Abstract
Objective:
To propose a new and practical MRI grading method for cervical neural foraminal stenosis and to evaluate its reproducibility.
Methods:
We evaluated 50 patients (37 males and 13 females, mean age 49 years) who visited our institution and underwent oblique sagittal MRI of the cervical spine. A total of 300 foramina and corresponding nerve roots in 50 patients were qualitatively analysed from C4–5 to C6–7. We assessed the grade of cervical foraminal stenosis at the maximal narrowing point according to the new grading system based on T2 weighted oblique sagittal images. The incidence of each of the neural foraminal stenosis grades according to the cervical level was analysed by χ2 tests. Intra- and interobserver agreements between two radiologists were analysed using kappa statistics. Kappa value interpretations were poor (κ<0.1), slight (0.1≤κ≤0.2), fair (0.2<κ≤0.4), moderate (0.4<κ≤0.6), substantial (0.6<κ≤0.8) and almost perfect (0.8<κ≤1.0).
Results:
Significant stenoses (Grades 2 and 3) were rarely found at the C4–5 level. The incidence of Grade 3 at the C5–6 level was higher than that at other levels, a difference that was statistically significant. The overall intra-observer agreement according to the cervical level was almost perfect. The agreement at each level was almost perfect, except for only substantial agreement at the right C6–7 by Reader 2. No statistically significant differences were seen according to the cervical level. Overall kappa values of interobserver agreement according to the cervical level were almost perfect. In addition, the agreement of each level was almost perfect. Overall intra- and interobserver agreement for the presence of foraminal stenosis (Grade 0 vs Grades 1, 2 and 3) and for significant stenosis (Grades 0 and 1 vs Grades 2 and 3) showed similar results and were almost perfect. However, only substantial agreement was seen in the right C6–7.
Conclusion:
A new grading system for cervical foraminal stenosis based on oblique sagittal MRI provides reliable assessment and good reproducibility. This new grading system is a useful and easy method for the objective evaluation of cervical neural foraminal stenosis by radiologists and clinicians.
Advances in knowledge:
The use of the new grading system for cervical foraminal stenosis based on oblique sagittal MRI can be a useful method for evaluating cervical neural foraminal stenosis.
Cervical radiculopathy resulting from neural foraminal stenosis is clinically important. The correct diagnosis of cervical neural foraminal stenosis can exclude the possibility of peripheral neuropathy, predict prognosis and determine appropriate treatment methods [1]. As the neural foramen in the cervical spine (C-spine) is oriented in a nearly 45° oblique position, and traditional cervical sagittal MRI does not offer clear views of the foramen, the 45° oblique sagittal view creates a cervical image similar to that of lumbar sagittal views [2].
Despite assessments made in previous studies, grading systems for cervical neural foramina based on MRI with corresponding studies of reproducibility are rare. In 2011, Kim et al [3] reported a new MRI grading system for cervical neural foraminal stenosis. However, their grading system has some limitations, as it is based on T2 weighted axial images, which cannot correctly reflect the anatomic relationship between the nerve root and the neural foramen and is based instead on quantitative measurements of the diameter of the foramen and the nerve root. These measurements are complicated and time-consuming and are therefore not practical for use in clinical situations.
In this study, we propose a new practical grading system for diagnosing and grading cervical neural foraminal stenosis that is based on oblique sagittal views of T2 weighted images and evaluate its reproducibility.
METHODS AND MATERIALS
MRI grading system for cervical neural foraminal stenosis
Cervical neural foraminal stenosis was classified into four grades according to MRI findings on T2 weighted oblique sagittal images (Figure 1). The grading system used was modified from a pre-existing grading system for lumbar neural foraminal stenosis suggested by Wildermuth et al [4]. Grade 0 refers to the absence of foraminal stenosis. Grade 1 refers to mild foraminal stenosis showing partial (<50% of root circumference) perineural fat obliteration surrounding the nerve root, where no evidence of morphological change in the nerve root is seen. Grade 2 refers to moderate (>50% of root circumference) foraminal stenosis showing nearly complete perineural fat obliteration surrounding the nerve root without morphological changes in the nerve root. Grade 3 refers to severe foraminal stenosis showing nerve root collapse or morphological changes.
Figure 1.
Schematic illustrations of grading systems for cervical foraminal stenosis. (a) Grade 0, oblique sagittal plane of the cervical neural foramen shows no significant stenosis and no perineural fat obliteration. (b) Grade 1, mild (below 50% of nerve root circumference) perineural fat obliteration. No morphological change of the nerve root is seen. (c) Grade 2, moderate (above 50% of nerve root circumference) perineural fat obliteration. No morphological change of the nerve root is seen. (d) Grade 3, collapsed nerve root and morphological change of the nerve root. Severe perineural fat obliteration is also combined.
Case selection
Our study sample included 37 (74%) males and 13 (26%) females who visited our institution and underwent MRI of the C-spine between January and April 2012. The age distribution was from 19 to 97 with a mean age of 49 years (standard deviation 15). Exclusion criteria were: infection, tumour, acute trauma, previous surgical history, central disc herniation, combined brain infarction or other intracranial lesion and peripheral neuropathy. This retrospective study was approved by the Institutional Ethics Review Board of Kangbuk Samsung Hospital, Seoul, Republic of Korea, and the requirement for informed patient consent was waived because of the retrospective study design.
Image analysis
MRI examinations were interpreted independently by two fellowship-trained musculoskeletal radiologists, one with 12 years and one with 10 years of experience, who were blinded to clinical information and radiological reports. A total of 300 foramina and corresponding nerve roots from C4–5 to C6–7 in 50 patients were qualitatively analysed. The radiologists assessed the presence and grade of cervical foraminal stenosis at the maximal narrowing point according to our new grading system based on T2 weighted oblique sagittal images.
MRI parameters
All MRI examinations were performed using the same protocol on a 1.5-T magnet (Intera; Philips Medical Systems, Best, Netherlands) using a syn-head coil and fast spin echo imaging. T2 weighted images were obtained in the axial and sagittal planes in the supine position with the following parameters: field of view, 24 cm; matrix, 360×270; slice thickness, 4 mm; signal average, 3; interslice gap, 0 mm (sagittal image); and field of view, 17 cm; matrix, 316×250; slice thickness, 3 mm; signal average, 1; interslice gap, 0.3 mm (axial image). The MRI sequences were as follows: sagittal T2 weighted spin echo (repetition time/echo time, 2500/100 ms) and axial T2 weighted turbo spin echo (2500/100 ms). To acquire oblique sagittal images, the medial edge of the neural foramen was identified and seven images progressing laterally through the foramen at 45° angled projections were taken with the following parameters: field of view, 24 cm; matrix, 370×270; slice thickness, 3 mm; interslice gap, 0 mm; repetition time/echo time, 3300–3700/100 ms; signal average, 3. The images included the medial margin of each pedicle, the isthmus of the foramen and the lateral edge of the foramen [5].
Statistical analysis
The incidence of each neural foraminal stenosis grade was analysed according to the cervical level by χ2 tests. Intra- and interobserver agreements between the two radiologists were analysed using kappa statistics. Kappa value interpretations were poor (κ<0.1), slight (0.1≤κ≤0.2), fair (0.2<κ≤0.4), moderate (0.4<κ≤0.6), substantial (0.6<κ≤0.8) and almost perfect (0.8<κ≤1.0). The agreement and kappa statistics were also calculated for distinctions between the four individual grades, the distinction between insignificant stenosis and significant stenosis (Grades 0 and 1 vs Grades 2 and 3) and the presence of foraminal stenosis (Grade 0 vs Grades 1, 2 and 3) [6,7]. Statistical analyses were performed using SPSS® software v. 10.1 (SPSS Inc., Chicago, IL); p≤0.05 was considered statistically significant.
RESULTS
Schematic illustrations and MR images illustrating the degrees of cervical foraminal stenosis are shown in Figures 1–5. The distribution of foraminal stenosis by cervical level is shown in Table 1. Grade 0 was most common (206–210, 69–70%), and Grade 2 was least common (11–13, 3.7–4.3%). Significant stenoses (Grades 2 and 3) were rarely found at the C4–5 level (1–2, 0.3–0.6%). The incidence of Grade 3 at the C5–6 level was significantly higher than at other levels (10–12 vs 1–7; p<0.05). The overall intra-observer agreements according to the cervical level were substantial and almost perfect (0.94, 0.84) (Table 2). The agreements of each level were almost perfect (0.81–0.99), except for only substantial agreement at the right C6–7 by Reader 2. No statistically significant differences were observed according to the cervical level. Overall kappa values of interobserver agreement according to the cervical level were almost perfect (0.81–0.93) (Table 2). The agreements at each level were substantial and almost perfect (0.71–0.99). Overall intra- and interobserver agreement for the presence of foraminal stenosis (Grade 0 vs Grades 1, 2 and 3) and for significant stenosis (Grades 0 and 1 vs Grades 2 and 3) showed similar results and were almost perfect (0.88–0.95) (Tables 3 and 4). However, only substantial agreement was seen in the right C6-7 (Tables 3 and 4).
Figure 5.
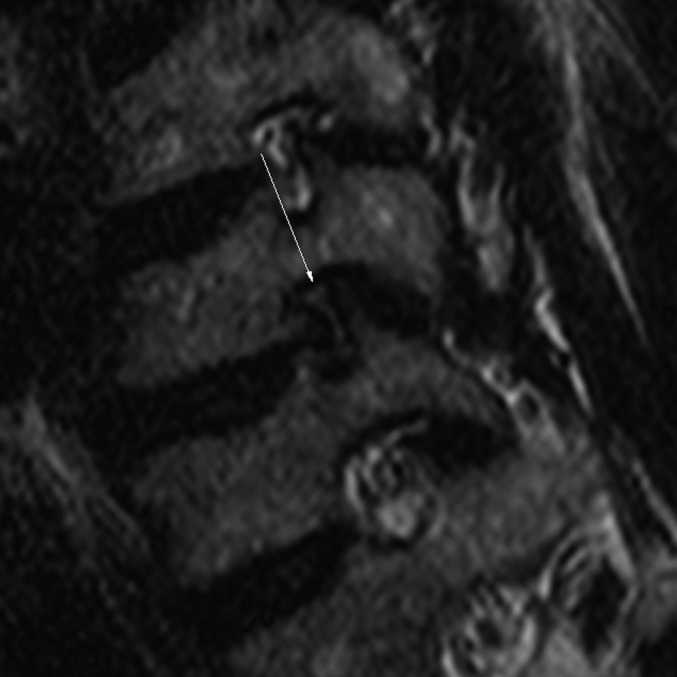
Grade 3: 51-year-old male with radiating pain in the right upper extremity. Oblique sagittal T2 weighted turbo spin echo image (repetition time/echo time, 3300/100 ms) shows a collapsed nerve root by the bony structures at the left C6–7 (arrow).
Table 1.
Distribution of foraminal stenoses by cervical level
| Cervical level | Grade 0 |
Grade 1 |
Grade 2 |
Grade 3 |
Total |
|||||
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | |
| C4–5 (left) | 39/38 | 40/41 | 9/10 | 8/7 | 1/1 | 1/1 | 1/1 | 1/1 | 50/50 | 50/50 |
| C4–5 (right) | 43/41 | 44/43 | 6/8 | 5/6 | 1/1 | 1/0 | 0/0 | 0/1 | 50/50 | 50/50 |
| C5–6 (left) | 26/27 | 28/26 | 15/14 | 13/15 | 3/3 | 3/2 | 6/6 | 6/7 | 50/50 | 50/50 |
| C5–6 (right) | 31/31 | 30/28 | 13/13 | 14/16 | 2/2 | 1/1 | 4/4 | 5/5 | 50/50 | 50/50 |
| C6–7 (left) | 33/33 | 33/32 | 8/7 | 9/8 | 4/4 | 3/6 | 5/6 | 5/4 | 50/50 | 50/50 |
| C6–7 (right) | 36/36 | 35/38 | 12/11 | 12/9 | 1/2 | 2/2 | 1/1 | 1/1 | 50/50 | 50/50 |
| Overall | 208/206 | 210/208 | 63/63 | 61/61 | 12/13 | 11/12 | 17/18 | 18/19 | 300/300 | 300/300 |
| p-value | 0.10/0.20 | 0.09/0.04 | 0.13/0.28 | 0.09/0.01 | 0.47/0.37 | 0.53/0.04 | 0.03/0.03 | 0.02/0.02 | ||
Data are number of patients detected by first reading/second reading. Values in bold are statistically significant.
Table 2.
Intra- and interobserver reliability of each level, kappa values
| Observer | C4–5 (left) | C4–5 (right) | C5–6 (left) | C5–6 (right) | C6–7 (left) | C6–7 (right) | Overall |
| Reader 1-1 vs 1-2 | 0.95 | 0.86 | 0.97 | 0.99 | 0.92 | 0.95 | 0.94 |
| Reader 2-1 vs 2-2 | 0.81 | 0.83 | 0.90 | 0.86 | 0.85 | 0.76 | 0.84 |
| Reader 1-1 vs 2-1 | 0.94 | 0.91 | 0.94 | 0.93 | 0.96 | 0.91 | 0.93 |
| Reader 1-2 vs 2-1 | 0.89 | 0.77 | 0.97 | 0.93 | 0.89 | 0.86 | 0.89 |
| Reader 1-1 vs 2-2 | 0.88 | 0.92 | 0.90 | 0.86 | 0.89 | 0.76 | 0.87 |
| Reader 1-2 vs 2-2 | 0.83 | 0.78 | 0.87 | 0.86 | 0.81 | 0.71 | 0.81 |
Reader 1–1, first reading of reader 1; 1–2, second reading of reader 1; 2–1, first reading of reader 2; 2–2, second reading of reader 2.
Table 3.
Intra- and interobserver reliability of stenosis (Grade 0 vs Grades 1–3), kappa values
| Observer | C4–5 (left) | C4–5 (right) | C5–6 (left) | C5–6 (right) | C6–7 (left) | C6–7 (right) | Overall |
| Reader 1-1 vs 1-2 | 0.94 | 0.85 | 0.96 | 0.99 | 0.99 | 0.99 | 0.95 |
| Reader 2-1 vs 2-2 | 0.81 | 0.91 | 0.92 | 0.92 | 0.96 | 0.75 | 0.88 |
| Reader 1-1 vs 2-1 | 0.94 | 0.91 | 0.92 | 0.96 | 0.99 | 0.95 | 0.95 |
| Reader 1-2 vs 2-1 | 0.88 | 0.94 | 0.96 | 0.99 | 0.95 | 0.95 | 0.95 |
| Reader 1-1 vs 2-2 | 0.88 | 0.99 | 0.92 | 0.88 | 0.96 | 0.79 | 0.90 |
| Reader 1-2 vs 2-2 | 0.82 | 0.85 | 0.88 | 0.96 | 0.97 | 0.79 | 0.88 |
Reader 1–1, first reading of reader 1; 1–2, second reading of reader 1; 2–1, first reading of reader 2; 2–2, second reading of reader 2.
Table 4.
Intra- and interobserver reliability of significance (Grades 0 and 1 vs Grades 2 and 3), kappa values
| Observer | C4–5 (left) | C4–5 (right) | C5–6 (left) | C5–6 (right) | C6–7 (left) | C6–7 (right) | Overall |
| Reader 1-1 vs 1-2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.94 | 0.79 | 0.95 |
| Reader 2-1 vs 2-2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.87 | 0.99 | 0.97 |
| Reader 1-1 vs 2-1 | 0.99 | 0.99 | 0.99 | 0.99 | 0.93 | 0.79 | 0.95 |
| Reader 1-2 vs 2-1 | 0.99 | 0.99 | 0.99 | 0.99 | 0.87 | 0.65 | 0.91 |
| Reader 1-1 vs 2-2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.94 | 0.79 | 0.95 |
| Reader 1-2 vs 2-2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.88 | 0.65 | 0.92 |
Reader 1–1, first reading of reader 1; 1–2, second reading of reader 1; 2–1, first reading of reader 2; 2–2, second reading of reader 2.
Figure 2.

Grade 0: 39-year-old male with posterior neck pain. Oblique sagittal T2 weighted turbo spin echo image (repetition time/echo time, 3300/100 ms) shows no cervical neural foraminal stenosis.
Figure 3.

Grade 1: 38-year-old male with radiating pain in both upper extremities. Oblique sagittal T2 weighted turbo spin echo image (repetition time/echo time, 3400/100 ms) shows mild (below 50% of root circumference) perineural fat obliteration in the neural foramen at the right C5–6 (arrow). Morphological change of the nerve root is not seen.
Figure 4.

Grade 2: 40-year-old male with radiating pain in the left upper extremity. Oblique sagittal T2 weighted turbo spin echo image (repetition time/echo time, 3300/100 ms) shows moderate (above 50% of root circumference) perineural fat obliteration in the neural foramen at the left C6–7 (arrow). Morphological change of the nerve root is not seen.
DISCUSSION
In this study, a new grading system for cervical foraminal stenosis is proposed, based on the oblique sagittal view. Oblique sagittal images provide much more information about the foramen than routine sagittal images because oblique images are oriented perpendicular to the course of the cervical neural foramen [5]. Facet overgrowth, herniated lateral discs and uncinate process osteophytes that may induce foraminal stenosis are more easily detectable in the oblique sagittal view [8]. A major disadvantage of the oblique sagittal view is that its use increases examination time. Shim et al [8] reported that the total scan time on the oblique sagittal view was 5 min. In the present study, we required 3.25 min for the acquisition of both oblique sagittal views, whereas the acquisition time for the routine T2 weighted sagittal view alone was 2.5 min. These differences might result from the fact that Shim et al [8] made four time signal acquisitions and averaged them, while we averaged only triple signal acquisitions. We used T2 weighted images for the acquisition of oblique sagittal views because T2 weighted images provide the high tissue contrast required to easily distinguish bone from soft tissue. On T1 weighted images, fat is enhanced and the cortical bone and cervical spinal fluid are more difficult to distinguish [5]. Our grading system is based on the Wildermuth classification of lumbar foraminal stenosis, modified to provide more objective grading [4]. The Wildermuth classification introduced semi-quantitative classification, focusing on the degree of epidural fat obliteration and the absence of characteristics of morphological nerve root change [4], but the evaluation of “slight” or “marked” stenosis in the Wildermuth classification remains subjective. We classified foraminal stenosis objectively as being above or below 50% based on the degree of fat obliteration, and the overall intra- and interobserver agreement of our grading system was very high. We found that the incidence of Grade 3 stenosis was higher at the C5–6 level than at other cervical levels (p<0.05). We attribute these results to the fact that the C5–6 level showed the largest range of movement, and, therefore, the most severe deformity of the neural foramen can be seen at this level [9].
Stenosis and significance showed substantial and almost perfect intra-observer agreement at each grade, and therefore our new grading system has good reproducibility. The high level of interobserver agreement implies that this new grading system is an objective method for the evaluation of cervical foraminal stenosis. Another recently reported grading system by Kim et al [3] that is based on axial images is less practical because it requires the measurement of the transverse diameter of the foramen and of the nerve root. Furthermore, the transverse diameter on an axial image cannot represent narrowed neural foramina accurately.
One limitation of this study was the lack of correlation between stenosis grade and clinical manifestation, and, therefore, further evaluations of the relationships between stenosis grade and clinical manifestations are needed. A second limitation was the single posture used for C-spine MRI, as the C-spine posture affects the dimensions of the neural foramen [10]. Bartlett et al [11] reported that cervical neural foramina are more likely to appear abnormal if the spine is extended and the foramina become narrowed. They suggested that MRI with neck extension might improve diagnostic accuracy. In this study, the C-spine was imaged in a neutral supine position, and no flexion or extension was applied. Nevertheless, as the same position was applied to every patient, the differences between patients were minimised.
In conclusion, our new grading system of cervical foraminal stenosis based on oblique sagittal MRI provides reliable assessment of cervical foraminal stenosis and good reproducibility. This new grading system is a useful and easy method for the objective evaluation of cervical neural foraminal stenosis.
ACKNOWLEDGMENT
This work was supported by medical research funds from Kangbuk Samsung Hospital.
REFERENCES
- 1.Yousem DM, Atlas SW, Goldberg HI, Grossman RI. Degenerative narrowing of the cervical spine neural foramina: evaluation with high-resolution 3DFT gradient-echo MRI imaging. AJNR Am J Neuroradiol 1991;12:229–36 [PMC free article] [PubMed] [Google Scholar]
- 2.Goodman BS, Geffen JF, Mallempati S, Noble BR. MRI images at a 45-degree angle through the cervical neural foramina: a technique for improved visualization. Pain Physician 2006;9:327–32 [PubMed] [Google Scholar]
- 3.Kim S, Lee JW, Chai JW. New MRI grading system for cervical neural foraminal stenosis based on T2-axial images. Proceedings of the Korean Congress of Radiology, 67th Annual Meeting, 2011; Scientific Session 12; 10th presentation. Republic of Korea; The Korean Society of Radiology, 2011. [Google Scholar]
- 4.Wildermuth S, Zanetti M, Duewell S, Schmid MR, Romanowski B, Benini A, et al. Lumbar spine: quantitative and qualitative assessment of positional (upright flexion and extension) MRI imaging and myelography. Radiology 1998;207:391–8 [DOI] [PubMed] [Google Scholar]
- 5.Humphreys SC, An HS, Eck JC, Coppes M, Lim TH, Estkowski L. Oblique MRI as a useful adjunct in evaluation of cervical foraminal impingement. J Spinal Disord 1998;11:295–9 [PubMed] [Google Scholar]
- 6.Kang Y, Lee JW, Kho YH, Hur S, Kim SJ, Chai JW, et al. New MRI grading system for the cervical canal stenosis. AJR Am J Roentgenol 2011;197:W134–40 doi: 10.2214/AJR.10.5560 [DOI] [PubMed] [Google Scholar]
- 7.Sim J, Wright CC. The kappa statistics in reliability studies: use, interpretation, and sample size requirements. Phys Ther 2005;85:257–68 [PubMed] [Google Scholar]
- 8.Shim JH, Park CK, Lee JH, Choi JW, Lee DC, Kim DH, et al. A comparison of angled sagittal MRI and conventional MRI in the diagnosis of herniated disc and stenosis in the cervical foramen. Eur Spine J 2009;18:1109–16 doi: 10.1007/s00586-009-0932-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pyhtimen J, Laitinen J. Cervical intervertebral foramen narrowing and myelographic nerve root sleeve deformities. Neuroradiology 1993;35:596–7 [DOI] [PubMed] [Google Scholar]
- 10.Park HJ, Kim SS, Lee SY, Park NH, Rho MH, Hong HP, et al. Clinical correlation of a new MRI imaging method for assessing lumbar foraminal stenosis. AJNR Am J Neuroradiol 2012;33:818–22 doi: 10.3174/ajnr.A2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartlett RJ, Hill CA, Rigby AS, Chandrasekaran S, Narayanamurthy H. MRI of the cervical spine with neck extension: is it useful? Br J Radiol 2012;85:1044–51 [DOI] [PMC free article] [PubMed] [Google Scholar]



