Abstract
Objectives:
Femoral neck fractures in older females resulting from decreased bone mineral density (BMD; osteopenia) are associated with increased morbidity and mortality. Bone mineralization inhibition is probably controlled by proteins which also foster vascular calcification. Therefore, we evaluated the relationship between calcified carotid artery plaque (CCAP) on panoramic images and BMD on dual energy X-ray absorptiometry (DXA) bone scans.
Methods:
Images and hospital records identified by dentists defined two study groups (20 white females and 24 black females) having CCAP and an incidentally obtained bone scan. Ethnically matched (age±7 years, body mass index ±3 units) control groups with panoramic images devoid of CCAP and accompanying DXA scan were likewise constituted. A physician determined the BMD on the DXA.
Results:
Females with CCAP had significantly (p = 0.03) poorer BMD at the femoral neck than those without CCAP. Although mean femoral neck BMD was significantly lower (p = 0.009) for white than for black females, there was no significant interaction between race and CCAP (p = 0.80).
Conclusion:
We observed a significant inverse association between the CCAP on panoramic images and femoral neck BMD in post-menopausal white females.
Keywords: atherosclerosis, panoramic images, osteopenia, fractures
Introduction
Hard artery–soft bone: a link?
Osteopenia is a skeletal disorder characterized by microarchitectural deterioration of bone which predisposes to fragility and increased risk of low-trauma fracture. A more severe form of low bone mass is termed osteoporosis. These maladies are most commonly seen in post-menopausal females and are usually diagnosed by dual energy X-ray absorptiometry (DXA) of bone mineral density (BMD) in the bilateral femoral necks, lumbar spine and hip (Figure 1). Severity of illness is quantified by comparing the number of standard deviations (SDs) the patient's BMD is below (–) the mean value for the reference population of young adult females. A T-score between −1 and −2.5 indicates osteopenia, and a T-score below −2.5 indicates osteoporosis.1 The majority of hip fractures in the USA sustained by white females (140 per 100 000) occur in those with osteopenia rather than osteoporosis because of the larger number of persons with that disorder.2 The prevalence of osteopenia and hip fracture is significantly less (50 per 100 000) in black females because of differences in the rate of bone loss after menopause, bone size, body size and hip geometry.3 Approximately one in four individuals with hip fractures require nursing-home care and within 1 year of the incident approximately 20% of them die.4 Low BMD is often underdiagnosed until fragility fractures occur because pre-clinical symptoms remain ill defined.
Figure 1.
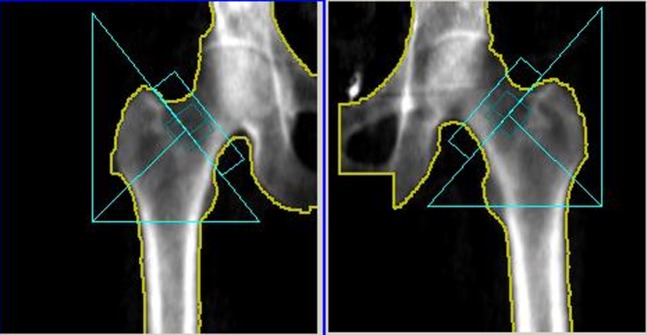
A dual energy X-ray absorptiometry bone mineral density scan of a 69-year-old black female with osteopenia (T-score −1.20) of the femoral neck
This “detection gap” has spurred clinicians to evaluate diagnostic tests used for other purposes in order to determine which of them might also be used to identify “at-risk” females with low BMD who would benefit from preventive education and possibly early therapeutic intervention. This construct is exemplified by an ultrasound study among an ethnically diverse population of older females which demonstrated that the greater the content of calcium in carotid artery plaques, the more severe was low BMD.5 Even more germane is another study which demonstrated that the risk of femoral neck fractures is significantly higher among white females with ultrasound-demonstrated calcified carotid artery plaque (CCAP) than among those without.6 Of significance, panoramic images obtained from large populations of females attending dental school clinics reveal that approximately 5% of those over the age of 50 years (ethnic distribution not defined) show evidence of CCAP (Figure 2).7,8 Confirmation of the presence of such lesions by ultrasound demonstrates that panoramic imaging is highly accurate with an 80% sensitivity, 81% specificity and 81% accurancy.9,10
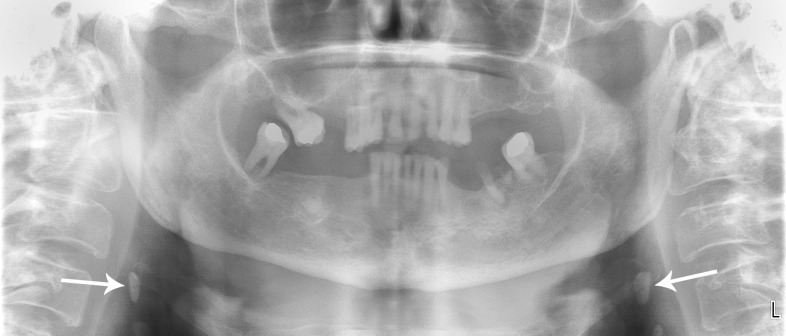
Figure 2.
A panoramic image of the maxillofacial complex (same patient as Figure 1) which has been digitally enhanced with the manufacturer's provided software and evidences bilateral calcified carotid artery plaques (arrows). Note the globular opacities that overlie the greater horns of the hyoid bone
The purpose of this study was to investigate whether CCAP on panoramic images of older white and black females is related to low BMD on DXA. We hypothesized that white females and possibly black females with CCAP on the panoramic images would have greater evidence of osteopenia on their DXA scan than females free of CCAP on their images.
Materials and methods
To address this research question, the investigators designed and implemented a retrospective study. Institutional review board approval was obtained from the VA Greater Los Angeles Healthcare System and the need for consent waived given the retrospective nature of the project. The Medical Center's panoramic and DXA digital libraries and electronic medical records were accessed and the studies of all female patients aged 50 years or older who had a panoramic image and a bone density study within 24 months of each other during the time frame 1 July 2007 to 30 June 2011 were reviewed. The panoramic images had been obtained with a Planmeca panorex unit and Dimaxis Pro 4.1.4 image transfer system (Planmeca OY, Helsinki, Finland) and the DXA scans with a Lunar Prodigy DXA dual energy X-ray absorptiometry bone densitometer (General Electric Healthcare, Waukesha, WI).
To be included in the study group patients had to have a panoramic image which evidenced CCAP as jointly determined by two dentists (AHF and TIC) certified by the American Board of Oral and Maxillofacial Surgery using the American Academy of Oral and Maxillofacial Radiology sponsored training packet for identification of carotid artery calcifications on panoramic radiographs.11 Consistent with these guidelines, heterogeneous radio-opacities in a vertical-linear orientation adjacent to or inferior to the hyoid bone, epiglottis and cervical vertebrae at, above or below the intervertebral space C3–C4 were diagnosed as CCAP after ruling out confounding radio-opacities that lie in close proximity to the vessel such as a calcified triticeous cartilage or superior cornu of calcified cartilage. Patients also had to have a bone densitometry study obtained within 24 months of the panoramic image. Excluded from the study were Hispanic and Asian patients given their paucity in the patient pool, and individuals whose medical record identified medications and systemic illnesses known to cause secondary bone loss: (1) oral/intramuscular administration of corticosteroids for more than 3 months, (2) long-term use of anticonvulsants (e.g. phenytoin), (3) use of oral anticoagulant (e.g. warfarin) medications known to antagonize vitamin K, (4) Type I diabetes mellitus, (5) total gastrectomy resulting in malabsorption syndrome, (6) chronic renal disease, (7) multiple myeloma, (8) rheumatoid arthritis, (9) hyperthyroidism, (10) hyperparathyroidism, (11) Cushing's syndrome. This cadre of individuals was then subdivided into white females (Group 1) and black females (Group 2). Members of the “control groups” were selected from the image pool of patients with a properly executed panoramic image which viewed the area of interest: 2.5 cm inferior and 2.5 cm posterior to the cortical rim of the mid-point of the mandibular angle but which was devoid of CCAP and matched to a member of the ethnically appropriate study group if they (1) were of similar age (±7 years), (2) had approximate body mass index (BMI) (±3 units) and (3) had a contemporaneously obtained DXA scan. Exclusion criteria were identical to those imposed upon membership in the study group.
Demographic variables collected included age and ethnicity (white/black). Atherogenic risk factors, including BMI, hypertension, dyslipidaemia, diabetes mellitus, and non-use of hormone replacement therapy, were also collected. Both the demographic data and the presence of comorbid illnesses (as determined by the list of physician-prescribed medications) were based on information garnered from the subjects' medical records.
The primary outcomes were derived from the bone density studies of the bilateral femoral necks, lumbar spine (L1–L4) and total hip. These scans were acquired and analysed using a bone densitometer as recommended by the US Preventive Services Task Force.1 The nuclear medicine physician (GRB) certified by the American Board of Nuclear Medicine assessed each study for the presence of osteoporosis/osteopenia. This individual had no previous knowledge of either the presence of CCAP on the panoramic images or the patients' medical histories.
Data analysis
Data were recorded, deidentified, entered into a standardized electronic database, and imported into PASW Statistics 18, release 18.0.02009 (IBM Corporation, New York, NY), and SAS v. 9.1 (SAS Institute Inc., Cary, NC). Descriptive statistics were run to include measures of central tendency and dispersion for age and BMI, and frequencies for the categorical atherosclerotic risk-factor variables. Cross-tabulations and Student's t-test were run to assess the comparability of the CCAP+/CCAP– groups for age and atherogenic risk factors. Analysis of variance (ethnicity × CCAP±) with post hoc comparisons (Tukey honestly significant difference) was used to evaluate the relationships between ethnicity and CCAP with BMD of the femoral neck. The α-value for each statistical comparison was 0.05.
Results
The medical records librarian identified all females who had both a panoramic image and a DXA study performed during the prescribed period of time. Approximately 7% of the satisfactory (proper patient positioning, etc.) panoramic images demonstrated CCAP (Table 1). The demographic data and medical histories evidenced by each ethnic group and their controls (CCAP–) did not reveal any statistically significant differences, although large percentages were taking hormone replacement therapy and medications to control hypertension, Type II diabetes mellitus and dyslipidaemia.
Table 1.
Patient demographics and comorbid medical diagnoses
| Variables | White females CCAP+ (n = 20) | White females CCAP– (n = 20) | p-value | Black females CCAP+ (n = 24) | Black females CCAP–(n = 24) | p-value |
| Age in years mean ±SD (range) | 66.8 ± 11.0 (51–85) | 66.5 ± 11.5 (53–90) | 0.7 | 59.5 ± 7.4 (51–80) | 59.8 ± 7.0 (53–82) | 0.64 |
| Body mass index mean ±SD (range) | 28 ± 5.4 (19–37) | 28.5 ± 6.4 (17–39) | 0.2 | 29.9 ± 6.2 (19–45) | 30.5 ± 6.8 (21–49) | 0.96 |
| Hypertension | 16 (80%) | 18 (91%) | 0.7 | 19 (79%) | 20 (83%) | 1.0 |
| Diabetes mellitus | 8 (40%) | 5 (25%) | 0.5 | 8 (33%) | 10 (41%) | 0.8 |
| Dyslipidaemia | 12 (60%) | 17 (85%) | 0.2 | 18 (75%) | 17 (71%) | 1.0 |
| Hormone replacement therapy prescribed within past 10 years | 9 (45%) | 8 (40%) | 1.0 | 10 (41%) | 7 (29%) | 0.5 |
CCAP, calcified carotid artery plaque; SD, standard deviation.
The T-score for the femoral neck (Table 2) was significantly lower for white females as a group, irrespective of the presence of CCAP status, than for black females. Furthermore, the mean T-score for the femoral neck in females with CCAP was significantly worse than females without CCAP, irrespective of ethnicity. There was no significant interaction between ethnicity on mean femoral neck T-scores.
Table 2.
Comparison of femoral neck mean T-scores between race and calcified carotid artery plaque (CCAP) status
| Race |
|||||
| CCAP status |
Black |
White |
Total |
||
| Mean | SD | Mean | SD | Mean | |
| CCAP+ | −0.45 | 1.16 | −1.19 | 1.23 | −0.79 |
| CCAP− | 0.05 | 1.06 | −0.57 | 1.16 | −0.23 |
| Total | −0.20 | 1.11 | −0.88 | 1.27 | |
ANOVA, analysis of variance; SD, standard deviation.
Main effect for race: ANOVA F = 7.18; p = 0.009.
Main effect for CCAP: ANOVA F = 4.94; p = 0.029.
Interaction race × CCAP: ANOVA F = 0.07; p = 0.789.
Comparisons of the L1–L4 mean T-scores (Table 3) did not reveal any statistical difference between ethnic and CCAP groups, although they were lower for white females than for black females and for the CCAP+ group than the CCAP– group. The same trend was seen for the total-hip mean T-scores (Table 4). Although the difference between CCAP groups was not statistically significant, the mean T-score for white females was significantly lower than for black females.
Table 3.
Comparison of L1–L4 mean T-scores between race and calcified carotid artery plaque (CCAP) status
| Race |
||||||
| CCAP status |
Black |
White |
Total |
|||
| Mean | SD | Mean | SD | Mean | SD | |
| CCAP+ | 0.05 | 1.53 | 0.02 | 1.90 | 0.03 | 1.69 |
| CCAP− | 0.60 | 1.57 | −0.05 | 1.60 | 0.30 | 1.60 |
| Total | 0.32 | 1.56 | −0.02 | 1.69 | ||
ANOVA, analysis of variance; SD, standard deviation.
Main effect for race: ANOVA F = 0.92; p = 0.341.
Main effect for CCAP: ANOVA F = 0.47; p = 0.493.
Interaction: ANOVA F = 0.74; p = 0.393.
Table 4.
Comparison of total-hip mean T-scores between race and calcified carotid artery plaque (CCAP) status
| Race |
||||||
| CCAP status |
Black |
White |
Total |
|||
| Mean | SD | Mean | SD | Mean | SD | |
| CCAP+ | 0.16 | 1.13 | −0.53 | 1.38 | −0.15 | 1.28 |
| CCAP− | 0.37 | 1.49 | −0.22 | 1.34 | −0.10 | 1.43 |
| Total | 0.28 | 1.32 | −0.41 | 1.34 | ||
ANOVA, analysis of variance; SD, standard deviation.
Main effect for race: ANOVA F = 4.92; p = 0.029.
Main effect for CCAP: ANOVA F = 0.84; p = 0.363.
Interaction: ANOVA F = 0.03; p = 0.853.
Of note, when the study participants were separated by ethnicity (Table 5), white CCAP+ females had significantly poorer bone quality than white CCAP– females. This finding did not hold true for black females, nor did an analysis of L1–L4 and total-hip T-scores among the ethnically matched study and control groups reveal any statistically significant differences.
Table 5.
Comparison of differences in T-scores of females with calcified carotid artery plaque (CCAP) with their age, ethnicity and body mass index matched control group
| T-scores | White females CCAP+ (n = 20) | White females CCAP– (n = 20) | t-test p-value | Black females CCAP+ (n = 24) | Black females CCAP– (n = 24) | t-test p-value |
| Femoral neck mean T-score | −1.19 | −0.57 | 0.05* | −0.45 | 0.05 | 0.1 |
| L1–L4 Mean T-score | 0.02 | −0.05 | 0.89 | 0.05 | 0.60 | 0.24 |
| Total-hip mean T-score | −0.53 | −0.22 | 0.30 | 0.16 | 0.37 | 0.57 |
A T-score of ≥−1.00 = normal; −1.01 to −2.49 = osteopenia; ≤−2.50 = osteoporosis
Discussion
The results of our study demonstrate that a biracial cohort of older females with CCAP on their panoramic images are significantly more likely to demonstrate lower femoral neck BMD on their accompanying DXA scans than are females free of atheromas after adjustment for age and cardiovascular risk factors. This finding is more apparent in white females than it is in black females. Our results are consistent with those of other researchers who in controlled studies utilized a variety of different imaging modalities to demonstrate a link between calcified atherosclerotic lesions in not only the carotid but also the aorta and coronary vessels with low BMD in post-menopausal females.12,13
BMD is frequently adequate in post-menopausal black females; however, the relationship between CCAP+ panoramic images and occult osteopenia permits the identification of a small number of individuals with occult disease and possibly at a uniquely high risk of adverse outcomes associated with fragility fractures of the femoral neck.3 black females, compared with white females, are more likely to die and have longer hospital stays and are less likely to be ambulatory at discharge.14 The factors contributing to these differences are not completely understood but may reflect the fact that black females are older at the time of fracture, have greater numbers of comorbid medical illnesses or suffer greater disparities in healthcare.15
There have been two prior studies which investigated the relationship between CCAP on panoramic images and low BMD. In a study of 50 Brazilian females (ethnicity not disclosed) with DXA-documented osteopenia/osteoporosis in at least two of three sites (wrist, vertebrae, femoral head) panoramic images were obtained.16 4 (8%) of these patients evidenced CCAP on their panoramic image. Of note, this project evaluated a much younger cohort of females than in our study, with almost 25% of the participants being under 50 years. A group of maxillofacial radiologists in Boston noted that 95% of individuals (n = 24; 18 males, 6 females; with neither age nor ethnicity disclosed) having CCAP on their panoramic image also demonstrated osteoporosis as defined by defects in endosteal margin and width of the mandibular inferior cortex in the region of the mental foramen and posterior body.17
The biological linkages between atherosclerosis and low bone mass remain speculative. However, it is known that the calcific deposits in the arterial wall can appear indistinguishable from bone with trabeculae and lacunae visible given that both contain the mineral hydroxyapatite. In addition, these vascular deposits often express several bone matrix proteins such as Gla protein, bone morphogenic protein-2, osteoprotegerin [receptor activator of nuclear factor kappa-B/receptor activator of nuclear factor kappa-B ligand (RANK/RANKL) system], osteocalcin and collagen.18 Other studies have implicated oestrogen and vitamin K deficiencies and vitamin D excess as possible metabolic linkages between vascular calcification and osteoporosis.19 Furthermore, lipid oxidization products (such as minimally oxidized low-density lipoprotein cholesterol) are known to promote calcification of vascular smooth muscle cells, inhibit differentiation of osteoblasts, induce differentiation of osteoclasts, whereas the pro-inflammatory cytokines interleukin-6 and tumour necrosis factor-α have been shown to exert proatherogenic effects on the vascular wall and promote osteoclastogenesis and bone resorption.20,21
Given the results of this study, we would suggest that dentists encountering females with an atheroma on their panoramic image refer the patient to their primary care physician for a BMD scan and an evaluation of their clinical risk factors [age, personal history of prior adult low trauma fractures, history of parental hip fracture, current smoking, oral glucocorticoid use (daily dose equivalent 5 mg or more prednisone) for more than 3 months, low body weight (under 57.6 kg) and three or more units of alcohol daily (one unit is 285 ml of beer, 120 ml of wine, 30 ml distilled)] as well as their cardiovascular status.22 For it must be noted that atheromas demonstrated on panoramic images and carotid artery ultrasound studies are associated with future myocardial infarction and stroke.23,24 Furthermore, epidemiological data indicate that females with low BMD are at increased risk of morbidity and mortality from coronary and cerebrovascular disease.25,26
The most significant strength of this study was the demonstration that panoramic images with CCAP identified a cohort of ethnically diverse females under 65 years with osteopenia of the femoral neck and thus at risk of a fragility fracture. This is uniquely relevant because current health care guidelines (National Osteoporosis Foundation,27 US Preventive Services Task Force28 and International Society for clinical Densitometry29) do not suggest measuring BMD until age 65 years. Furthermore, it is likely that approximately 10% of the females with moderate osteopenia (T-score, −1.50 to −1.99) will in less than 5 years transition to osteoporosis and be at even greater risk of hip fracture.30
The study's most significant weaknesses were our inability to ascertain from each participant's medical record the presence of other osteopenic risk factors (e.g. smoking status, daily alcohol use) and a history of having received antiresorptive medications (e.g. bisphosphonates, parathyroid hormone, raloxifene, calcitonin) from non-Veterans Affairs clinicians. Furthermore, ultrasound studies were not used to confirm the presence of CCAP on the panoramic images because of resource limitations. Thus, some calcifications other than those caused by carotid atheroma may have been included. This caveat should be kept in mind when considering the results. Currently, another project is being planned, which, if approved, will provide the necessary financial support to immediately obtain the corroborating ultrasound studies.
Conclusion
This study demonstrated that CCAP on the panoramic images of older females is a surrogate marker for low BMD. This is a significant finding because prior research efforts have validated that individuals with osteopenia and FRAX-documented criteria are at high risk for low-trauma fractures with a related increased risk of all-cause mortality and that treating screening-detected osteopenia reduces fracture risk.1 We suggest that the dental profession participate in the medical communities effort to close the “detection gap” and redress the ethnic disparities involved in identifying females with occult low BMD and concomitant atherosclerosis. This effort is uniquely important given that the proportion of females who have had BMD measurement is only 30% in white females and 15% in black females.31
References
- 1.US Preventive Services Task Force. Screening for osteoporosis: US Preventive Services Task Force recommendation statement. Ann Intern Med 2011; 154: 356–364 [DOI] [PubMed] [Google Scholar]
- 2.Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 2011; 286: 2815–2822 [DOI] [PubMed] [Google Scholar]
- 3.Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Rel Res 2011; 469: 1891–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leibson CL, Tosteson AN, Gabriel SE, Ransom JE, Melton LJ. Mortaility, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc 2002; 50: 1644–1650 [DOI] [PubMed] [Google Scholar]
- 5.Hyder JA, Allison MA, Barrett-Connor E, Detrano R, Wong ND, Sirlin C, et al. Bone mineral density and atherosclerosis: the multi-ethnic study of atherosclerosis, abdominal aortic calcium study. Atherosclerosis 2010; 209: 283–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jorgensen L, Jaakimsen O, Mathiesen EB, Ahmed L, Berntsen GK, Fonnebo V, et al. Carotid plaque echogenicity and risk of nonvertebral fractures in women: a longitudinal population-based study. Calcif Tissue Int 2006; 79: 207–213 [DOI] [PubMed] [Google Scholar]
- 7.Carter LC, Haller AD, Nadarajah V, Calamel AD, Aguirre A. Use of panoramic radiographs among ambulatory dental population to dental patients at risk of stroke. J Am Dent Assoc 1997; 128: 977–984 [DOI] [PubMed] [Google Scholar]
- 8.Lewis DA, Brooks SL. Carotid artery calcification in a general dental population: a retrospective study of panoramic radiographs. Gen Dent 1999; 47: 98–103 [PubMed] [Google Scholar]
- 9.Romano-Sousa CM, Krejci L, Medeiros FM, Graciosa-Filho RG, Martins MF, Guedes VN. Diagnostic agreement between panoramic radiographs and color Doppler images of carotid atheroma. J Appl Oral Sci 2009; 171: 45–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ertas ET, Sisman Y. Detection of incidental carotid artery calcifications during dental examination: panoramic radiography is an important aid in dentistry. Oral Surg Oral Med Oral Pathol Oral Radiol Endodon 2011; 112: e11–e17 [DOI] [PubMed] [Google Scholar]
- 11.Almog DM, Tsimidis K, Moss ME, Gottlieb RH, Carte LC. Evaluation of a teaching program for detection of carotid artery calcifications on panoramic radiographs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 90: 111–117 [DOI] [PubMed] [Google Scholar]
- 12.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab 2004; 89: 4246–4253 [DOI] [PubMed] [Google Scholar]
- 13.Choi SH, An JH, Lim S, Koo BK, Park SE, Chang HJ, et al. Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre-and postmenopausal women. Clin Endocrinol (Oxf) 2009; 71: 644–651 [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Race and sex differences in mortality following fracture of the hip. Am J Public Health 1992; 82: 1147–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furstenberg Al, Mezey MD. Differences in outcome between black and White elderly hip fracture patients. J Chronic Dis 1987; 40: 931–938 [DOI] [PubMed] [Google Scholar]
- 16.Watanabe PC, Dias FC, Issa JP, Monteiro SA, de Paula FJ, Tiossi R. Elongated styloid process and atheroma in panoramic radiography and its relationship with systemic osteoporosis and osteopenia. Osteoporosis Int 2010; 21: 831–836 [DOI] [PubMed] [Google Scholar]
- 17.Pabla T, Ramesh A. Prevalence of osteoporetic changes in panoramic radiographs of patients showing carotid calcifications. J Oral Health Comm Dent 2007; 1: 49–51 [Google Scholar]
- 18.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 2004; 24: 1161–1170 [DOI] [PubMed] [Google Scholar]
- 19.Watson K, Abrolat M, Lonzetta L, Hoeg J, Doherty T, Detrano R. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation 1997; 96: 1755–1760 [DOI] [PubMed] [Google Scholar]
- 20.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A Possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol 1997; 17: 680–687 [DOI] [PubMed] [Google Scholar]
- 21.Tintut Y, Parhami F, Tsingotjidou A, Tetradis S, Territo M, Demer LL. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway. J Biol Chem 2002; 277: 14221–14226 [DOI] [PubMed] [Google Scholar]
- 22.Siris ES, Baim S, Nattiv A. Primary care use of FRAX: absolute fracture risk assessment in post menopausal women and older men. Post Grad Med 2010; 122: 82–89 [DOI] [PubMed] [Google Scholar]
- 23.Friedlander AH, Cohen SN. Panoramic radiographic atheromas portend adverse vascular events. Oral Surg Med Oral Pathol Oral Radiol Endod 2007; 103: 830–835 [DOI] [PubMed] [Google Scholar]
- 24.Prabhakaran S, Singh R, Zhou X, Ramas R, Sacco RL, Rundek T. Presence of calcified carotid plaque predicts vascular events: the Northern Manhattan Study. Atherosclerosis 2007; 195: e197–e201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kado DM, Browner WS, Blackwell T, Gore R, Cummings SR. Rate of bone loss is associated with mortality in older women: a prospective study. J Bone Miner Res 2000; 15: 1974–1980 [DOI] [PubMed] [Google Scholar]
- 26.Varma R, Aronow WS, Basis Y, Singh T, Kalapatapu K, Weiss MB, et al. Relation of bone mineral density to frequency of coronary artery diseases. Am J Cardio 2008; 101: 1103–1104 [DOI] [PubMed] [Google Scholar]
- 27.National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. [cited 14 March 2010]. Available from: http://www.nof.org/professionals/N0F_clinicians_guide.pdf. [Google Scholar]
- 28.Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for postmenopausal osteoporosis: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002; 137: 529–541 [DOI] [PubMed] [Google Scholar]
- 29.Leib ES, Lewiecki EM, Binkley N, Hamdy RC; International Society for Clinical Densitometry Official positions of the International Society for Clinical Densitometry. J Clin Densitom 2004; 7: 1–6 [DOI] [PubMed] [Google Scholar]
- 30.Gourlay ML, Fine JP, Preisser JS, May RC, Chenxi L, Li-Yung L, et al. Bone-testing interval and transition to osteoporosis in older women. N Engl J Med 2012; 366: 225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curtis JR, Carbone L, Cheng H, Hayes B, Laster A, Matthews R, et al. Longitudinal trends in use of bone mass measurement among older Americans, 1999–2005. J Bone Miner Res 2008; 23: 1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]