Abstract
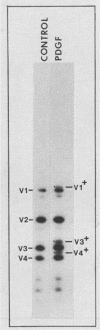
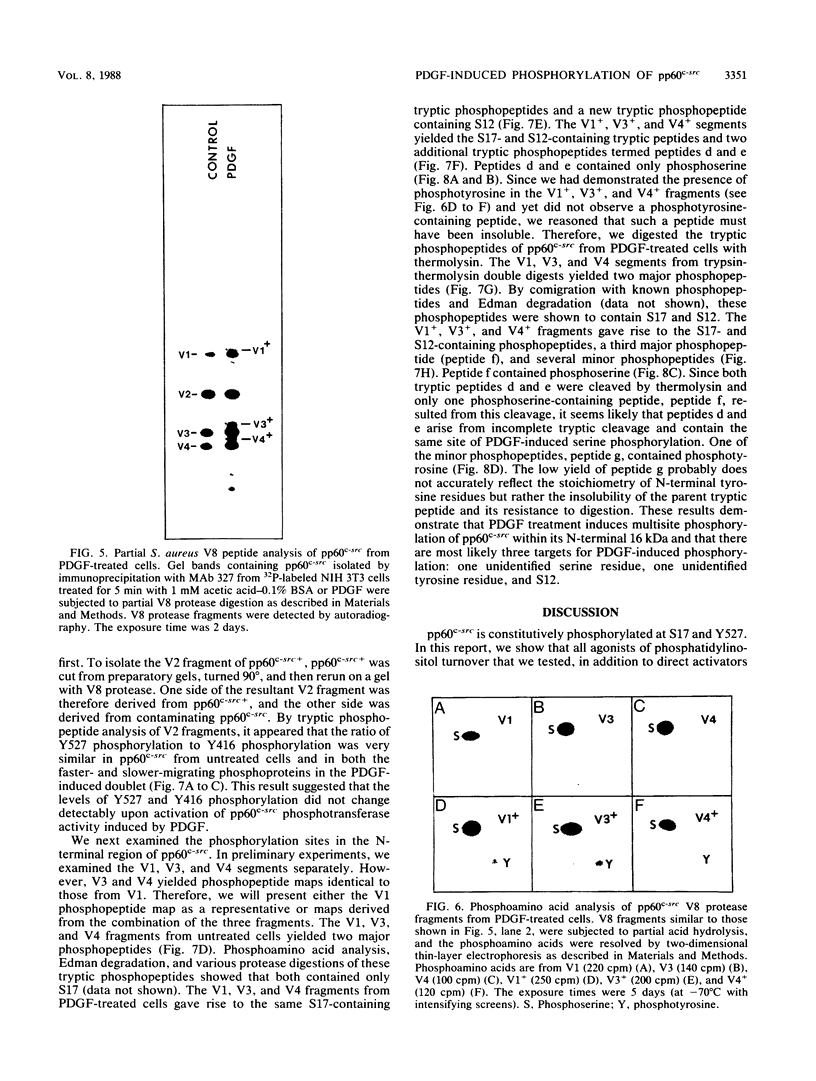
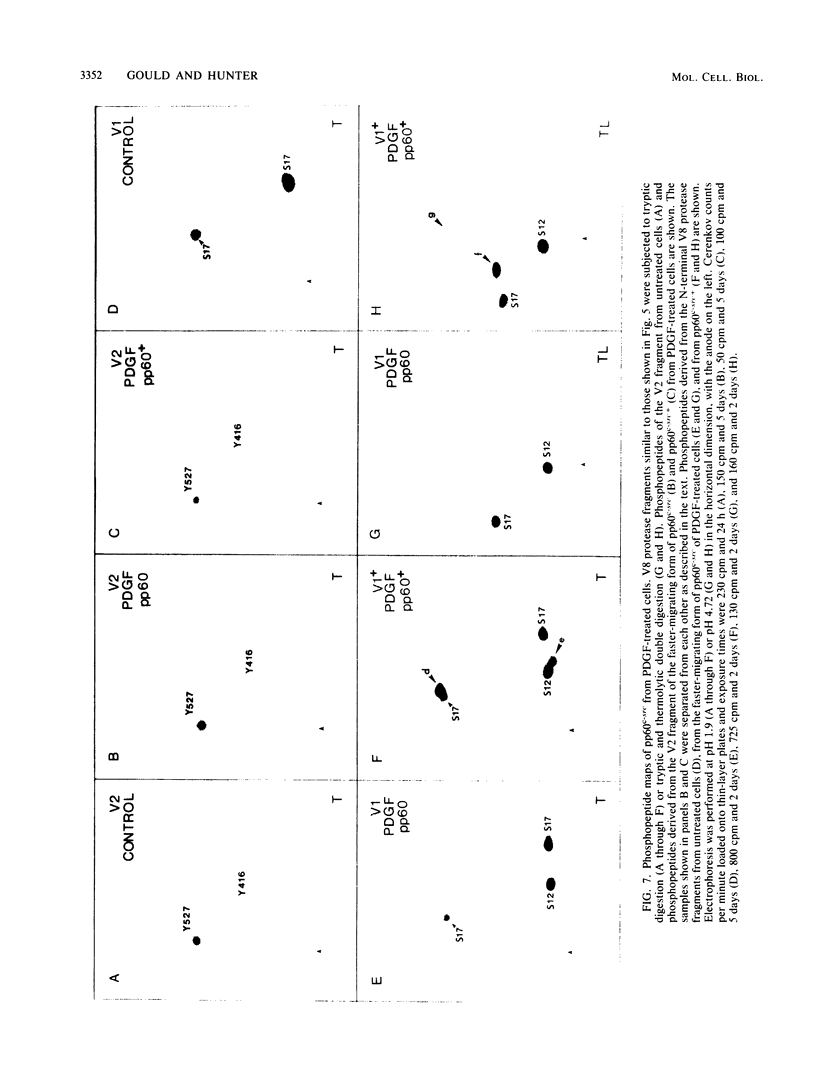
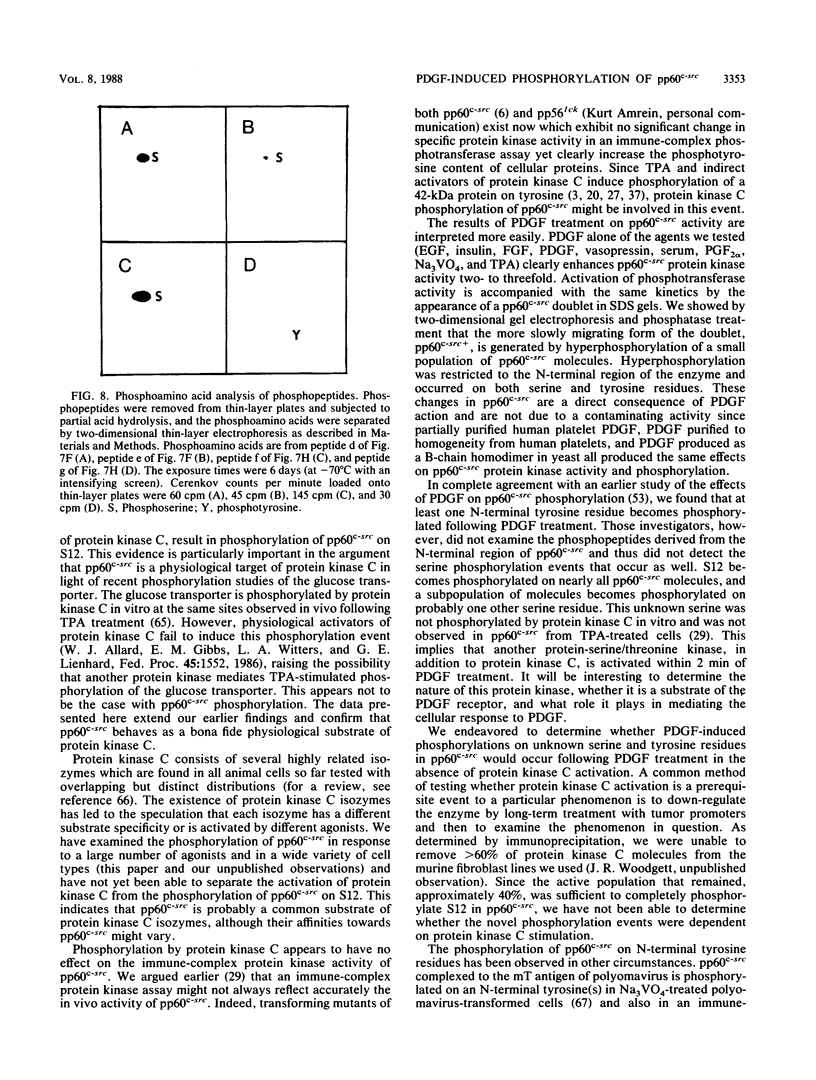
We have shown previously that pp60c-src is a substrate for protein kinase C in vivo and that the target of protein kinase C phosphorylation in mammalian pp60c-src is serine 12. We now demonstrate that in addition to tumor promoters, all activators of phosphatidylinositol turnover that we have tested in fibroblasts (platelet-derived growth factor, fibroblast growth factor, serum, vasopressin, sodium orthovanadate, and prostaglandin F2 alpha) lead to the phosphorylation of pp60c-src at serine 12. In addition to stimulating serine 12 phosphorylation in pp60c-src, platelet-derived growth factor treatment of quiescent fibroblasts induces phosphorylation of one or two additional serine residues and one tyrosine residue within the N-terminal 16 kilodaltons of the enzyme and activates its immune complex protein-tyrosine kinase activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol trisphosphate formation and calcium mobilization in Swiss 3T3 cells in response to platelet-derived growth factor. Biochem J. 1984 Aug 15;222(1):195–201. doi: 10.1042/bj2220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman J. M., Watson S. P., Cuatrecasas P. Lack of association of epidermal growth factor-, insulin-, and serum-induced mitogenesis with stimulation of phosphoinositide degradation in BALB/c 3T3 fibroblasts. J Biol Chem. 1986 Jan 15;261(2):723–727. [PubMed] [Google Scholar]
- Bishop R., Martinez R., Nakamura K. D., Weber M. J. A tumor promoter stimulates phosphorylation on tyrosine. Biochem Biophys Res Commun. 1983 Sep 15;115(2):536–543. doi: 10.1016/s0006-291x(83)80178-8. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Blay J., Irvine R. F., Heslop J. P., Berridge M. J. Reduction of epidermal growth factor receptor affinity by heterologous ligands: evidence for a mechanism involving the breakdown of phosphoinositides and the activation of protein kinase C. Biochem Biophys Res Commun. 1984 Aug 30;123(1):377–384. doi: 10.1016/0006-291x(84)90424-8. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Cotton P. C., Queral A. E., Barrett J. N., Nonner D., Keane R. W. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985 Aug 8;316(6028):554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Eckhart W., Simon S., Kaplan P. L. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell. 1987 Apr 10;49(1):83–91. doi: 10.1016/0092-8674(87)90758-6. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Hutchinson M. A., Eckhart W. Structural and functional modification of pp60c-src associated with polyoma middle tumor antigen from infected or transformed cells. Mol Cell Biol. 1985 Oct;5(10):2647–2652. doi: 10.1128/mcb.5.10.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Kaplan P. L., Cooper J. A., Hunter T., Eckhart W. Altered sites of tyrosine phosphorylation in pp60c-src associated with polyomavirus middle tumor antigen. Mol Cell Biol. 1986 May;6(5):1562–1570. doi: 10.1128/mcb.6.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Simantov R., Kaplan P. L., Hunter T., Eckhart W. Alterations in pp60c-src accompany differentiation of neurons from rat embryo striatum. Mol Cell Biol. 1987 May;7(5):1830–1840. doi: 10.1128/mcb.7.5.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chackalaparampil I., Shalloway D. Altered phosphorylation and activation of pp60c-src during fibroblast mitosis. Cell. 1988 Mar 25;52(6):801–810. doi: 10.1016/0092-8674(88)90422-9. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collett M. S., Belzer S. K., Purchio A. F. Structurally and functionally modified forms of pp60v-src in Rous sarcoma virus-transformed cell lysates. Mol Cell Biol. 1984 Jul;4(7):1213–1220. doi: 10.1128/mcb.4.7.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Wells S. K., Purchio A. F. Physical modification of purified Rous sarcoma virus pp60v-src protein after incubation with ATP/Mg2+. Virology. 1983 Jul 30;128(2):285–297. doi: 10.1016/0042-6822(83)90256-8. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., King C. S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986 Dec;6(12):4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Diverse mitogenic agents induce the phosphorylation of two related 42,000-dalton proteins on tyrosine in quiescent chick cells. Mol Cell Biol. 1984 Jan;4(1):30–37. doi: 10.1128/mcb.4.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton P. C., Brugge J. S. Neural tissues express high levels of the cellular src gene product pp60c-src. Mol Cell Biol. 1983 Jun;3(6):1157–1162. doi: 10.1128/mcb.3.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Coussens P. M., Cooper J. A., Hunter T., Shalloway D. Restriction of the in vitro and in vivo tyrosine protein kinase activities of pp60c-src relative to pp60v-src. Mol Cell Biol. 1985 Oct;5(10):2753–2763. doi: 10.1128/mcb.5.10.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Gentry L. E., Chaffin K. E., Shoyab M., Purchio A. F. Novel serine phosphorylation of pp60c-src in intact cells after tumor promoter treatment. Mol Cell Biol. 1986 Feb;6(2):735–738. doi: 10.1128/mcb.6.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore T., Martin G. S. Phorbol ester and diacylglycerol induce protein phosphorylation at tyrosine. Nature. 1983 Dec 1;306(5942):487–490. doi: 10.1038/306487a0. [DOI] [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Woodgett J. R., Cooper J. A., Buss J. E., Shalloway D., Hunter T. Protein kinase C phosphorylates pp60src at a novel site. Cell. 1985 Oct;42(3):849–857. doi: 10.1016/0092-8674(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Hepler J. R., Nakahata N., Lovenberg T. W., DiGuiseppi J., Herman B., Earp H. S., Harden T. K. Epidermal growth factor stimulates the rapid accumulation of inositol (1,4,5)-trisphosphate and a rise in cytosolic calcium mobilized from intracellular stores in A431 cells. J Biol Chem. 1987 Mar 5;262(7):2951–2956. [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Cross F. R., Garber E. A., Hanafusa H. Low level of cellular protein phosphorylation by nontransforming overproduced p60c-src. Mol Cell Biol. 1985 May;5(5):1058–1066. doi: 10.1128/mcb.5.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J. Y., Takeya T., Grandori C., Iba H., Levy J. B., Hanafusa H. Amino acid substitutions sufficient to convert the nontransforming p60c-src protein to a transforming protein. Mol Cell Biol. 1986 Dec;6(12):4155–4160. doi: 10.1128/mcb.6.12.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiecik T. E., Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987 Apr 10;49(1):65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- Kohno M. Diverse mitogenic agents induce rapid phosphorylation of a common set of cellular proteins at tyrosine in quiescent mammalian cells. J Biol Chem. 1985 Feb 10;260(3):1771–1779. [PubMed] [Google Scholar]
- L'Allemain G., Pouysségur EGF and insulin action in fibroblasts. Evidence that phosphoinositide hydrolysis is not an essential mitogenic signalling pathway. FEBS Lett. 1986 Mar 3;197(1-2):344–348. doi: 10.1016/0014-5793(86)80354-4. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Gentry L. E., June C. H., Rabinovitch P. S., Purchio A. F. Stimulation of T cells through the CD3/T-cell receptor complex: role of cytoplasmic calcium, protein kinase C translocation, and phosphorylation of pp60c-src in the activation pathway. Mol Cell Biol. 1987 Feb;7(2):650–656. doi: 10.1128/mcb.7.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. B., Iba H., Hanafusa H. Activation of the transforming potential of p60c-src by a single amino acid change. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4228–4232. doi: 10.1073/pnas.83.12.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macphee C. H., Drummond A. H., Otto A. M., Jimenez de Asua L. Prostaglandin F2 alpha stimulates phosphatidylinositol turnover and increases the cellular content of 1,2-diacylglycerol in confluent resting Swiss 3T3 cells. J Cell Physiol. 1984 Apr;119(1):35–40. doi: 10.1002/jcp.1041190107. [DOI] [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Further evidence for a phospholipase C-coupled G protein in hamster fibroblasts. Induction of inositol phosphate formation by fluoroaluminate and vanadate and inhibition by pertussis toxin. J Biol Chem. 1987 Feb 15;262(5):1970–1976. [PubMed] [Google Scholar]
- Parker P. J., Stabel S., Waterfield M. D. Purification to homogeneity of protein kinase C from bovine brain--identity with the phorbol ester receptor. EMBO J. 1984 May;3(5):953–959. doi: 10.1002/j.1460-2075.1984.tb01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Varmus H. E., Bishop J. M. Expression of v-src and chicken c-src in rat cells demonstrates qualitative differences between pp60v-src and pp60c-src. Cell. 1984 May;37(1):131–139. doi: 10.1016/0092-8674(84)90308-8. [DOI] [PubMed] [Google Scholar]
- Parsons S. J., Creutz C. E. p60c-src activity detected in the chromaffin granule membrane. Biochem Biophys Res Commun. 1986 Jan 29;134(2):736–742. doi: 10.1016/s0006-291x(86)80482-x. [DOI] [PubMed] [Google Scholar]
- Patschinsky T., Hunter T., Esch F. S., Cooper J. A., Sefton B. M. Analysis of the sequence of amino acids surrounding sites of tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1982 Feb;79(4):973–977. doi: 10.1073/pnas.79.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patschinsky T., Hunter T., Sefton B. M. Phosphorylation of the transforming protein of Rous sarcoma virus: direct demonstration of phosphorylation of serine 17 and identification of an additional site of tyrosine phosphorylation in p60v-src of Prague Rous sarcoma virus. J Virol. 1986 Jul;59(1):73–81. doi: 10.1128/jvi.59.1.73-81.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Herschman H. R. Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3918–3921. doi: 10.1073/pnas.74.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Gentry L., Shoyab M. Phosphorylation of pp60v-src by the TPA receptor kinase (protein kinase C). Virology. 1986 Apr 30;150(2):524–529. doi: 10.1016/0042-6822(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Shoyab M., Gentry L. E. Site-specific increased phosphorylation of pp60v-src after treatment of RSV-transformed cells with a tumor promoter. Science. 1985 Sep 27;229(4720):1393–1395. doi: 10.1126/science.2994221. [DOI] [PubMed] [Google Scholar]
- Ralston R., Bishop J. M. The product of the protooncogene c-src is modified during the cellular response to platelet-derived growth factor. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7845–7849. doi: 10.1073/pnas.82.23.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D., Erikson R. L. Characterization of pp60src phosphorylation in vitro in Rous sarcoma virus-transformed cell membranes. Mol Cell Biol. 1985 May;5(5):916–922. doi: 10.1128/mcb.5.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D., Erikson R. L. Highly specific antibody to Rous sarcoma virus src gene product recognizes a novel population of pp60v-src and pp60c-src molecules. J Cell Biol. 1985 Feb;100(2):409–417. doi: 10.1083/jcb.100.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. B., Vila J., Lansing T. J., Potts W. M., Weber M. J., Parsons J. T. Activation of the oncogenic potential of the avian cellular src protein by specific structural alteration of the carboxy terminus. EMBO J. 1987 Aug;6(8):2359–2364. doi: 10.1002/j.1460-2075.1987.tb02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Serum, like phorbol esters, rapidly activates protein kinase C in intact quiescent fibroblasts. EMBO J. 1985 Jan;4(1):71–76. doi: 10.1002/j.1460-2075.1985.tb02319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder J. W., Gordon J. A. In vivo effect of sodium orthovanadate on pp60c-src kinase. Mol Cell Biol. 1987 Mar;7(3):1139–1147. doi: 10.1128/mcb.7.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai A., Smith K. B., Panneerselvam M., Salomon D. S. Activation of calcium and phospholipid-dependent protein kinase by epidermal growth factor (EGF) in A431 cells: attenuation by 12-0-tetradecanoylphorbol-13-acetate (TPA). Biochem Biophys Res Commun. 1982 Dec 31;109(4):1206–1214. doi: 10.1016/0006-291x(82)91905-2. [DOI] [PubMed] [Google Scholar]
- Sawyer S. T., Cohen S. Enhancement of calcium uptake and phosphatidylinositol turnover by epidermal growth factor in A-431 cells. Biochemistry. 1981 Oct 13;20(21):6280–6286. doi: 10.1021/bi00524a057. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Oppermann H., Czernilofsky A. P., Purchio A. F., Erikson R. L., Bishop J. M. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc Natl Acad Sci U S A. 1981 Oct;78(10):6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Friis R. R., Bauer H. pp60c-src is a substrate for phosphorylation when cells are stimulated to enter cycle. FEBS Lett. 1984 Nov 5;177(1):151–156. doi: 10.1016/0014-5793(84)81001-7. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Kaibuchi K., Kawahara Y., Fukuzaki H., Takai Y. Induction of protein kinase C activation and Ca2+ mobilization by fibroblast growth factor in Swiss 3T3 cells. FEBS Lett. 1985 Oct 28;191(2):205–210. doi: 10.1016/0014-5793(85)80009-0. [DOI] [PubMed] [Google Scholar]
- Vicentini L. M., Villereal M. L. Serum, bradykinin and vasopressin stimulate release of inositol phosphates from human fibroblasts. Biochem Biophys Res Commun. 1984 Sep 17;123(2):663–670. doi: 10.1016/0006-291x(84)90280-8. [DOI] [PubMed] [Google Scholar]
- Witters L. A., Vater C. A., Lienhard G. E. Phosphorylation of the glucose transporter in vitro and in vivo by protein kinase C. 1985 Jun 27-Jul 3Nature. 315(6022):777–778. doi: 10.1038/315777a0. [DOI] [PubMed] [Google Scholar]
- Yonemoto W., Filson A. J., Queral-Lustig A. E., Wang J. Y., Brugge J. S. Detection of phosphotyrosine-containing proteins in polyomavirus middle tumor antigen-transformed cells after treatment with a phosphotyrosine phosphatase inhibitor. Mol Cell Biol. 1987 Feb;7(2):905–913. doi: 10.1128/mcb.7.2.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemoto W., Jarvis-Morar M., Brugge J. S., Bolen J. B., Israel M. A. Tyrosine phosphorylation within the amino-terminal domain of pp60c-src molecules associated with polyoma virus middle-sized tumor antigen. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4568–4572. doi: 10.1073/pnas.82.14.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]