko YV, Taguchi T, Eisenberg B, von Mehren M, Godwin AK. Therapeutic effect of imatinib in gastrointestinal stromal tumors: AKT signaling dependent and independent mechanisms. Cancer Res. 2006;66:5477–5486. doi: 10.1158/0008-5472.CAN-05-3906. [DOI] [PubMed] [Google Scholar]
- 35.Rink L, Skorobogatko Y, Kossenkov AV, et al. Gene expression signatures and response to imatinib mesylate in gastrointestinal stromal tumor. Mol Cancer Ther. 2009;8:2172–2182. doi: 10.1158/1535-7163.MCT-09-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 37.Debiec-Rychter M, Dumez H, Judson I, et al. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2004;40:689–695. doi: 10.1016/j.ejca.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 38.Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42:1093–1103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.