Abstract
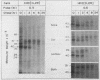
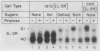
The synthesis and intracellular sorting of the interleukin-2 (IL-2) receptor were studied with a line of mutant Chinese hamster ovary (CHO) cells with a reversible defect in protein O glycosylation. Under normal culture conditions the mutant ldlD cannot add N-acetylgalactosamine (Ga1NAc) to proteins. Ga1NAc is the first sugar of mucin-type O-linked oligosaccharides attached to protein. This O-glycosylation defect is rapidly corrected when Ga1NAc is added to the culture mediu. An expression vector for the p55 human IL-2 receptor was transfected into wild-type CHO and ldlD cells and the structure, stability, and cell surface expression of the receptor were examined by immunoprecipitation and antibody-binding assays. Essentially all of the mature form of the normally glycosylated IL-2 receptor in both wild-type CHO cells and ldlD cells incubated with Ga1NAc was expressed on the cell surface. The stability of O-linked carbohydrate-deficient (Od) IL-2 receptors (in ldlD cells without Ga1NAc) was normal; however, missorting of the Od receptors resulted in very little cell surface expression. The sialidase sensitivity and endoglycosidase H resistance of mature Od IL-2 receptors suggest that Od receptor missorting occurred in or beyond the trans Golgi apparatus. The abnormal sorting of the Od IL-2 receptor is compared with the O-glycosylation dependence of the surface expression and stability of the low-density lipoprotein receptor, decay-accelerating factor, and the major antigen envelope protein of Epstein-Barr virus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Cosman D., Cerretti D. P., Larsen A., Park L., March C., Dower S., Gillis S., Urdal D. Cloning, sequence and expression of human interleukin-2 receptor. Nature. 1984 Dec 20;312(5996):768–771. doi: 10.1038/312768a0. [DOI] [PubMed] [Google Scholar]
- Cosman D., Wignall J., Lewis A., Alpert A., Cerretti D. P., Park L., Dower S. K., Gillis S., Urdal D. L. High level stable expression of human interleukin-2 receptors in mouse cells generates only low affinity interleukin-2 binding sites. Mol Immunol. 1986 Sep;23(9):935–941. doi: 10.1016/0161-5890(86)90123-9. [DOI] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S., Schneider W. J., Hobgood K. K., Tolleshaug H., Brown M. S., Goldstein J. L. Biosynthesis of N- and O-linked oligosaccharides of the low density lipoprotein receptor. J Biol Chem. 1983 Dec 25;258(24):15261–15273. [PubMed] [Google Scholar]
- Davis C. G., Elhammer A., Russell D. W., Schneider W. J., Kornfeld S., Brown M. S., Goldstein J. L. Deletion of clustered O-linked carbohydrates does not impair function of low density lipoprotein receptor in transfected fibroblasts. J Biol Chem. 1986 Feb 25;261(6):2828–2838. [PubMed] [Google Scholar]
- Dower S. K., Hefeneider S. H., Alpert A. R., Urdal D. L. Quantitative measurement of human interleukin 2 receptor levels with intact and detergent-solubilized human T-cells. Mol Immunol. 1985 Aug;22(8):937–947. doi: 10.1016/0161-5890(85)90080-x. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Brands R., Rothman J. E. Attachment of terminal N-acetylglucosamine to asparagine-linked oligosaccharides occurs in central cisternae of the Golgi stack. Cell. 1985 Feb;40(2):463–472. doi: 10.1016/0092-8674(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Duprez V., Dautry-Varsat A. Receptor-mediated endocytosis of interleukin 2 in a human tumor T cell line. Degradation of interleukin 2 and evidence for the absence of recycling of interleukin receptors. J Biol Chem. 1986 Nov 25;261(33):15450–15454. [PubMed] [Google Scholar]
- Gallis B., Lewis A., Wignall J., Alpert A., Mochizuki D. Y., Cosman D., Hopp T., Urdal D. Phosphorylation of the human interleukin-2 receptor and a synthetic peptide identical to its C-terminal, cytoplasmic domain. J Biol Chem. 1986 Apr 15;261(11):5075–5080. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greene W. C., Robb R. J., Svetlik P. B., Rusk C. M., Depper J. M., Leonard W. J. Stable expression of cDNA encoding the human interleukin 2 receptor in eukaryotic cells. J Exp Med. 1985 Jul 1;162(1):363–368. doi: 10.1084/jem.162.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Minamoto S., Uchiyama T., Hardy R. R., Yamada G., Taniguchi T. Reconstitution of functional receptor for human interleukin-2 in mouse cells. Nature. 1985 Dec 5;318(6045):467–470. doi: 10.1038/318467a0. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Hobbie L., Krieger M. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell. 1986 Mar 14;44(5):749–759. doi: 10.1016/0092-8674(86)90841-x. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Segal M., Krieger M. Three types of low density lipoprotein receptor-deficient mutant have pleiotropic defects in the synthesis of N-linked, O-linked, and lipid-linked carbohydrate chains. J Cell Biol. 1986 May;102(5):1576–1585. doi: 10.1083/jcb.102.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Shimizu A., Saito Y., Kinoshita M., Honjo T. Molecular basis for two different affinity states of the interleukin 2 receptor: affinity conversion model. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9026–9029. doi: 10.1073/pnas.83.23.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky K. F., Brush H. A., Krieger M. Unusual forms of low density lipoprotein receptors in hamster cell mutants with defects in the receptor structural gene. J Cell Biol. 1986 May;102(5):1567–1575. doi: 10.1083/jcb.102.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky K., Kingsley D., Krieger M. Use of a mutant cell line to study the kinetics and function of O-linked glycosylation of low density lipoprotein receptors. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4335–4339. doi: 10.1073/pnas.85.12.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M. Complementation of mutations in the LDL pathway of receptor-mediated endocytosis by cocultivation of LDL receptor-defective hamster cell mutants. Cell. 1983 Jun;33(2):413–422. doi: 10.1016/0092-8674(83)90423-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Krönke M., Robb R. J., Waldmann T. A., Greene W. C. The human receptor for T-cell growth factor. Evidence for variable post-translational processing, phosphorylation, sulfation, and the ability of precursor forms of the receptor to bind T-cell growth factor. J Biol Chem. 1985 Feb 10;260(3):1872–1880. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Robb R. J., Waldmann T. A., Greene W. C. Characterization of the human receptor for T-cell growth factor. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6957–6961. doi: 10.1073/pnas.80.22.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk M. M., Krieger M., Corless C. L., Boime I. Effects of preventing O-glycosylation on the secretion of human chorionic gonadotropin in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6354–6358. doi: 10.1073/pnas.84.18.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T., Yachie A., Uwadana N., Ohzeki S., Nagaoki T., Taniguchi N. Functional significance of Tac antigen expressed on activated human T lymphocytes: Tac antigen interacts with T cell growth factor in cellular proliferation. J Immunol. 1982 Dec;129(6):2474–2478. [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Robb R. J. Conversion of low-affinity interleukin 2 receptors to a high-affinity state following fusion of cell membranes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3992–3996. doi: 10.1073/pnas.83.11.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Rusk C. M., Yodoi J., Greene W. C. Interleukin 2 binding molecule distinct from the Tac protein: analysis of its role in formation of high-affinity receptors. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2002–2006. doi: 10.1073/pnas.84.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W., Schneider W. J., Yamamoto T., Luskey K. L., Brown M. S., Goldstein J. L. Domain map of the LDL receptor: sequence homology with the epidermal growth factor precursor. Cell. 1984 Jun;37(2):577–585. doi: 10.1016/0092-8674(84)90388-x. [DOI] [PubMed] [Google Scholar]
- Sege R. D., Kozarsky K., Nelson D. L., Krieger M. Expression and regulation of human low-density lipoprotein receptors in Chinese hamster ovary cells. Nature. 1984 Feb 23;307(5953):742–745. doi: 10.1038/307742a0. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Trowbridge I. S. Induction of expression and phosphorylation of the human interleukin 2 receptor by a phorbol diester. J Biol Chem. 1984 Oct 10;259(19):11706–11712. [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Contribution of a p75 interleukin 2 binding peptide to a high-affinity interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4215–4218. doi: 10.1073/pnas.84.12.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdal D. L., March C. J., Gillis S., Larsen A., Dower S. K. Purification and chemical characterization of the receptor for interleukin 2 from activated human T lymphocytes and from a human T-cell lymphoma cell line. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6481–6485. doi: 10.1073/pnas.81.20.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986 May 9;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Harford J. B., Svetlik P. B., Leonard W. L., Depper J. M., Waldmann T. A., Greene W. C., Klausner R. D. Only high-affinity receptors for interleukin 2 mediate internalization of ligand. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1463–1466. doi: 10.1073/pnas.83.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Bishop R. W., Brown M. S., Goldstein J. L., Russell D. W. Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science. 1986 Jun 6;232(4755):1230–1237. doi: 10.1126/science.3010466. [DOI] [PMC free article] [PubMed] [Google Scholar]