Abstract
Objective:
To describe CT findings of non-tuberculous mycobacteria (NTM) pulmonary infection in non-AIDS immunocompromised patients (ICPs) and to compare these findings with those in immunocompetent patients.
Methods:
From July 2000 to August 2007, 369 patients (mean age 58.3 years; 169 males and 200 females) with pulmonary NTM infection were retrospectively reviewed. Of these 369 patients, 24 ICPs (mean age 64.8 years; 15 males and 9 females) were identified. 16 patients had diabetes mellitus, and 6 patients had received long-term steroid therapy. One had received solid organ transplantation and one had received high-dose chemotherapy for haematological disease. 24 age- and sex-matched immunocompetent patients (mean age 64.6 years; 15 males and 9 females) were selected as the control group from the same registry. CT images were reviewed in consensus by three chest radiologists, who were blinded to immune status. Each lung lobe was evaluated in terms of extent of the lesion, bronchiectasis, parenchymal opacity and the presence of ancillary findings.
Results:
A total of 287 lobes were evaluated in ICPs and the control group. The ICPs showed a higher prevalence of ill-defined nodules, with cavities and large opacity >2 cm with/without cavity (p=0.03, 0.04 and 0.02, respectively). Regardless of the immune status, the most common CT findings were bronchiectasis and ill-defined nodules without cavity.
Conclusion:
The most common CT findings of pulmonary NTM infection in ICPs were bronchiectasis and ill-defined nodules, similar to those in the control group. Ill-defined nodules with cavity and large opacity >2 cm with/without cavity were more frequently found in ICPs.
Advances in knowledge:
In patients affected by NTM infection, large opacities and cavitation in pulmonary nodules are more frequent in ICPs than in immunocompetent patients.
The non-tuberculous mycobacteria (NTM) species are common environmental organisms [1,2]. As they commonly colonise the human respiratory system, identification of these bacteria in human samples is not always considered a pathogenic condition [3,4]. Since the 1950s, when Timpe, Runyon and others established the potential pathogenicity of NTM, the suspected role of these organisms in the pathogenesis of pulmonary disease has increased [5,6]. As acquired immunodeficiency syndrome (AIDS) has become epidemic, pulmonary and disseminated infection caused by the more common NTM has emerged as one of the frequent complications in patients with AIDS. These organisms were termed Mycobacterium avium complex (MAC) infections [7,8]. Since then, the pulmonary involvement of NTM in AIDS has been more exactly described [9,10]. Pulmonary infection caused by MAC in patients with other illnesses, including chronic obstructive pulmonary disorder, previous pulmonary tuberculosis, bronchiectasis and pneumoconiosis, has been described [11,12]. Subsequently, pulmonary MAC infection in middle-aged females having neither predisposing lung disease nor immunodeficiency has been reported [13–15].
Previous reports describing the radiological findings of NTM pulmonary infection were limited to NTM infection in AIDS patients or in immunocompetent hosts. Despite the increasing incidence and diagnoses of NTM infection in both immunocompromised patients (ICPs) and immunocompetent patients, to the best of our knowledge, there have been no comparisons of the radiological findings in ICPs and immunocompetent patients. In this study, we sought to analyse the CT findings of NTM pulmonary infection in non-AIDS ICPs compared with those in immunocompetent patients (control).
METHODS AND MATERIALS
Subjects
This study was approved by the institutional review board of the Asan Medical Center, Seoul, Republic of Korea. Between July 2000 and August 2007, 369 patients (mean age 58.3 years; 169 males and 200 females) with pulmonary disease caused by NTM were retrospectively reviewed. All patients fulfilled the 2007 American Thoracic Society (ATS) criteria for pulmonary NTM infection [16]. There were no patients with Mycobacterium tuberculosis infection among the 369 patients. Of these patients, 24 ICPs (15 males and 9 females) were identified. The mean age of the patients at the time of diagnosis was 64.8 years (range 34–83 years). We selected ICPs and a control group by medical chart review. The diagnoses of the ICPs included diabetes mellitus (n=16, mean duration 13.1 years), autoimmune disease or lung fibrosis receiving long-term steroid therapy (n=6, mean duration 2.8 years), liver transplantation recipient (n=1) and a patient undergoing high-dose chemotherapy for haematological disease (n=1). The mean dose of steroid therapy was 6.5 mg day−1 (range 5–10 mg). From the same registry, 24 age- and sex-matched immunocompetent patients (mean age 64.6 years; range 35–81 years) were selected as the control group. They had no significant underlying disease including M. tuberculosis. Patients were proved to have NTM pulmonary infection either on the basis of positive culture results from sputum (n=38), from bronchial washing (n=5), from both sputum and bronchial washing (n=3) or on the basis of positive histopathological findings from percutaneous needle biopsy (n=1) and from pneumonectomy (n=1). Microbiological findings were regarded as positive when sputum cultures, bronchoalveolar lavage fluid or transbronchial biopsy specimens were positive for NTM organisms. Histopathological findings were regarded as positive when the biopsy specimen showed granulomatous inflammation and was positive for NTM organisms.
In the ICP group of 24 patients, 8 were found to have M. avium infection, 8 to have M. intracellulare, 5 to have M. abscessus and 3 to have M. kansasii. In the control group of 24 patients, 8 were found to have M. avium, 7 to have M. intracellulare, 7 to have M. abscessus and 2 to have M. kansasii. Micro-organisms detected in both groups were not different from each other and are described along with the other clinical characteristics in Table 1.
Table 1.
Demographic information and clinical characteristics of both groups
| Characteristics | ICPs (n=24) | Controls (n=24) |
| Age, years | 64.8±12.5 | 64.6±12.1 |
| Gender, n (%) | ||
| Male | 15 (62.5) | 15 (62.5) |
| Female | 9 (37.5) | 9 (37.5) |
| Symptoms,a n (%) | ||
| Cough | 7 (29.2) | 11 (45.8) |
| Sputum | 8 (33.3) | 11 (45.8) |
| Dyspnoea | 2 (8.3) | 4 (16.7) |
| Haemoptysis | 2 (8.3) | 2 (8.3) |
| Weight loss | 0 (0) | 3 (12.5) |
| Fever | 5 (20.8) | 1 (4.2) |
| Organisms, n (%) | ||
| Mycobacterium avium | 8 (33.3) | 8 (33.3) |
| M. intracellulare | 8 (33.3) | 7 (29.2) |
| M. abscessus | 5 (20.8) | 7 (29.2) |
| M. kansasii | 3 (12.5) | 2 (8.3) |
ICP, immunocompromised patients.
Symptoms lasting longer than 2 weeks are indicated. Some patients had more than one symptom.
CT scanning
CT scans were obtained using various helical CT scanners with a pitch of 1.5 or 2. The volumetric data were reconstructed with a 5–10-mm section thickness using a soft kernel. The CT data were also reconstructed with a 1-mm section thickness with a 10-mm interval using a sharp kernel. The scanning parameters varied depending on the indications. All scans were obtained 40 s after administration of 100 ml of a 300 mgI per ml intravenous contrast medium at a rate of 2.5 ml s–1.
Analysis of CT findings
A total 187 CT scans were obtained in 48 patients (mean 7.6 CT scans per patient). We chose one CT for each patient, and it was obtained at approximately the first time of organism isolation. The time interval between the first isolation of the NTM bacteria and the CT examinations ranged from 0 to 695 days (mean 91.4 days; median 22 days). There was no significant difference in time interval between the ICPs and the control group (p=0.12).
The CT images were reviewed in consensus by three chest radiologists, one with 15, one with 7 and one with 3 years of clinical experience. These radiologists were blinded to the patients’ immune status. Six lobes were evaluated in each patient, i.e. the right upper lobe, right middle lobe, right lower lobe, superior division of the left upper lobe, lingular division of the left upper lobe and left lower lobe. One right upper lobe of one ICP was excluded from evaluation because of previous lobectomy owing to lung cancer. A total of 287 lobes in the 48 patients were evaluated in terms of extent of the lesion, bronchiectasis, parenchymal opacity and the presence of ancillary findings.
Lesion extent
For each lobe, the extent of disease was graded using a five-point scale: 0, no involvement; 1, involving <25% of the lobe; 2, 25–50%; 3, 50–75%; and 4, involving >75% of the lobe. The score of the total involvement was calculated by totalling the scores of each lobe. The maximum score for each patient was 24.
Bronchiectasis
For bronchiectasis, both severity and extent were evaluated. The severity of bronchiectasis was assessed according to the CT scoring system for cystic fibrosis [17,18]. The severity was defined as Score 1 if the luminal diameter was greater than but did not exceed twice the diameter of the adjacent vessel, Score 2 if the luminal diameter was two to three times the diameter of an adjacent vessel and Score 3 if the luminal diameter exceeded three times the diameter of the adjacent vessel. The grade of the extent of bronchiectasis was the same as the grade of extent of the lesion. The final bronchiectasis score was then calculated by multiplying the severity score by the extent score.
Parenchymal opacity
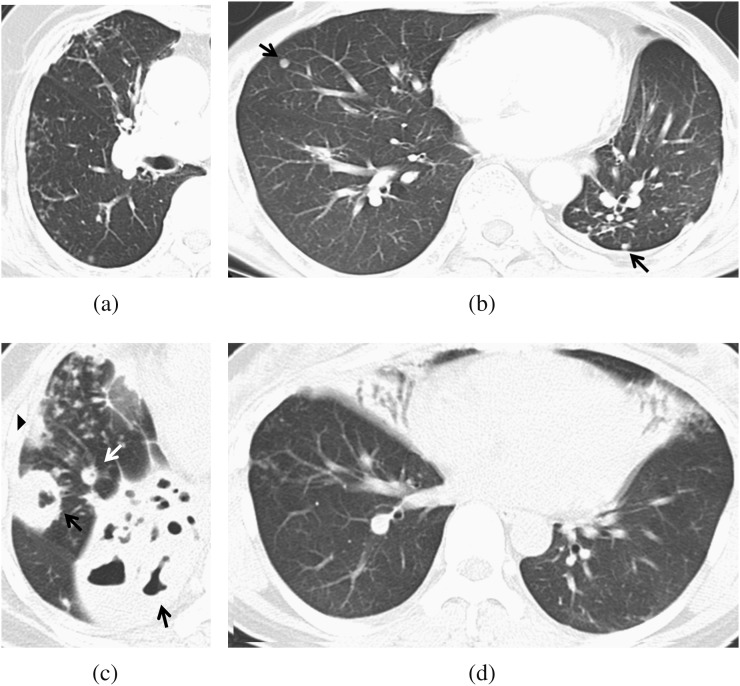
Parenchymal opacity was classified as small opacity (<1 cm) and large opacity (≥1 cm) by 1 cm in diameter. Small opacity was subdivided into ill-defined nodules with or without cavity and well-defined nodules with or without cavity. Nodules showing a branching pattern or a faint margin were regarded as ill-defined nodules. Calcified nodules, nodules with discrete margins and nodules with adjacent fibrosis were regarded as well-defined nodules. Large opacity was subdivided into opacity ≤2 cm in diameter with or without cavity and opacity >2 cm in diameter with or without cavity (Figure 1). The grade of the extent of the parenchymal opacity was the same as the grade of the extent of the lesion.
Figure 1.
Types of parenchymal opacities. (a) A 77-year-old female undergoing long-term steroid therapy; ill-defined nodules. CT scan shows multiple, ill-defined nodules in the right middle lobe and right lower lobe. (b) A 54-year-old male with diabetes mellitus (DM); well-defined nodules. CT scan shows well-defined nodules (black arrows) in the right middle lobe and left lower lobe. (c) A 54-year-old female with DM; large opacities. CT scan shows a large opacity >2 cm (black arrows), a large opacity ≤2 cm (arrowhead) and a well-defined nodule (white arrow) in the right lower lobe. Ill-defined nodules are also seen. (d) A 48-year old female with DM; large opacities. CT scan shows a large opacity >2 cm in the right middle lobe and left lingular segment.
Scoring of bronchiectasis and parenchymal opacity are summarised in Table 2.
Table 2.
Scoring system of bronchiectasis and parenchymal opacity
| Severity of bronchiectasis | |
| 0 | Absent |
| 1 | Mild (luminal diameter slightly greater than adjacent vessel) |
| 2 | Moderate (lumen 2–3 times the diameter of the vessel) |
| 3 | Severe (lumen >3 times diameter of vessel) |
| Extent of bronchiectasis and parenchymal opacity | |
| 0 | Absent |
| 1 | <25% |
| 2 | 25–50% |
| 3 | 50–75% |
| 4 | >75% |
The bronchiectasis score was calculated by multiplying the severity score with the extent score. The parenchymal opacity score was calculated by totalling the extent scores of each lobe.
Ancillary findings
The presence of ancillary findings such as lymphadenopathy, pleural effusion and pleural thickening was evaluated, and the number of lymph nodes was counted.
Statistics
Commercial statistical package SPSS® v. 12.1.1 (SPSS, Chicago, IL) was used. The results were expressed as mean ± standard deviation. The paired t-test was performed to assess the differences in the scores of the extent of the lesion, bronchiectasis and parenchymal opacity between the ICPs and the control group and between those with diabetes and the control group. A p-value <0.05 was considered to indicate statistical significance, and all p-values were two-tailed.
RESULTS
The most common CT findings were the presence of bronchiectasis (ICPs 53%, 76/143 lobes; controls 49%, 71/144 lobes) and ill-defined nodules without cavity (ICPs 49%, 70/143 lobes; controls 51%, 74/144 lobes), which was seen in all patients (Tables 3 and 4). There was no predominance in the zonal distribution of any of the findings in either the ICPs or the control group. In the ICPs, ill-defined nodules with cavity were seen in 15 lobes (10.4%) among 143 evaluated lobes, well-defined nodules without cavity were seen in 28 (19.5%) lobes, well-defined nodules with cavity were seen in 1 lobe, large opacity between 1 and 2 cm in diameter without cavity was seen in 36 (25.1%) lobes, large opacity between 1 and 2 cm in diameter with cavity was seen in 5 (3.4%) lobes, large opacity >2 cm in diameter without cavity was seen in 20 (13.9%) lobes and large opacity >2 cm in diameter with cavity was seen in 25 (17.4%) lobes.
Table 3.
CT findings seen in 143 lobes of 24 immunocompromised patients
| Findings | RUL | RML | RLL | LUL | Lingula | LLL | Total |
| Lesion extent | 17 (23) | 20 (24) | 19 (24) | 21 (24) | 20 (24) | 19 (24) | 116 (143) |
| Bronchiectasis | 11 (23) | 17 (24) | 9 (24) | 15 (24) | 15 (24) | 9 (24) | 76 (143) |
| Small opacity | |||||||
| Ill-defined nodule | |||||||
| Cavity (−) | 8 (23) | 12 (24) | 14 (24) | 9 (24) | 12 (24) | 15 (24) | 70 (143) |
| Cavity (+) | 6 (23) | 1 (24) | 1 (24) | 1 (24) | 2 (24) | 4 (24) | 15 (143) |
| Well-defined nodule | |||||||
| Cavity (−) | 4 (23) | 4 (24) | 4 (24) | 5 (24) | 4 (24) | 7 (24) | 28 (143) |
| Cavity (+) | 1 (23) | 0 (24) | 0 (24) | 0 (24) | 0 (24) | 0 (24) | 1 (143) |
| Large opacity | |||||||
| 1–2 cm | |||||||
| Cavity (−) | 9 (23) | 7 (24) | 5 (24) | 4 (24) | 7 (24) | 4 (24) | 36 (143) |
| Cavity (+) | 2 (23) | 0 (24) | 0 (24) | 2 (24) | 1 (24) | 0 (24) | 5 (143) |
| >2 cm | |||||||
| Cavity (−) | 2 (23) | 5 (24) | 2 (24) | 3 (24) | 6 (24) | 2 (24) | 20 (143) |
| Cavity (+) | 5 (23) | 2 (24) | 1 (24) | 8 (24) | 6 (24) | 3 (24) | 25 (143) |
LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
Number in parenthesis indicates the number of lobes evaluated in the 24 patients.
Table 4.
CT findings seen in 144 lobes of 24 control patients
| Findings | RUL | RML | RLL | LUL | Lingula | LLL | Total |
| Lesion extent | 20 (24) | 18 (24) | 19 (24) | 13 (24) | 16 (24) | 12 (24) | 98 (144) |
| Bronchiectasis | 19 (24) | 17 (24) | 11 (24) | 6 (24) | 14 (24) | 4 (24) | 71 (144) |
| Small opacity | |||||||
| Ill-defined nodule | |||||||
| Cavity (−) | 14 (24) | 14 (24) | 17 (24) | 8 (24) | 12 (24) | 9 (24) | 74 (144) |
| Cavity (+) | 0 (24) | 0 (24) | 0 (24) | 1 (24) | 0 (24) | 0 (24) | 1 (144) |
| Well-defined nodule | |||||||
| Cavity (−) | 10 (24) | 4 (24) | 8 (24) | 5 (24) | 2 (24) | 5 (24) | 34 (144) |
| Cavity (+) | 0 (24) | 0 (24) | 0 (24) | 0 (24) | 0 (24) | 0 (24) | 0 (144) |
| Large opacity | |||||||
| 1–2 cm | |||||||
| Cavity (−) | 2 (24) | 11 (24) | 6 (24) | 2 (24) | 11 (24) | 4 (24) | 36 (144) |
| Cavity (+) | 4 (24) | 0 (24) | 0 (24) | 0 (24) | 0 (24) | 0 (24) | 4 (144) |
| >2 cm | |||||||
| Cavity (−) | 2 (24) | 3 (24) | 2 (24) | 0 (24) | 0 (24) | 0 (24) | 7 (144) |
| Cavity (+) | 5 (24) | 0 (24) | 2 (24) | 3 (24) | 0 (24) | 0 (24) | 10 (144) |
LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
The number in parenthesis indicates the number of lobes evaluated in the 24 patients.
Table 5 shows the comparison of the CT findings between the ICPs and the control group. The mean score of the total extent was slightly higher in the ICPs than in the control group (p=0.04). ICPs showed a slightly higher bronchiectasis score than did the control group; however, the difference was not statistically significant. For parenchymal opacity, ill-defined nodules with cavity and large opacity >2 cm in diameter with/without cavity were more frequent in the ICPs than in the control group (Figure 2). For ill-defined nodules without cavity and well-defined nodules with or without cavity, neither group showed significant difference.
Table 5.
Comparison of the CT findings in the immunocompromised patients and the control group
| Findings | ICPs | Controls | p-value | |
| Lesion extent | 10.1 | 7.8 | 0.04 | |
| Bronchiectasis | Severity | 4.5 | 4.3 | 0.71 |
| Extent | 6.0 | 4.6 | 0.08 | |
| Score | 9.8 | 7.3 | 0.23 | |
| Small opacity | ||||
| Ill-defined nodule | ||||
| Cavity (−) | Extent | 4.58 | 4.16 | 0.64 |
| Cavity (+) | Extent | 0.75 | 0.04 | 0.03 |
| Well-defined nodule | ||||
| Cavity (−) | Extent | 1.17 | 1.42 | 0.92 |
| Cavity (+) | Extent | 0.04 | 0.00 | 0.33 |
| Large opacity | ||||
| 1–2 cm | ||||
| Cavity (−) | Extent | 1.79 | 1.75 | 0.92 |
| Cavity (+) | Extent | 0.25 | 0.17 | 0.53 |
| >2 cm | ||||
| Cavity (−) | Extent | 1.75 | 0.54 | 0.04 |
| Cavity (+) | Extent | 2.50 | 0.58 | 0.02 |
ICP, immunocompromised patients.
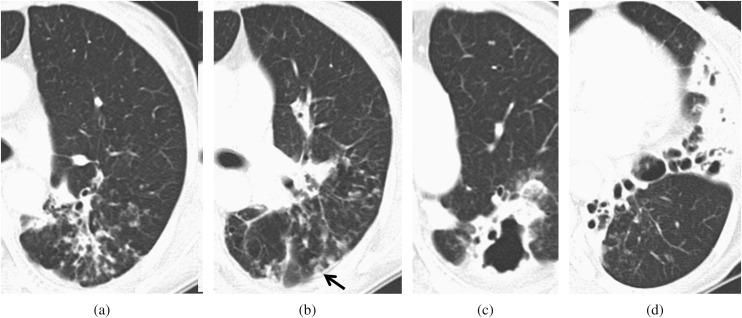
Figure 2.
A 56-year-old female on steroid therapy for 1 year. The total disease extent was 10. (a, b) CT scan shows bronchiectasis with ill-defined nodules in the superior division of the left upper lobe. A cavity (black arrow) in an ill-defined nodule was also seen. The bronchiectasis score was 4. (c, d) CT scan shows a large opacity >2 cm, with a cavity and bronchiectasis in the left lower lobe. The bronchiectasis severity was 3. The bronchiectasis score was 9.
Table 6 shows the comparison of the CT findings between diabetes patients (n=16) and the control group. Ill-defined nodules with cavity and large opacity >2 cm in diameter with or without cavity were more frequent in diabetes patients than in the control group (Figure 3). Although there were no statistically significant differences, cavitation was more frequently seen in all types of nodules in the patients with diabetes.
Table 6.
Comparison of CT findings in diabetes patients and control group.
| Findings | DM | Controls | p-value | |
| Lesion extent | 10.6 | 8.0 | 0.09 | |
| Bronchiectasis | Severity | 4.5 | 4.6 | 0.89 |
| Extent | 6.3 | 4.7 | 0.14 | |
| Score | 10.1 | 7.8 | 0.36 | |
| Small opacity | ||||
| Ill-defined nodule | ||||
| Cavity (−) | Extent | 4.00 | 4.00 | 1.00 |
| Cavity (+) | Extent | 0.93 | 0.00 | 0.04 |
| Well-defined nodule | ||||
| Cavity (−) | Extent | 1.13 | 1.81 | 0.18 |
| Cavity (+) | Extent | 0.06 | 0.00 | 0.33 |
| Large opacity | ||||
| 1–2 cm | ||||
| Cavity (−) | Extent | 1.56 | 1.75 | 0.74 |
| Cavity (+) | Extent | 0.25 | 0.19 | 0.75 |
| >2 cm | ||||
| Cavity (−) | Extent | 2.44 | 0.31 | 0.01 |
| Cavity (+) | Extent | 3.00 | 0.81 | 0.05 |
DM, patients with diabetes mellitus.
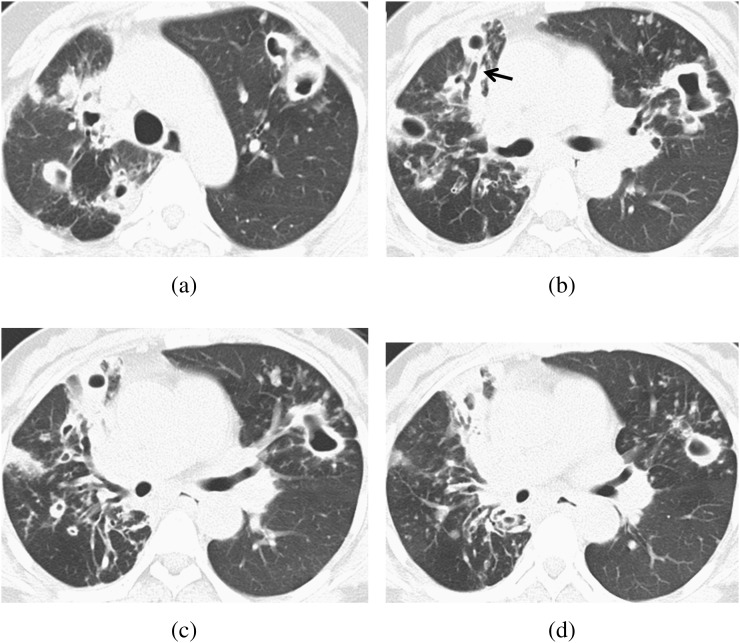
Figure 3.
A 54-year-old female with diabetes mellitus. The total disease extent was 20. (a–d) CT scan shows multiple large opacities and well-defined nodules, with a cavity in both lobes. Bronchiectasis was also seen. The bronchiectasis (black arrow in b) severity in the right middle lobe was 2.
Lymphadenopathy was seen in 12 of the ICPs and in 8 patients of the control group. The mean number of lymph nodes was 4.3 in the ICPs and 3.0 in the control group. Pleural effusion was seen in four of the ICPs and in one of the patients in the control group. Pleural thickening was seen in three of the ICPs, although there was no pleural thickening in the control group patients.
DISCUSSION
Our study is the first report to compare the radiological findings in non-AIDS ICPs and immunocompetent patients. Regardless of a patient’s immune status, an ill-defined nodule without cavity and bronchiectasis were the most common CT findings. Ill-defined nodules with cavity and a large opacity >2 cm with or without cavity were more frequent in the ICPs including those with diabetes mellitus than in immunocompetent patients.
It has been reported that the upper lobe cavitary and the nodular bronchiectatic forms are responsible for most NTM infections in immunocompetent patients [19–24]. The cavitary form is usually seen in older, white males with underlying chronic pulmonary disease. CT findings of the cavitary form include upper lobe cavitary lesions and endobronchial spread, as evidenced by nodules adjacent to the foci of disease [23,25]. The most common organisms of this form are MAC and M. kansasii, and M. xenopi, M. abscessus and M. malmoense are less frequent. The cavities are usually small and thin walled. The nodular bronchiectatic form is usually seen in middle-aged or older females with no predisposing factors [14,19,25,26]. CT findings of the nodular bronchiectatic form include small centrilobular nodules with bronchiectasis, usually occurring in the same lobe [13,23,24,27–31]. Similar findings were seen in the immunocompetent patients and ICPs in our study. However, a large opacity >2 cm and cavitation were more frequently found in the ICPs than in the control group. Considering our results and previous reports, there was a substantial similarity in the radiological findings between M. tuberculosis infection in patients with diabetes and NTM infection in the ICPs. In previous studies [32–34], it was seen that diabetic patients with tuberculosis had a higher number of multiple cavities than did patients without underlying disease. In our study, the ICP group included a large proportion of diabetic patients. When we analysed diabetes separately in all the ICPs, the diabetic patients also showed a higher score of small, ill-defined nodules with cavity and large opacity with or without cavity than did the control group patients. Therefore, these similarities may be attributed to the similar immunosuppressive mechanisms of diabetes mellitus on tuberculosis and NTM infection.
The radiological features of NTM infection in AIDS patients differ from those seen in non-AIDS patients. NTM infection in AIDS patients is characterised by disseminated disease [35]. Carpenter and Parks [36] reviewed M. kansasii infections in patients seropositive for human immunodeficiency virus and reported the diversity of the radiological findings, i.e. alveolar and interstitial infiltration as well as masses, nodules and nodes. El-Solh et al [37] reported that the most common findings included interstitial and mixed disease and that cavitary disease was seen in one patient. Other previous reports [38,39] regarding the radiographic finding of AIDS patients with NTM infection also noted that there was neither cavitary disease nor pleural effusion in the AIDS patients.
In contrast, in our study, the presence of cavitary nodules was a frequent finding in non-AIDS ICPs. Although both AIDS patients and non-AIDS ICPs have an immunocompromised status, the radiological features differ between the two patient groups. Compared with previous reports [35–39], the presence of a cavitary lesion is the feature that would most often indicate NTM infection in non-AIDS ICPs rather than NTM infection in immunocompetent or AIDS patients. MAC infection occurs in the setting of low CD4 counts (usually <100 cells per mm3) in AIDS patients [40]. As impaired immune function prevents destructive change, cavitary change is rare in AIDS patients. However, in our study, there was no significant neutropenia (usually <100 cells per mm3) in non-AIDS ICPs, thus indicating that immune function is less impaired in non-AIDS patients than in AIDS patients. Therefore, destructive change can occur in non-AIDS ICPs, and it causes cavitary change and more large opacities in non-AIDS ICPs.
Our study has several limitations. First, it was a retrospective study with a relatively small number of patients. As we only included patients who fulfilled the ATS criteria, we might have omitted patients with NTM infection owing to weak evidence, such as a limited number of positive microbiological findings. Another limitation is that non-AIDS ICPs in our study consisted of a relatively large proportion of patients with diabetes and a small proportion of other types of immunocompromised hosts. Although our centre has a huge number of cases of transplantation including solid organ and bone marrow as a tertiary referral centre, only eight patients were proved to have NTM infection in 7 years. This rarity of NTM infection in transplanted patients results in the deviation of proportion of diabetes and non-diabetes ICPs in our study. Therefore, further investigation with a large number of non-diabetic ICPs or meta-analysis of reports would be required in the future. Because our nation has a high prevalence of M. tuberculosis, the sequelae of previous tuberculosis infection might be present and might interfere with the description of findings resulting from NTM disease. This is one of the inherent limitations of our study.
In conclusion, in our study, regardless of the specific mycobacterial species, ill-defined small nodules and large opacities accompanying cavitary change were more common findings in the non-AIDS ICPs, including those with diabetes mellitus, than in the immunocompetent controls. When radiologists encounter both cavitary nodules and large opacities in ICPs, NTM infection can be included in the differential diagnoses.
ACKNOWLEDGMENTS
This study was supported by a grant (2009-439) from the Asan Institute for Life Sciences, Seoul, Republic of Korea.
References
- 1.O'Brien RJ, Geiter LJ, Snider DE., Jr The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis 1987;135:1007–14 [DOI] [PubMed] [Google Scholar]
- 2.Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis 1979;119:107–59 [DOI] [PubMed] [Google Scholar]
- 3.Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am Rev Respir Dis 1990;142:940–53 [DOI] [PubMed] [Google Scholar]
- 4.Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med 1997;156:S1–25 [DOI] [PubMed] [Google Scholar]
- 5.Runyon EH. Anonymous mycobacteria in pulmonary disease. Med Clin North Am 1959;43:273–90 [DOI] [PubMed] [Google Scholar]
- 6.Timpe A, Runyon EH. The relationship of atypical acid-fast bacteria to human disease; a preliminary report. J Lab Clin Med 1954;44:202–9 [PubMed] [Google Scholar]
- 7.Horsburgh CR, Jr, Mason UG, 3rd, Farhi DC, Iseman MD. Disseminated infection with Mycobacterium avium-intracellulare. A report of 13 cases and a review of the literature. Medicine (Baltimore) 1985;64:36–48 [DOI] [PubMed] [Google Scholar]
- 8.Young LS, Inderlied CB, Berlin OG, Gottlieb MS. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis 1986;8:1024–33 [DOI] [PubMed] [Google Scholar]
- 9.Murray JF, Garay SM, Hopewell PC, Mills J, Snider GL, Stover DE. NHLBI workshop summary. Pulmonary complications of the acquired immunodeficiency syndrome: an update. Report of the second National Heart, Lung and Blood Institute workshop. Am Rev Respir Dis 1987;135:504–9 [DOI] [PubMed] [Google Scholar]
- 10.Teirstein AS, Damsker B, Kirschner PA, Krellenstein DJ, Robinson B, Chuang MT. Pulmonary infection with Mycobacterium avium-intracellulare: diagnosis, clinical patterns, treatment. Mt Sinai J Med 1990;57:209–15 [PubMed] [Google Scholar]
- 11.Miller WT, Jr, Miller WT. Pulmonary infections with atypical mycobacteria in the normal host. Semin Roentgenol 1993;28:139–49 [DOI] [PubMed] [Google Scholar]
- 12.Rosenzweig DY. Pulmonary mycobacterial infections due to Mycobacterium intracellulare-avium complex. Clinical features and course in 100 consecutive cases. Chest 1979;75:115–19 [DOI] [PubMed] [Google Scholar]
- 13.Kubo K, Yamazaki Y, Hachiya T, Hayasaka M, Honda T, Hasegawa M, et al. Mycobacterium avium-intracellulare pulmonary infection in patients without known predisposing lung disease. Lung 1998;176:381–91 [DOI] [PubMed] [Google Scholar]
- 14.Prince DS, Peterson DD, Steiner RM, Gottlieb JE, Scott R, Israel HL, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med 1989;321:863–8 doi: 10.1056/NEJM198909283211304 [DOI] [PubMed] [Google Scholar]
- 15.Wallace RJ, Jr, Zhang Y, Brown BA, Dawson D, Murphy DT, Wilson R, et al. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am J Respir Crit Care Med 1998;158:1235–44 [DOI] [PubMed] [Google Scholar]
- 16.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416 doi: 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 17.Bhalla M, Turcios N, Aponte V, Jenkins M, Leitman BS, McCauley DI, et al. Cystic fibrosis: scoring system with thin-section CT. Radiology 1991;179:783–8 [DOI] [PubMed] [Google Scholar]
- 18.Helbich TH, Heinz-Peer G, Eichler I, Wunderbaldinger P, Gotz M, Wojnarowski C, et al. Cystic fibrosis: CT assessment of lung involvement in children and adults. Radiology 1999;213:537–44 [DOI] [PubMed] [Google Scholar]
- 19.Albelda SM, Kern JA, Marinelli DL, Miller WT. Expanding spectrum of pulmonary disease caused by nontuberculous mycobacteria. Radiology 1985;157:289–96 [DOI] [PubMed] [Google Scholar]
- 20.Christensen EE, Dietz GW, Ahn CH, Chapman JS, Murry RC, Anderson J, et al. Initial roentgenographic manifestations of pulmonary Mycobacterium tuberculosis, M kansasii, and M intracellularis infections. Chest 1981;80:132–6 [DOI] [PubMed] [Google Scholar]
- 21.Christensen EE, Dietz GW, Ahn CH, Chapman JS, Murry RC, Anderson J, et al. Pulmonary manifestations of Mycobacterium intracellularis. AJR Am J Roentgenol 1979;133:59–66 [DOI] [PubMed] [Google Scholar]
- 22.Levin DL. Radiology of pulmonary Mycobacterium avium-intracellulare complex. Clin Chest Med 2002;23:603–12 [DOI] [PubMed] [Google Scholar]
- 23.Martinez S, McAdams HP, Batchu CS. The many faces of pulmonary nontuberculous mycobacterial infection. AJR Am J Roentgenol 2007;189:177–86 doi: 10.2214/AJR.07.2074 [DOI] [PubMed] [Google Scholar]
- 24.Ellis SM, Hansell DM. Imaging of non-tuberculous (atypical) mycobacterial pulmonary infection. Clin Radiol 2002;57:661–9 [DOI] [PubMed] [Google Scholar]
- 25.Koh WJ, Kwon OJ, Lee KS. Nontuberculous mycobacterial pulmonary diseases in immunocompetent patients. Korean J Radiol 2002;3:145–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller WT., Jr Spectrum of pulmonary nontuberculous mycobacterial infection. Radiology 1994;191:343–50 [DOI] [PubMed] [Google Scholar]
- 27.Han D, Lee KS, Koh WJ, Yi CA, Kim TS, Kwon OJ. Radiographic and CT findings of nontuberculous mycobacterial pulmonary infection caused by Mycobacterium abscessus. AJR Am J Roentgenol 2003;181:513–17 [DOI] [PubMed] [Google Scholar]
- 28.Hartman TE, Swensen SJ, Williams DE. Mycobacterium avium-intracellulare complex: evaluation with CT. Radiology 1993;187:23–6 [DOI] [PubMed] [Google Scholar]
- 29.Moore EH. Atypical mycobacterial infection in the lung: CT appearance. Radiology 1993;187:777–82 [DOI] [PubMed] [Google Scholar]
- 30.Obayashi Y, Fujita J, Suemitsu I, Kamei T, Nii M, Takahara J. Successive follow-up of chest computed tomography in patients with Mycobacterium avium-intracellulare complex. Respir Med 1999;93:11–15 [DOI] [PubMed] [Google Scholar]
- 31.Swensen SJ, Hartman TE, Williams DE. Computed tomographic diagnosis of Mycobacterium avium-intracellulare complex in patients with bronchiectasis. Chest 1994;105:49–52 [DOI] [PubMed] [Google Scholar]
- 32.Ikezoe J, Takeuchi N, Johkoh T, Kohno N, Tomiyama N, Kozuka T, et al. CT appearance of pulmonary tuberculosis in diabetic and immunocompromised patients: comparison with patients who had no underlying disease. AJR Am J Roentgenol 1992;159:1175–9 [DOI] [PubMed] [Google Scholar]
- 33.Perez-Guzman C, Torres-Cruz A, Villarreal-Velarde H, Vargas MH. Progressive age-related changes in pulmonary tuberculosis images and the effect of diabetes. Am J Respir Crit Care Med 2000;162:1738–40 [DOI] [PubMed] [Google Scholar]
- 34.Umut S, Tosun GA, Yildirim N. Radiographic location of pulmonary tuberculosis in diabetic patients. Chest 1994;106:326. [PubMed] [Google Scholar]
- 35.Aronchick JM, Miller WT., Jr Disseminated nontuberculous mycobacterial infections in immunosuppressed patients. Semin Roentgenol 1993;28:150–7 [DOI] [PubMed] [Google Scholar]
- 36.Carpenter JL, Parks JM. Mycobacterium kansasii infections in patients positive for human immunodeficiency virus. Rev Infect Dis 1991;13:789–96 [DOI] [PubMed] [Google Scholar]
- 37.El-Solh AA, Nopper J, Abdul-Khoudoud MR, Sherif SM, Aquilina AT, Grant BJ. Clinical and radiographic manifestations of uncommon pulmonary nontuberculous mycobacterial disease in AIDS patients. Chest 1998;114:138–45 [DOI] [PubMed] [Google Scholar]
- 38.Hawkins CC, Gold JW, Whimbey E, Kiehn TE, Brannon P, Cammarata R, et al. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1986;105:184–8 [DOI] [PubMed] [Google Scholar]
- 39.Marinelli DL, Albelda SM, Williams TM, Kern JA, Iozzo RV, Miller WT. Nontuberculous mycobacterial infection in AIDS: clinical, pathologic, and radiographic features. Radiology 1986;160:77–82 [DOI] [PubMed] [Google Scholar]
- 40.Benson CA, Ellner JJ. Mycobacterium avium complex infection and AIDS: advances in theory and practice. Clin Infect Dis 1993;17:7–20 [DOI] [PubMed] [Google Scholar]