Abstract
Purpose
This clinical trial evaluated standard-dose radioimmunotherapy with a chemotherapy-based transplantation regimen followed by autologous hematopoietic cell transplantation versus rituximab with the same regimen in patients with relapsed diffuse large B-cell lymphoma (DLBCL).
Patients and Methods
Patients with chemotherapy-sensitive persistent or relapsed DLBCL were randomly assigned to receive iodine-131 tositumomab (dosimetric dose of 5 mCi on day −19 and therapeutic dose of 0.75 Gy on day −12), carmustine 300 mg/m2 (day −6), etoposide 100 mg/m2 twice daily (days −5 to −2), cytarabine 100 mg/m2 twice daily (days −5 to −2), and melphalan 140 mg/m2 (day −1; B-BEAM) or rituximab 375 mg/m2 on days −19 and −12 and the same chemotherapy regimen (R-BEAM).
Results
Two hundred twenty-four patients were enrolled, with 113 patients randomly assigned to R-BEAM and 111 patients assigned to B-BEAM. Two-year progression-free survival (PFS) rates, the primary end point, were 48.6% (95% CI, 38.6% to 57.8%) for R-BEAM and 47.9% (95% CI, 38.2% to 57%; P = .94) for B-BEAM, and the 2-year overall survival (OS) rates were 65.6% (95% CI, 55.3% to 74.1%) for R-BEAM and 61% (95% CI, 50.9% to 69.9%; P = .38) for B-BEAM. The 100-day treatment-related mortality rates were 4.1% (95% CI, 0.2% to 8.0%) for R-BEAM and 4.9% (95% CI, 0.8% to 9.0%; P = .97) for B-BEAM. The maximum mucositis score was higher in the B-BEAM arm (0.72) compared with the R-BEAM arm (0.31; P < .001).
Conclusion
The B-BEAM and R-BEAM regimens produced similar 2-year PFS and OS rates for patients with chemotherapy-sensitive relapsed DLBCL. No differences in toxicities other than mucositis were noted.
INTRODUCTION
The Parma study established the use of high-dose chemotherapy with autologous bone marrow transplantation as the standard of care for relapsed chemotherapy-sensitive diffuse large B-cell lymphoma (DLBCL).1 However, even in patients with chemotherapy-sensitive DLBCL, relapse of lymphoma remains the major cause of transplantation failure.2–4 To address this problem, different chemotherapeutic agents have been combined such as carmustine, etoposide, cytarabine, and melphalan (BEAM); carmustine, etoposide, cytarabine, and cyclophosphamide; and cyclophosphamide, etoposide, and carmustine.5–7 Total-body irradiation (TBI) has been combined with cyclophosphamide or with cyclophosphamide and etoposide in various studies.8,9 Although lymphoma is a radiation-sensitive tumor, the TBI used in many of these regimens has proven to be more toxic, especially in older patients.9 None of these chemotherapy-only or TBI-containing regimens has proven to be superior.
In an attempt to further improve outcome, the addition of monoclonal antibodies to the transplantation regimen has been explored. Initially, the use of rituximab in the peritransplantation period seemed to improve the progression-free survival (PFS) compared with patients who did not receive rituximab.10,11 However, as the use of rituximab in first-line therapy was extended to all patients, the advantage of peritransplantation rituximab faded.12,13
Radioimmunotherapy has properties that would make it an ideal candidate for addition to a transplantation regimen. The major adverse effect of radioimmunotherapy is myelosuppression, which can be overcome with the infusion of hematopoietic stem cells. Therefore, several phase I and II studies have been performed using either high doses of yttrium-90 (90Y) –ibritumomab tiuxetan (Zevalin; Spectrum Pharmaceuticals, Henderson, NV)14,15 or iodine-131 (131I) –tositumomab (Bexxar; GlaxoSmithKline, Philadelphia, PA)16 as part of the transplantation regimen. Alternatively, phase I and II studies of standard outpatient doses of 90Y–ibritumomab tiuxetan17 or 131I-tositumomab18 added to standard transplantation regimens have been performed. With promising results in the phase I and II studies, standard-dose 131I-tositumomab with BEAM (B-BEAM) was included in this phase III trial.
Herein, we report the results of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0401 study, which was a phase III trial comparing outcomes of patients with relapsed chemotherapy-sensitive DLBCL receiving rituximab plus BEAM (R-BEAM) versus B-BEAM with autologous hematopoietic cell transplantation (AHCT).
PATIENTS AND METHODS
Study Design
From January 2006 to July 2009, a prospective phase III multicenter trial was conducted in 37 transplantation centers of the BMT CTN (Appendix Table A1, online only). Patients who met eligibility criteria were randomly assigned to receive either tositumomab and 131I-tositumomab (dosimetric dose of 5 mCi on day −19 and therapeutic total-body dose of 0.75 Gy on day −12), carmustine 300 mg/m2 (day −6), etoposide 100 mg/m2 twice daily (days −5 to −2), cytarabine 100 mg/m2 twice daily (days −5 to −2), and melphalan 140 mg/m2 (day −1; B-BEAM) or rituximab (375 mg/m2 on days −19 and −12) with the BEAM regimen (R-BEAM). The primary hypothesis to be tested in patients with chemotherapy-sensitive persistent or relapsed DLBCL was that the addition of 131I-tositumomab to a standard high-dose chemotherapy transplantation regimen with BEAM would improve the 2-year PFS compared with the addition of rituximab to the same chemotherapy transplantation protocol. Secondary end points included overall survival (OS), time to progression, complete response (CR) and partial response (PR) at day +100, relapse rates, time to hematologic recovery, maximum mucositis score on day +21, 100-day treatment-related mortality, and the development of secondary myelodysplastic syndrome (MDS) or acute myelogenous leukemia (AML).
The protocol was approved by the protocol review committee of the National Heart, Lung, and Blood Institute, the local institutional review board, and appropriate radiation safety and scientific review boards. All patients signed the approved informed consent in effect for the study in accordance with the Declaration of Helsinki.
Eligibility Criteria
Patients enrolled onto this study were age 18 to 80 years; had a Karnofsky score ≥ 70, persistent or recurrent DLBCL, and chemotherapy-sensitive disease; and had received one to three prior chemotherapy regimens. Patients were eligible who had persistent DLBCL after induction chemotherapy but were chemotherapy sensitive (first PR), had experienced relapse after an initial CR but had a PR to salvage chemotherapy, or had a CR to salvage chemotherapy (second [CR2]). Patients also needed ≤ 20% involvement of their bone marrow with lymphoma with no evidence of MDS in the pretransplantation bone marrow. All patients had their pathology reviewed locally and had a specimen that was CD20+ with no evidence of transformed follicular lymphoma. Mobilization therapy was used as per institutional guidelines, but all patients received at least one dose of rituximab 375 mg/m2 within 3 months of the first apheresis collection. Patients were required to have an adequate autograft collection (target ≥ 2.0 × 106 CD34+ cells/kg; minimum 1.5 × 106 CD34+ cells/kg) to be eligible for the protocol.
Patients
Two hundred twenty-four patients with persistent or relapsed DLBCL were enrolled onto the clinical trial. Median follow-up of the study population is 25.5 months (range, 13.8 to 55.8 months).
Treatment
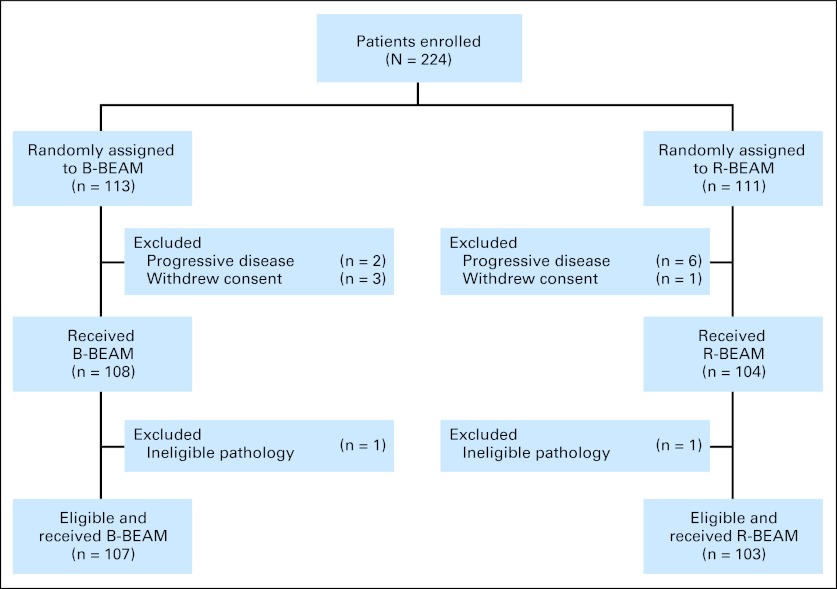
The CONSORT diagram is outlined in Figure 1. Enrolled patients were randomly assigned to R-BEAM or B-BEAM with AHCT infusion 24 hours later. The patients assigned to the B-BEAM arm received orally administered saturated solution of potassium iodide, two drops given three times daily starting 1 day before the dosimetric dose, and this was continued for 14 days after the therapeutic dose. The murine monoclonal anti-CD20 (tositumomab) was radioiodinated with sodium 131I by the iodogen method, purified, and tested as previously described.19 Within 1 hour after the dosimetric dose of 131I-tositumomab and before urination, a whole-body quantitative gamma camera image was obtained for baseline readings. Additional scans were performed on day 2, 3, or 4 and day 6 or 7 after the dosimetric dose. Using this information, the radioactive clearance from each patient was obtained to determine the radioactive millicurie activity of 131I-tositumomab required to deliver the desired therapeutic dose 1 week later. The methodology for determining the patient-specific millicurie activity was performed in accordance with the Medical Internal Radiation Dose Primer for Absorbed Dose Calculations.20 The day of AHCT was designated as day 0. Filgrastim 5 μg/kg subcutaneously was administered starting on day +5 and continued until neutrophil recovery of ≥ 500/μL for 3 consecutive days was obtained. Patients received supportive care during the transplantation as per institutional guidelines.
Fig 1.
CONSORT diagram outlining the number of patients at each step of the trial. B-BEAM, iodine-131 tositumomab plus carmustine, etoposide, cytarabine, and melphalan; R-BEAM, rituximab plus carmustine, etoposide, cytarabine, and melphalan.
End Points
The study had an 80% power to detect a 20% difference in the primary end point with an α = .05. The primary end point of the clinical trial was 2-year PFS. PFS was defined as time to disease relapse or progression, initiation of nonprotocol antilymphoma therapy, or death, measured from the time of AHCT, with patients censored at time of last contact. Supporting data for disease status and nonprotocol antilymphoma therapy during the trial were centrally reviewed by an outcome adjudication committee blinded to treatment assignment. Secondary end points were OS, time to progression, CR and PR at day + 100, relapse rates, time to hematologic recovery, incidence of grade 3 to 5 adverse events (Common Terminology Criteria for Adverse Events, version 3),21 maximum mucositis score on day +21 measured by the Oral Mucositis Assessment Scale,22 100-day treatment-related mortality, and the development of secondary MDS or AML. Hematologic recovery was defined as follows: neutrophil recovery was defined as the first of 3 consecutive days of an absolute neutrophil count ≥ 500/μL; platelet recovery was defined as the first of 7 consecutive days with a platelet count ≥ 20,000/μL with no transfusions; and RBC recovery was defined as the first of 30 days with a hemoglobin ≥ 8.0 g/dL without a transfusion. OS was defined as the time from transplantation to death from any cause.
Definition of disease response was based on the 2007 Cheson criteria.23 Disease assessments were performed before AHCT, at 100 days after AHCT, and at 1 and 2 years after AHCT. Patients received computed tomography and/or positron emission tomography/computed tomography of the chest, abdomen, and pelvis; physical examinations; CBC; chemistry profile; and a bone marrow biopsy to confirm CR at these time points.
Statistical Analysis
The data cutoff for analysis was November 14, 2011. Primary analysis was performed using the intent-to-treat principle (ie, patients were classified according to their original assigned treatment, even if they did not receive all prescribed interventions). Both PFS and OS were calculated using the Kaplan-Meier method.24 CIs were calculated using the logit transformation and the Greenwood variance estimate.25 Differences between the Kaplan-Meier curves were assessed using the log-rank test.26 The significance of demographic and treatment features was assessed using stratified survival analysis and univariate, multivariable Cox proportional hazards regression analysis or the corresponding hazard analysis for competing risks.27 All calculations were analyzed using SAS (version 9.2; SAS Institute, Cary, NC) and R (version 2.10.0; www.r-project.org/) statistical software. Statistical significance was set at P < .05; all P values were two-sided. Covariates used for the Cox regression model of PFS and OS were treatment arm, sex, ethnicity, race, age at transplantation, performance status, interval from diagnosis to transplantation, disease in CR at transplantation, number of prior chemotherapy regimens, total bilirubin, ALT, AST, and pulmonary diffusion capacity (DLCO). Backward selection and stepwise selection methods were used for model building. Proportional hazards were tested for all the variables in the model. Interactions were tested between treatment arm and any covariates, and no significant interactions were found.
Monitoring for accrual and toxicity according to sequential probability ratio stopping guidelines was performed by the National Heart, Lung, and Blood Institute–appointed Data and Safety Monitoring Board. No stopping rules were reached during the protocol accrual period.
RESULTS
Patients
Of the randomly assigned patients, 104 (93.7%) of 111 assigned to B-BEAM received a transplantation and 108 (95.6%) of 113 assigned to R-BEAM received a transplantation. Of the 12 patients who did not receive a transplantation, eight had progressive disease and four withdrew consent (three patients on the B-BEAM arm and one patient on the R-BEAM arm; Fig 1). The median age of the patients was 58 years, and 63% of the patients were male. Patient characteristics were similar in the two random assignment groups (Table 1).
Table 1.
Demographic and Clinical Characteristics of Patients With DLBCL Enrolled Onto the BMT CTN 0401 Trial
| Characteristic | B-BEAM (n = 111) |
R-BEAM (n = 113) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Received transplantation | 104 | 108 | ||
| Eligible for study | 103 | 107 | ||
| Male | 68 | 61.3 | 74 | 65.5 |
| Age, years | ||||
| Median | 56.8 | 58.5 | ||
| Range | 19.8-74.9 | 24.0-76.6 | ||
| Race, white | 99 | 89.2 | 103 | 91.2 |
| KPS | ||||
| 100 | 29 | 26.1 | 26 | 23 |
| 90 | 67 | 60.4 | 63 | 55.8 |
| 80 | 12 | 10.8 | 19 | 16.8 |
| 70 | 3 | 2.7 | 5 | 4.4 |
| Disease status at transplantation | ||||
| PR1 | 21 | 18.9 | 15 | 13.3 |
| REL1 | 35 | 31.5 | 45 | 39.8 |
| CR2 | 55 | 49.5 | 53 | 46.9 |
| No. of prior therapies | ||||
| 1 | 2 | 1.8 | 7 | 6.2 |
| 2 | 93 | 83.8 | 83 | 73.5 |
| 3 | 16 | 14.4 | 23 | 20.4 |
Abbreviations: B-BEAM, iodine-131 tositumomab plus carmustine, etoposide, cytarabine, and melphalan; BMT CTN, Blood and Marrow Transplant Clinical Trials Network; CR2, second complete remission; DLBCL, diffuse large B-cell lymphoma; KPS, Karnofsky performance score; PR1, first partial response; R-BEAM, rituximab plus carmustine, etoposide, cytarabine, and melphalan; REL1, first relapse (chemotherapy sensitive).
PFS and OS
Probabilities of 2-year PFS were 48.6% (95% CI, 38.6% to 57.8%) and 47.9% (95% CI, 38.2% to 57%) for the R-BEAM and B-BEAM arms, respectively (P = .94; Fig 2A). The probabilities of 2–year OS were 65.6% (95% CI, 55.3% to 74.1%) and 61% (95% CI, 50.9% to 69.6%) for the R-BEAM and B-BEAM arms, respectively (P = .38; Fig 2B).
Fig 2.
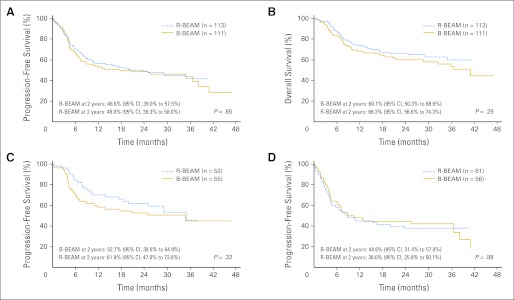
(A) Progression-free survival (PFS) according to random assignment groups. (B) Overall survival according to random assignment groups. (C) PFS of patients in second complete response according to random assignment groups. (D) PFS of patients with chemotherapy-sensitive relapse according to random assignment groups. B-BEAM, iodine-131 tositumomab plus carmustine, etoposide, cytarabine, and melphalan; R-BEAM, rituximab plus carmustine, etoposide, cytarabine, and melphalan.
Patients in CR after salvage chemotherapy (CR2) had an improved 2-year OS and PFS compared with patients with chemotherapy-sensitive first PR or chemotherapy-sensitive relapse. However, there were no differences in any of the groups by treatment arm. The 2-year PFS for CR2 patients receiving R-BEAM was 61.9% (95% CI, 47% to 73.8%) compared with 52.7% (95% CI, 38.8% to 64.9%) for patients receiving B-BEAM (P = .61; Fig 2C). The 2-year PFS for patients with chemotherapy-sensitive disease at relapse was 38.0% (95% CI, 25.8% to 50.1%) for patients receiving R-BEAM compared with 44.6% (95% CI, 31.4% to 57.0%) for patients receiving B-BEAM (P = .88; Fig 2D).
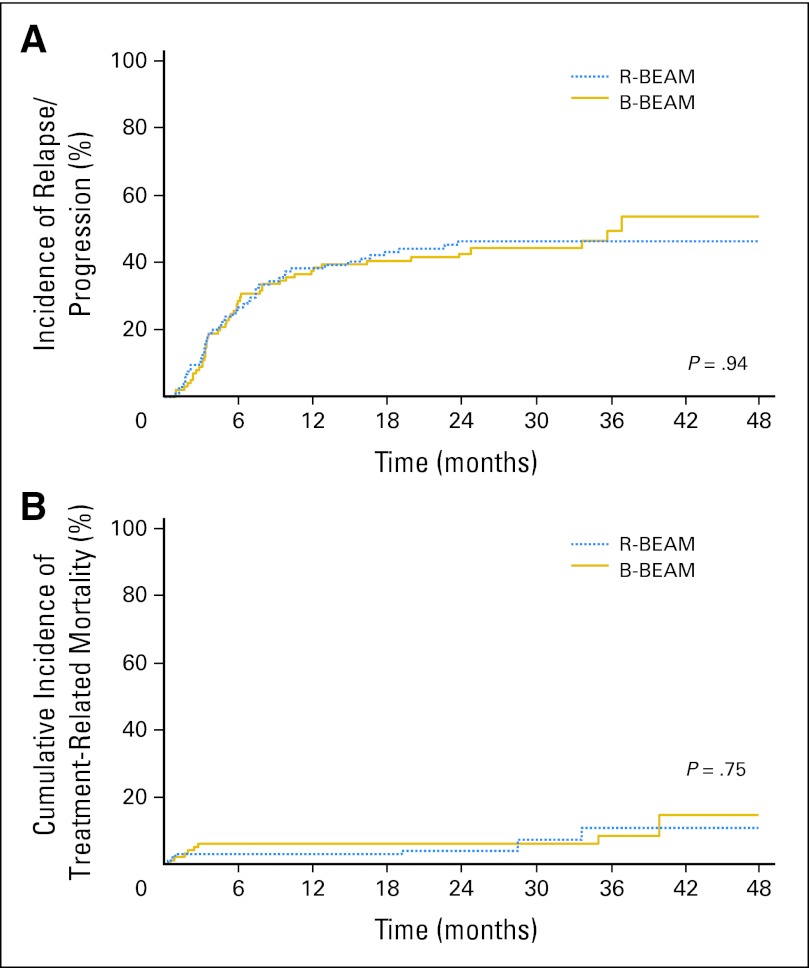
Disease Progression/Relapse and Treatment-Related Mortality
The most common cause of treatment failure was progression/relapse of lymphoma, with a cumulative incidence of relapse/progression at 2 years after transplantation of 48.1% (95% CI, 38.1% to 58.1%) in the R-BEAM arm compared with 45% (95% CI, 35.2% to 54.8%) in the B-BEAM arm (P = .68; Fig 3A). The 100-day treatment-related mortality was low in both arms, with a rate of 4.1% (95% CI, 0.2% to 8.0%) in the R-BEAM arm compared with 4.9% (95% CI, 0.8% to 9.0%) in the B-BEAM arm (P = .97; Fig 3B).
Fig 3.
(A) Cumulative incidence of disease progression or relapse according to random assignment groups. (B) Cumulative incidence of transplantation-related mortality according to random assignment groups. B-BEAM, iodine-131 tositumomab plus carmustine, etoposide, cytarabine, and melphalan; R-BEAM, rituximab plus carmustine, etoposide, cytarabine, and melphalan.
Engraftment
Engraftment was similar, with an absolute neutrophil count ≥ 500/μL by day +28 in 93.5% (95% CI, 88.6% to 98.4%) of patients in the R-BEAM arm compared with 96.1% (95% CI, 92.2% to 100%) of patients in the B-BEAM arm (P = .40). Platelet recovery to ≥ 20,000/μL with transfusion independence by day +100 was present in 81.3% (95% CI, 73.9% to 88.7%) of patients in the R-BEAM arm compared with 84.5% (95% CI, 77.4% to 91.6%) of patients in the B-BEAM arm (P = .58).
Toxicities
Grade 3 to 5 toxicities are listed in Table 2. The only toxicity that was different between the treatment groups was the mucositis score as measured by Oral Mucositis Assessment Scale.28 The median maximum mucositis score was 0.72 in the B-BEAM arm compared with 0.31 in the R-BEAM arm (P < .001). One case of MDS was reported in each arm of the trial, and one additional case of AML was reported in the R-BEAM arm.
Table 2.
Grade 3 to 5 Nonhematologic Toxicities Within 2 Years After Transplantation (> 10% of patients)
| Toxicity | B-BEAM |
R-BEAM |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Any grade 3-5 toxicity | 67 | 65 | 46 | 43 | < .01 |
| Mucositis WHO grade 3-5 | 53 | 52 | 19 | 18 | < .01 |
| Hypotension | 11 | 10.7 | 13 | 12.1 | .83 |
| Hypoxia | 20 | 19.4 | 17 | 15.9 | .59 |
| Dyspnea | 29 | 28.2 | 25 | 23.4 | .44 |
| Diarrhea | 9 | 8.7 | 14 | 17.8 | .38 |
Abbreviations: B-BEAM, iodine-131 tositumomab plus carmustine, etoposide, cytarabine, and melphalan; R-BEAM, rituximab plus carmustine, etoposide, cytarabine, and melphalan.
Causes of Death
At the time of data analysis, deaths had been reported in 81 patients—42 patients (52%) in the B-BEAM arm and 39 patients (48%) in the R-BEAM arm. The most common causes of death were progression/relapse (n = 64), organ failure (n = 4), and adult respiratory distress syndrome (n = 3). There was no significant difference in the distribution of primary causes of death between the two arms (P = .86).
Multivariate Cox Model for PFS and OS
The multivariate analysis for the primary end point of PFS is shown in Table 3. The only significant covariate in the model was disease status at the time of transplantation. The patients in CR at transplantation had an improved PFS compared with patients with a chemotherapy-sensitive persistent or relapsed lymphoma (P = .008). The treatment arm was not significant in the model (P = .66). The multivariate analysis for OS is shown in Table 4. The disease status at the time of transplantation was again the most important characteristic predicting for OS (P < .001).
Table 3.
Multivariate Analysis of PFS
| Covariate | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Treatment arm | |||
| R-BEAM | 1.00 | .66 | |
| B-BEAM | 1.08 | 0.76 to 1.54 | |
| Disease status at transplantation | |||
| CR | 1.00 | .008 | |
| Not in CR | 1.63 | 1.14 to 2.33 |
Abbreviations: B-BEAM, iodine-131 tositumomab plus carmustine, etoposide, cytarabine, and melphalan; CR, complete remission; PFS, progression-free survival; R-BEAM, rituximab plus carmustine, etoposide, cytarabine, and melphalan.
Table 4.
Multivariate Analysis for OS
| Covariate | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Treatment arm | |||
| R-BEAM | 1.00 | .55 | |
| B-BEAM | 1.14 | 0.73 to 1.78 | |
| Disease status at transplantation | |||
| CR | 1.00 | < .001 | |
| Not in CR | 2.42 | 1.47 to 3.96 | |
| Age at transplantation, years | |||
| < 50 | 1.00 | .027 | |
| ≥ 50 | 1.93 | 1.08 to 3.47 | |
| Interval from diagnosis to transplantation, months | |||
| < 15 | 1.00 | .001 | |
| ≥ 15 | 0.47 | 0.30 to 0.75 | |
| Sex | |||
| Female | 1.00 | .045 | |
| Male | 1.65 | 1.01 to 2.70 | |
| AST or ALT, units/L | |||
| < 25 | 1.00 | .042 | |
| ≥ 25 | 0.62 | 0.39 to 0.98 |
Abbreviations: B-BEAM, iodine-131 tositumomab plus carmustine, etoposide, cytarabine, and melphalan; CR, complete remission; OS, overall survival; R-BEAM, rituximab plus carmustine, etoposide, cytarabine, and melphalan.
DISCUSSION
The use of high-dose chemotherapy and AHCT has been the standard of care for chemotherapy-sensitive relapsed DLBCL since the Parma Trial demonstrated the improved outcomes compared with standard salvage chemotherapy.1 The results of the Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL) study demonstrated that for patients with relapsed DLBCL in the rituximab era, the benchmarks have been modified.29,30 In the CORAL study, only one half of the patients with relapse were able to proceed on to transplantation after salvage rituximab-containing chemotherapy. Therefore, on an intent-to-treat basis, patients with prior rituximab exposure and early relapse (< 12 months after diagnosis) who were enrolled onto the study had a 2-year PFS of only 23%. However, for such patients who responded to salvage therapy and then went on to transplantation, the 2-year event-free survival was only 40%.30 Similar outcomes have been reported in other studies of patients who fail a rituximab-containing regimen.11
With low PFS and OS after standard high-dose chemotherapy and AHCT, new areas of research have focused on the modification of the transplantation regimen. One major focus has been the addition of radioimmunotherapy as part of the conditioning regimen for transplantation. Multiple phase I and II studies using either 131I-tositumomab or 90Y–ibritumomab tiuxetan combined with chemotherapy have been published.14–18 On the basis of these favorable early results, the BMT CTN developed the current study, which randomly assigned patients to rituximab/BEAM versus 131I-tositumomab and BEAM.
Despite the promising phase I and II studies using radioimmunotherapy as part of the transplantation preparative regimen, this phase III study did not demonstrate a benefit for the addition this radioimmunotherapy to the transplantation regimen for patients with chemotherapy-sensitive relapsed DLBCL. However, the study was only powered to detect a large difference in PFS. The only variable that was predictive for an improved outcome with transplantation was the documentation of CR of the DLBCL before the transplantation procedure. The major cause of failure on this trial was relapse of the DLBCL. Although few cases of MDS were identified, further follow-up will be needed to monitor for late cases.
The other phase I and II radioimmunotherapy-containing transplantation regimens that have demonstrated the best outcomes have been those using higher doses of radioimmunotherapy. Studies evaluating higher doses of 90Y–ibritumomab tiuxetan used either a higher set dose of 0.8 to 1.2 mCi/kg31 or a dose calculated to deliver a set dose to critical organs ranging from 10 to 15 Gy.14,15 Higher doses of 131I-tositumomab have also been used either alone32 or in association with high-dose etoposide and cyclophosphamide16 for transplantation. In these studies, the patients received a median dose of 20 to 27 Gy to critical organs. These studies had patients with a variety of histologic types of lymphomas and were small studies; however, the CR rates were higher. A phase III randomized trial using one of these regimens would be needed to evaluate the efficacy of the higher doses of radioimmunotherapy in the transplantation regimen using a homogeneous population of patients.
Future efforts for the improvement of AHCT for patients with relapsed DLBCL will need to focus on improved salvage therapy to get a higher proportion of patients into CR, as well as consideration of post-transplantation consolidation or maintenance therapy to reduce relapse rates. Few phase III maintenance studies in the post-transplantation setting have been completed. One study by Thompson et al33 evaluated interkeukin-2 in the post-transplantation setting. This study showed that post-transplantation interleukin-2 given at the published dose and schedule had no significant effect on PFS or OS. More recently, the results of the second random assignment from the CORAL study also demonstrated no benefit from rituximab maintenance in the post-transplantation setting.12,30
There have recently been several novel agents that have demonstrated activity in relapsed DLBCL that may be candidates for oral maintenance therapy in the post-transplantation setting. These include agents inhibiting the B-cell receptor signaling pathway such as spleen tyrosine kinase34 or Bruton's tyrosine kinase.35 Another possible oral agent for consideration of use for post-transplantation maintenance therapy is lenalidomide because it does have single-agent activity for DLBCL and affects the microenvironment of the lymph node and bone marrow.36 Future clinical trials will be needed to evaluate these options for improving the outcome of transplantation for patients with relapsed DLBCL.
Supplementary Material
Appendix
Table A1.
List of Participating Transplantation Centers
| Centers |
|---|
| Baylor College of Medicine/The Methodist Hospital |
| Blood and Marrow Transplant Program at Northside Hospital |
| City of Hope National Medical Center |
| City of Hope Samaritan |
| Dana-Farber Cancer Institute/Brigham and Women's Hospital |
| Duke University Medical Center–Adult BMT |
| Emory University |
| Fox Chase–Temple University–BMT Program |
| Fred Hutchinson Cancer Research Center |
| Hackensack University Medical Center |
| Indiana BMT at Beech Grove |
| Jewish Hospital BMT Program |
| Loyola University Medical Center |
| Medical College of Wisconsin |
| Memorial Sloan-Kettering Cancer Center |
| Moffitt Cancer Center |
| Oregon Health and Science University |
| Rocky Mountain BMT Program |
| Scripps Clinic/Green Hospital |
| Stanford Hospital and Clinics |
| Texas Transplant Institute |
| Tufts Medical Center |
| University of California San Diego Medical Center |
| University Hospitals of Cleveland/University Hospitals Case Medical Center |
| University of Alabama at Birmingham |
| University of Florida College of Medicine (Shands) |
| University of Kansas Hospital |
| University of Michigan Medical Center |
| University of Minnesota |
| University of Nebraska Medical Center |
| University of Oklahoma Medical Center |
| University of Pennsylvania Abramson Cancer Center |
| The University of Texas MD Anderson Cancer Research Center |
| University of Wisconsin Hospital and Clinics |
| University of Utah Medical School |
| Vanderbilt University Medical Center/VA Tennessee Valley Healthcare System |
| Virginia Commonwealth University MCV Hospitals |
| Wichita CCOP |
Abbreviations: BMT, bone marrow transplantation; CCOP, Community Clinical Oncology Program; MCV, Medical College of Virginia; VA, Veterans Affairs.
Footnotes
Supported in part by Grant No. U01HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute, by the Southwest Oncology Group, and by contributions from GlaxoSmithKline to the Blood and Marrow Transplant Clinical Trials Network.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00329030
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Craig H. Moskowitz, Pharmacyclics (C), Seattle Genetics (C); Richard Fisher, Genentech (C) Stock Ownership: None Honoraria: None Research Funding: Julie M. Vose, Genentech, GlaxoSmithKline; Craig H. Moskowitz, Cephalon, Genentech, Seattle Genetics Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Julie M. Vose, Linda J. Burns, Oliver W. Press, Craig H. Moskowitz, Edward A. Stadtmauer, Mary Horowitz, Marcie Tomblyn
Administrative support: Shelly Carter, Mary Horowitz, Marcie Tomblyn
Provision of study materials or patients: Julie M. Vose, Linda J. Burns, Ernesto Ayala, Oliver W. Press, Craig H. Moskowitz, Edward A. Stadtmauer, Shin Mineshi, Richard Ambinder, Timothy Fenske, Richard Fisher, Marcie Tomblyn
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 2.Vose JM, Zhang MJ, Rowlings PA, et al. Autologous transplantation for diffuse aggressive non-Hodgkin's lymphoma in patients never achieving remission: A report from the autologous blood and marrow transplant registry. J Clin Oncol. 2001;19:406–413. doi: 10.1200/JCO.2001.19.2.406. [DOI] [PubMed] [Google Scholar]
- 3.Nademanee A, Molina A, Dagis A, et al. Autologous stem-cell transplantation for poor-risk and relapsed intermediate- and high-grade non-Hodgkin's lymphoma. Clin Lymphoma. 2000;1:46–54. doi: 10.3816/clm.2000.n.004. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz N, Buske C, Gisselbrecht C. Autologous stem cell transplantation in lymphoma. Semin Hematol. 2007;44:234–245. doi: 10.1053/j.seminhematol.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Caballero MD, Rubio V, Rifon J, et al. BEAM chemotherapy followed by autologous stem cell support in lymphoma patients: Analysis of efficacy, toxicity and prognostic factors. Bone Marrow Transplant. 1997;20:451–458. doi: 10.1038/sj.bmt.1700913. [DOI] [PubMed] [Google Scholar]
- 6.Philip T, Armitage JO, Spitzer G, et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin's lymphoma. N Engl J Med. 1987;316:1493–1498. doi: 10.1056/NEJM198706113162401. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez-Delgado F, Maloney DG, Press OW, et al. Autologous stem cell transplantation for non-Hodgkin's lymphoma: Comparison of radiation-based and chemotherapy-only preparative regimens. Bone Marrow Transplant. 2001;28:455–461. doi: 10.1038/sj.bmt.1703179. [DOI] [PubMed] [Google Scholar]
- 8.Horning SJ, Negrin RS, Chao JC, et al. Fractionated total-body irradiation, etoposide, and cyclophosphamide plus autografting in Hodgkin's disease and non-Hodgkin's lymphoma. J Clin Oncol. 1994;12:2552–2558. doi: 10.1200/JCO.1994.12.12.2552. [DOI] [PubMed] [Google Scholar]
- 9.Caballero MD, Pérez-Simón JA, Iriondo A, et al. High-dose therapy in diffuse large cell lymphoma: Results and prognostic factors in 452 patients from the GEL-TAMO Spanish Cooperative Group. Ann Oncol. 2003;14:140–151. doi: 10.1093/annonc/mdg008. [DOI] [PubMed] [Google Scholar]
- 10.Fenske TS, Hari PN, Carreras J, et al. Impact of pre-transplant rituximab on survival after autologous hematopoietic stem cell transplantation for diffuse large B cell lymphoma. Biol Blood Marrow Transplant. 2009;15:1455–1464. doi: 10.1016/j.bbmt.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoerr AL, Gao F, Hidalgo J, et al. Effects of pretransplantation treatment with rituximab on outcomes of autologous stem-cell transplantation for non-Hodgkin's lymphoma. J Clin Oncol. 2004;22:4561–4566. doi: 10.1200/JCO.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 12.Gisselbrecht C, Glass B, Fournier M, et al. Salvage regimen with autologous stem cell transplantation with or without rituximab maintenance for relapsed diffuse large B-cell lymphoma (DLBCL): CORAL study final report. Ann Oncol. 2011;22:75a. abstr. [Google Scholar]
- 13.Smith SD, Bolwell BJ, Rybicki LA, et al. Comparison of outcomes after auto-SCT for patients with relapsed diffuse large B-cell lymphoma according to previous therapy with rituximab. Bone Marrow Transplant. 2011;46:262–266. doi: 10.1038/bmt.2010.95. [DOI] [PubMed] [Google Scholar]
- 14.Winter JN, Inwards DJ, Spies S, et al. Yttrium-90 ibritumomab tiuxetan doses calculated to deliver up to 15 Gy to critical organs may be safely combined with high-dose BEAM and autologous transplantation in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2009;27:1653–1659. doi: 10.1200/JCO.2008.19.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nademanee A, Forman S, Molina A, et al. A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood. 2005;106:2896–2902. doi: 10.1182/blood-2005-03-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Press OW, Eary JF, Gooley T, et al. A phase I/II trial of iodine-131-tositumomab (anti-CD20), etoposide, cyclophosphamide, and autologous stem cell transplantation for relapsed B-cell lymphomas. Blood. 2000;96:2934–2942. [PubMed] [Google Scholar]
- 17.Decaudin D, Mounier N, Tilly H, et al. (90)Y Ibritumomab tiuxetan (Zevalin) combined with BEAM (Z-BEAM) conditioning regimen plus autologous stem cell transplantation in relapsed or refractory low-grade CD20-positive B-cell lymphoma: A GELA phase II prospective study. Clin Lymphoma Myeloma Leuk. 2011;11:212–218. doi: 10.1016/j.clml.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Vose JM, Bierman PJ, Enke C, et al. Phase I trial of iodine-131 tositumomab with high-dose chemotherapy and autologous stem-cell transplantation for relapsed non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:461–467. doi: 10.1200/JCO.2005.05.117. [DOI] [PubMed] [Google Scholar]
- 19.Kaminski MS, Zelenetz AD, Press OW, et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin's lymphomas. J Clin Oncol. 2001;19:3918–3928. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 20.Loevinger R, Budinger TF, Watson EE. Reston, VA: The Society of Nuclear Medicine; 1988. MIRD Primer for Absorbed Dose Calculations. [Google Scholar]
- 21.National Cancer Institute: Common Terminology Criteria for Adverse Events. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 22.Sonis ST, Eilers JP, Epstein JB, et al. Validation of a new scoring system for assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy: Mucositis Study Group. Cancer. 1999;85:2103–2113. doi: 10.1002/(sici)1097-0142(19990515)85:10<2103::aid-cncr2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Anderson JR, Bernstein L, Pike MC. Appropriate confidence intervals for probabilities of survival and quantiles in life-table analysis. Biometrics. 1982;38:407–416. [PubMed] [Google Scholar]
- 26.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. Part II: Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–200. [Google Scholar]
- 28.Sonis ST, Oster G, Fuchs H, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol. 2001;19:2201–2205. doi: 10.1200/JCO.2001.19.8.2201. [DOI] [PubMed] [Google Scholar]
- 29.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gisselbrecht C, Glass B, Laurent G, et al. Maintenance with rituximab after autologous stem cell transplantation in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL): CORAL final analysis. JCO. 2011;29(suppl):505s. abstr 8004. [Google Scholar]
- 31.Devizzi L, Guidetti A, Tarella C, et al. High-dose yttrium-90-ibritumomab tiuxetan with tandem stem-cell reinfusion: An outpatient preparative regimen for autologous hematopoietic cell transplantation. J Clin Oncol. 2008;26:5175–5182. doi: 10.1200/JCO.2008.16.8294. [DOI] [PubMed] [Google Scholar]
- 32.Gopal AK, Gooley TA, Maloney DG, et al. High-dose radioimmunotherapy versus conventional high-dose therapy and autologous hematopoietic stem cell transplantation for relapsed follicular non-Hodgkin lymphoma: A multivariable cohort analysis. Blood. 2003;102:2351–2357. doi: 10.1182/blood-2003-02-0622. [DOI] [PubMed] [Google Scholar]
- 33.Thompson JA, Fisher RI, Leblanc M, et al. Total body irradiation, etoposide, cyclophosphamide, and autologous peripheral blood stem-cell transplantation followed by randomization to therapy with interleukin-2 versus observation for patients with non-Hodgkin lymphoma: Results of a phase 3 randomized trial by the Southwest Oncology Group (SWOG 9438) Blood. 2008;111:4048–4054. doi: 10.1182/blood-2007-09-111708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Advani RH, Sharman JP, Smith SM, et al. The BTK inhibitor PCI-32765 is highly active and well tolerated in patients with relapsed/refractory B-cell malignancies: Final results from a phase I study. Ann Oncol. 2011;22:153a. abstr. [Google Scholar]
- 36.Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, et al. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer. 2011;117:5058–5066. doi: 10.1002/cncr.26135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.