Abstract
Objective:
We evaluated the diagnostic role of ultrasonography-guided core needle biopsy (CNB) according to ultrasonography features of thyroid nodules that had inconclusive ultrasonography-guided fine-needle aspiration (FNA) results.
Methods:
A total of 88 thyroid nodules in 88 patients who underwent ultrasonography-guided CNB because of previous inconclusive FNA results were evaluated. The patients were classified into three groups based on ultrasonography findings: Group A, which was suspicious for papillary thyroid carcinoma (PTC); Group B, which was suspicious for follicular (Hurthle cell) neoplasm; and Group C, which was suspicious for lymphoma. The final diagnoses of the thyroid nodules were determined by surgical confirmation or follow-up after ultrasonography-guided CNB.
Results:
Of the 88 nodules, the malignant rate was 49.1% in Group A, 12.0% in Group B and 90.0% in Group C. The rates of conclusive ultrasonography-guided CNB results after previous incomplete ultrasonography-guided FNA results were 96.2% in Group A, 64.0% in Group B and 90.0% in Group C (p=0.001). 12 cases with inconclusive ultrasonography-guided CNB results were finally diagnosed as 8 benign lesions, 3 PTCs and 1 lymphoma. The number of previous ultrasonography-guided FNA biopsies was not significantly different between the conclusive and the inconclusive result groups of ultrasonography-guided CNB (p=0.205).
Conclusion:
Ultrasonography-guided CNB has benefit for the diagnosis of thyroid nodules with inconclusive ultrasonography-guided FNA results. However, it is still not helpful for the differential diagnosis in 36% of nodules that are suspicious for follicular neoplasm seen on ultrasonography.
Advances in knowledge:
This study shows the diagnostic contribution of ultrasonography-guided CNB as an alternative to repeat ultrasonography-guided FNA or surgery.
Ultrasonography-guided fine-needle aspiration (FNA) biopsy is the most cost-effective and safe method for diagnostic evaluation of thyroid nodules. However, there are some limitations to ultrasonography-guided FNA cytology, including those results that are categorised as non-diagnostic or indeterminate aspirates [1–3]. Earlier studies reported that the malignancy rates for non-diagnostic and indeterminate aspirates of the ultrasonography-guided FNA samples were up to 10.9% and 60.0%, respectively [1–3]. In addition, the cytological results obtained from ultrasonography-guided FNA are occasionally discordant with imaging findings [4,5]. In one study of imaging–pathology discordant thyroid nodules after ultrasonography-guided FNA, the malignancy rate for those nodules was reported to be 62.8% [5]. Therefore, uncertainty about the presence of thyroid malignancy still remains a clinical problem after the non-diagnostic, indeterminate or imaging–pathology discordant aspirates.
Although ultrasonography-guided core needle biopsy (CNB) is reported to be a safe and well-tolerated procedure for obtaining a larger tissue sample [6,7], the Bethesda system for reporting thyroid cytopathology does not discuss the role of ultrasonography-guided CNB in the evaluation of thyroid nodules [8]. Several reports have suggested the potential use of ultrasonography-guided CNB, and have recommended its use after inconclusive FNAs [9–12]. However, they did not find any diagnostic contribution of ultrasonography-guided CNB according to ultrasonography features of thyroid nodules.
Therefore, we evaluated the diagnostic role of ultrasonography-guided CNB according to the ultrasonography features of thyroid nodules that had inconclusive results based on ultrasonography-guided FNA cytology.
MATERIALS AND METHODS
This study was granted institutional review board approval, and informed consent was received from patients before they underwent the ultrasonography-guided FNA and ultrasonography-guided CNB procedures.
Patients
From April 2006 to December 2010, 109 patients who had 109 thyroid lesions underwent ultrasonography-guided CNB. We excluded 21 thyroid nodules involving 21 patients who did not have ultrasonography-guided FNA or who underwent ultrasonography-guided FNA at another hospital. The remaining 88 patients, whose initial ultrasonography-guided FNA reading was inconclusive, were consecutively enrolled in this study. We retrospectively reviewed the medical records to obtain information, including age, gender, cytological results after ultrasonography-guided FNA, histological results after ultrasonography-guided CNB, final diagnoses after surgery and follow-up results. Of the 88 patients (age range 15–86 years; mean age 49.9±14.2 years), 72 (81.8%) were females and 16 (18.2%) were males. 61 patients (69.3%) had follow-up imaging for at least 12 months (range 18–72 months; mean 38.1 months), and 27 (30.7%) patients were surgically treated through total thyroidectomy (n=21) or lobectomy (n=6).
Ultrasonography-guided FNA and CNB procedures
Ultrasonography was performed using high-resolution ultrasonography equipment (IU22® or HDI® 5000; Philips–Advanced Technology Laboratories, Bothell, WA), with 5–12-MHz linear array transducers. Ultrasonography-guided FNA was performed by one of the seven board-certified radiologists with more than 2 years of experience in thyroid ultrasonography. All ultrasonography-guided FNAs were accomplished using the freehand technique, with a 23-gauge needle tip and a 2-ml syringe. In general, multiple excursions were made through the lesion.
The Bethesda system was not routinely used at the time of this study in our hospital. With respect to cytological results, “inconclusive” was defined as “non-diagnostic”, “indeterminate nodule (i.e. follicular neoplasm vs nodular hyperplasia)”, “lesions that are cytologically benign but malignant ultrasonography features” and “suspicious for malignancy”, for which the histological subtype or surgical event had to be determined. The sonographic findings of these nodules were retrospectively reviewed by two experienced radiologists. We classified 88 cytologically inconclusive thyroid nodules into three groups based on ultrasonography features: Group A, which was suspicious for papillary thyroid carcinoma (PTC) and had at least one of the malignant ultrasonography features including microcalcifications, taller-than-wide shape, infiltrative margin and marked hypoechogenicity [13]; Group B, which was suspicious for follicular (Hurthle cell) neoplasm and showed oval-to-round iso- to hypoechoic nodules with or without halo and had no evidence of malignant ultrasonography features; and Group C, which was suspicious for lymphoma and showed diffuse heterogeneous hypoechoic parenchyma with intervening echogenic septa-like structures and a history of a rapidly growing mass [14].
The ultrasonography-guided CNB was performed using the freehand technique with a spring-activated, short-throw (1.1-cm excursion) 18-gauge trucut-type needle (Acecut®; TSK Laboratory, Tochigi-ken, Japan) by one of the seven board-certified radiologists with more than 2 years of experience in thyroid biopsy. At least three biopsy cores were obtained. The specimen was fixed in a formalin solution. The biopsy specimens were sent to the pathology department. The category “persistent inconclusive CNB” based on reports was defined as “lacking the definite diagnostic term (benign vs malignant)” and “the same results as the previous inconclusive cytology (e.g. follicular neoplasm vs nodular hyperplasia)”.
Data and statistical analysis
The final diagnosis after surgery or follow-up imaging served as the standard reference for the results of ultrasonography-guided CNB. We analysed and compared the diagnostic efficacy and malignancy detection rates of ultrasonography-guided CNB among Groups A, B and C. Statistical analyses were performed using SPSS® v. 19 for Windows (SPSS Inc., Chicago, IL). The χ2 and Fisher exact tests were used for the comparative analysis between the study groups; p<0.05 was considered to be statistically significant.
RESULTS
Histological diagnosis after ultrasonography-guided CNB
Of a total of 88 nodules examined by previous inconclusive ultrasonography-guided FNA, 76 (86.4%) were conclusive and 12 (13.6%) were persistently inconclusive with ultrasonography-guided CNB. Finally, the prevalence of malignancy in the 88 nodules was 49.1% (26 of 53) in Group A, 12.0% (3 of 25) in Group B and 90.0% (9 of 10) in Group C. The prevalence of persistently inconclusive ultrasonography-guided CNB results after previous incomplete ultrasonography-guided FNA results was 3.8% (2 of 53) in Group A, 36.0% (9 of 25) in Group B and 10.0% (1 of 10) in Group C (p=0.001) (Table 1).
Table 1.
Ultrasonography-guided core needle biopsy (CNB) and final results of 88 thyroid nodules with inconclusive ultrasonography-guided fine-needle aspiration (FNA) results
| Variable | Group A (n=53) | Group B (n=25) | Group C (n=10) | p-value |
| Ultrasonography-guided CNB results | 0.001 | |||
| Benign | 25 (47.1) | 15 (60.0) | 1 (10.0) | |
| Malignant | 26 (49.1) | 1 (4.0) | 8 (80.0) | |
| Persistent inconclusive | 2 (3.8) | 9 (36.0) | 1 (10.0) | |
| Final results | ||||
| Benign | 26 (49.1) | 22 (88.0) | 1 (10.0) | |
| Malignant | 27 (50.9) | 3 (12.0) | 9 (90.0) | |
| Mean size (cm) | 1.4±1.2 (range 0.3–6.0) | 1.7±0.9 (range 0.4–3.5) | 8.6±3.0 (range 2.0–10.0) | |
Data are the number of lesions. Numbers in parentheses are percentages.
Group A (suspicious for papillary thyroid carcinoma)
In Group A, there were 53 thyroid nodules with inconclusive results of ultrasonography-guided FNA. Of these 53 nodules, 51 thyroid nodules (96.2%) showed conclusive results after ultrasonography-guided CNB (Figure 1). Among the 51 conclusive thyroid nodules, 26 nodules (49.1%) were malignant (16 PTCs, 5 anaplastic carcinomas, 3 metastases, 1 medullary carcinoma and 1 poorly differentiated carcinoma) and 25 nodules (47.2%) were benign (14 nodular hyperplasia, 6 chronic lymphocytic thyroiditis, 2 dystrophic calcification with fibrous tissue, 1 Hashimoto’s thyroiditis, 1 granuloma and 1 cyst). The remaining two nodules (3.8%) showed persistently inconclusive results after ultrasonography-guided CNB. Finally, these two nodules were confirmed as nodular hyperplasia and PTC. The mean size of thyroid nodules in Group A was 1.4±1.2 cm (range 0.3–6.0 cm). We found no statistical difference between the benign and the malignant nodules with regard to size (1.1±0.6 cm vs 2.0±2.2 cm, p=0.355). Surgery was performed in 18 (34.0%) of the 53 nodules.
Figure 1.
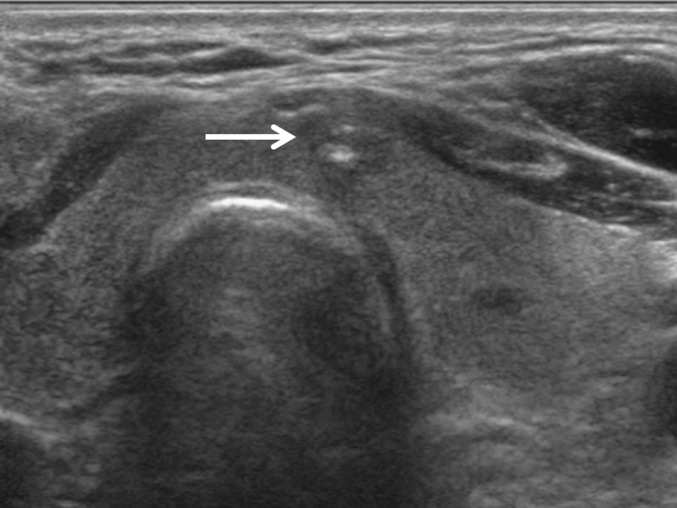
Group A. Suspicious for papillary thyroid carcinoma (PTC) seen on ultrasonography. A 55-year-old male had a 0.7-cm-sized irregular hypoechoic nodule (arrow) with microcalcification in the paraisthmic portion of the left thyroid gland, which was inconclusive at repeated ultrasonography-guided fine-needle aspiration cytology. Ultrasonography-guided core needle biopsy finally revealed PTC and the patient underwent surgery.
Group B (suspicious for follicular neoplasm)
After ultrasonography-guided FNA, 25 inconclusive thyroid nodules were categorised as Group B. In Group B, there were 16 conclusive thyroid nodules (64.0%) after ultrasonography-guided CNB. Among the 16 conclusive thyroid nodules, 15 nodules (60.0%) were diagnosed as nodular hyperplasia and 1 nodule (4.0%) as medullary thyroid carcinoma. The remaining 9 (36.0%) nodules showed persistently inconclusive results after ultrasonography-guided CNB, which still failed to differentiate nodular hyperplasia from follicular neoplasm with biopsy specimens (Figure 2). Of nine nodules, six were confirmed as nodular hyperplasia (three), PTCs (two) and chronic lymphocytic thyroiditis (one). The other three nodules still remained inconclusive after ultrasonography-guided CNB, which showed no interval changes on 23.4-month follow-up. The mean size of the thyroid nodules in Group B was 1.7±0.9 cm (range 0.4–3.5 cm). There was no significant size difference between conclusive and persistently inconclusive thyroid nodules (1.9±0.7 cm vs 2.9±3.5 cm, p=0.545). 9 (36.0%) out of 25 nodules were confirmed surgically.
Figure 2.

Group B. Suspicious for follicular (Hurthle cell) neoplasm seen on ultrasonography. A 43-year-old female had a 1.1-cm-sized round isoechoic nodule (arrow) with a peripheral halo in the right thyroid gland on ultrasonography, which was inconclusive at ultrasonography-guided fine-needle aspiration cytology. Ultrasonography-guided core needle biopsy was still not conclusive in that nodular hyperplasia and Hurthle cell neoplasm were considered in the differential diagnosis. Surgery confirmed nodular hyperplasia.
Group C (suspicious for lymphoma)
In Group C, there were 10 thyroid nodules with inconclusive results on ultrasonography-guided FNA. 9 out of 10 nodules (90.0%) had conclusive results after ultrasonography-guided CNB (Figure 3). These conclusive nodules revealed 8 (80.0%) lymphomas and 1 (10.0%) Hashimoto’s thyroiditis. The mean size of the thyroid nodules in Group C was 8.6±3.0 cm (range 2.0–10.0 cm). The diagnosis of only one lymphoma with an inconclusive CNB result was achieved with surgical specimens.
Figure 3.
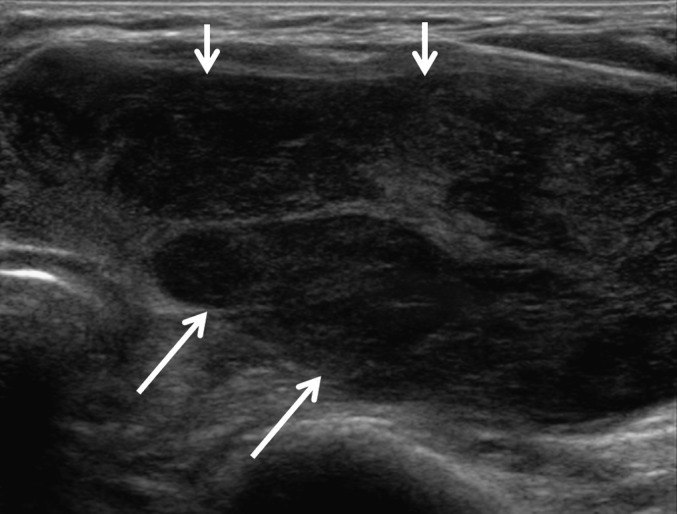
Group C. Suspicious for primary lymphoma seen on ultrasonography. An 82-year-old female had diffuse heterogeneous hypoechoic parenchyma (arrows) with intervening echogenic septa-like structures in the left thyroid gland. She had a history of a rapidly growing mass. The cytological result showed the differential diagnosis of florid lymphoid hyperplasia and non-Hodgkin’s lymphoma and was inconclusive. Ultrasonography-guided core needle biopsy diagnosed diffuse large B-cell lymphoma.
Previous ultrasonography-guided FNA and ultrasonography-guided CNB results
The relationship between the number of previous ultrasonography-guided FNA and ultrasonography-guided CNB results is summarised in Table 2. A previous ultrasonography-guided FNA was performed once in 60 nodules (68.2%; 35 nodules in Group A, 15 nodules in Group B and 10 nodules in Group C), twice in 18 nodules (20.5%; 11 nodules in Group A and 7 nodules in Group B) and at least 3 times in 10 nodules (11.3%; 7 nodules in Group A and 3 nodules in Group B). The number of previous ultrasonography-guided FNA biopsies was not significantly different between conclusive and persistently inconclusive results after ultrasonography-guided CNB (p=0.205). There were no significant complications during or after CNB.
Table 2.
Relation between the number of previous ultrasonography-guided fine-needle aspiration (FNA) and ultrasonography-guided core needle biopsy (CNB) results
| Number of ultrasonography-guided FNAs | Number of nodules (n=88) | Ultrasonography-guided CNB results |
Ultrasonography-guided CNB results |
p-value | |||
| Group A | Group B | Group C | Conclusive (n=76) | Inconclusive (n=12) | |||
| 1 | 60 (68.2) | 35 (66.0) | 15 (60.0) | 10 (100.0) | 54 (71.1) | 6 (50.0) | 0.205 |
| 2 | 18 (20.5) | 11 (20.8) | 7 (28.0) | 0 | 14 (18.4) | 4 (33.3) | |
| ≥3 | 10 (11.3) | 7 (13.2) | 3 (12.0) | 0 | 8 (10.5) | 2 (16.7) | |
Data are numbers of lesions. Numbers in parentheses are percentages.
DISCUSSION
The ultrasonography-guided FNA analysis of the thyroid gland will continue to have a central role in the investigation of patients with nodular disease of the thyroid gland. However, there remains the question of how to approach the 5–20% of patients with non-diagnostic results [15] and the 1–11% of patients with false-negative results [16,17]. Repeat ultrasonography-guided FNA is recommended for thyroid nodules with initially non-diagnostic cytology results to avoid unnecessary surgery [15,18,19]. Nevertheless, the persistently non-diagnostic rates for nodules with initially non-diagnostic results on FNA are reported to be 20–38% [15,20,21]. In addition, the third FNA for thyroid nodules with two consecutive non-diagnostic cytology results is less likely to be diagnostic [15]. Although the guidelines of the American Thyroid Association and the American Association of Clinical Endocrinologists recommend surgery for thyroid nodules with persistently non-diagnostic cytology results, it is true that surgery for all thyroid nodules with persistently non-diagnostic cytology results is not cost-effective, not to mention the increased morbidity and unnecessary post-operative medication for patients. However, the malignancy rate for thyroid nodules with one or two consecutive non-diagnostic results on FNA is reported to be 12–14% [16,20,22,23]. Thus, besides repeat ultrasonography-guided FNA or surgery, there is a need for further guidelines for thyroid nodules with persistently inconclusive cytology results. Several studies have demonstrated that ultrasonography-guided CNB of the thyroid gland is a safe technique with high yield and accuracy [24–26] and can effectively reduce non-diagnostic readings when compared with repeat ultrasonography-guided FNA of thyroid nodules with non-diagnostic cytology results [10,11,27].
Although the malignancy rate demonstrated the lowest value in Group B (Group A, 49.1%; Group B, 12.0%; and Group C, 90.0%), persistently inconclusive results after ultrasonography-guided CNB were most frequently obtained in Group B (36.0%). The reason for this is because a follicular adenoma cannot be differentiated from a low-grade follicular carcinoma without examination of the entire nodule for evidence of capsular or vascular invasion [26]. Many studies have attempted to improve the pre-operative diagnosis of follicular neoplasm by FNA or imaging characteristics on ultrasonography, but there is still no accurate way for predicting the risk of malignancy [10,28–31]. In this study, we found that the use of CNB would have reduced the inconclusive rate for Group B by 36.0%. For these 36% of indeterminate nodules, surgery still plays an important role by allowing a confirmative diagnosis. We also found that ultrasonography-guided CNB could be useful for accurate diagnosis in cases suspicious for PTC (i.e. Group A) or thyroid lymphoma (i.e. Group C). These were consistent with other reports, suggesting that ultrasonography-guided CNB might be a suitable replacement for repeat FNA [10,11] or diagnostic thyroid surgery [14].
In our study, the numbers of thyroid nodules with persistently inconclusive results after ultrasonography-guided CNB were 2 (3.8%) in Group A, 9 (36.0%) in Group B and 1 (10.0%) in Group C (p=0.001), respectively. These 12 persistently inconclusive thyroid nodules were finally diagnosed as benign thyroid lesions, including nodular hyperplasia and chronic lymphocytic thyroiditis (n=8), PTC (n=3) and lymphoma (n=1). Although ultrasonography-guided CNB performed by experienced radiologists is a safe and well-tolerated procedure with advantages including larger tissue sample, less operator dependency if the needle successfully penetrates the nodule, capability of assessment of the histological architecture and relation to the adjacent thyroid tissue [6,7], there are still certain possible complications and technical difficulties. Compared with FNA, ultrasonography-guided CNB may be technically difficult in some cases (especially in small nodules located in the posterior portion of the thyroid gland or very close to the carotid artery or trachea). In our 12 cases with persistently inconclusive results, 5 nodules were <1 cm and 4 nodules were located in the posterior portion of the thyroid gland or very close to the main vascular structures. These small sizes and particular locations could lead to persistently inconclusive results owing to inaccurate targeting. The remaining three nodules showed mainly fibrosis and a few benign follicular cells in the specimen. The confirmation failure of core biopsy for the fibrotic portion within the relatively large nodule could also produce inconclusive results. When a large nodule shows heterogeneous components on ultrasonography, biopsies should be performed in different areas to avoid the inconclusive results [32].
In this study, the number of previous ultrasonography-guided FNA biopsies was not significantly different between the conclusive and the inconclusive result groups of ultrasonography-guided CNB (p=0.205). In other words, inconclusive results can be persistently obtained despite the repeat ultrasonography-guided FNAs for the definitive diagnosis. However, CNB produces a histological sample that retains its cytological appearances and its tissue architecture. The histological sample is familiar to most pathologists, and the larger amount of tissue permits the use of a range of immunohistochemical stains and may provide a more precise histological diagnosis [6,33,34].
There are some limitations in this study. First, our study was designed as a retrospective study. Thus, there could be selection bias owing to inclusion of only cytologically inconclusive cases because indications for ultrasonography-guided CNB had not been flexible at the time of study. This may cause underestimation of the ultrasonography-guided CNB performance. Second, we did not consider the differences in experience levels of the radiologists performing ultrasonography-guided CNB. Finally, surgical confirmation was not obtained in 39 patients with benign thyroid nodules and in 3 patients with inconclusive thyroid nodules. Even though we set a standard follow-up period of at least 1 year in these study populations, this follow-up period may not be long enough to exclude a slow-growing malignancy.
In conclusion, the rates of conclusive ultrasonography-guided CNB results after previous incomplete ultrasonography-guided FNA results were 96.2% in Group A (suspicious for PTC), 64.0% in Group B (suspicious for follicular neoplasm) and 90.0% in Group C (suspicious for lymphoma). Ultrasonography-guided CNB has benefit for the diagnosis of thyroid nodules with inconclusive ultrasonography-guided FNA results. However, it is still not helpful for the differential diagnosis in 36% of nodules that are suspicious for follicular neoplasm seen on ultrasonography.
REFERENCES
- 1.Yassa L, Cibas ES, Benson CB, Frates MC, Doubilet PM, Gawande AA, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer 2007;111:508–16 doi: 10.1002/cncr.23116 [DOI] [PubMed] [Google Scholar]
- 2.Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer 2007;111:306–15 doi: 10.1002/cncr.22955 [DOI] [PubMed] [Google Scholar]
- 3.Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer 2009;117:195–202 doi: 10.1002/cncy.20029 [DOI] [PubMed] [Google Scholar]
- 4.Ko MS, Jeong KS, Shong YK, Gong GY, Baek JH, Lee JH. Collapsing benign cystic nodules of the thyroid gland: sonographic differentiation from papillary thyroid carcinoma. AJNR Am J Neuroradiol 2012;33:124–7 doi: 10.3174/ajnr.A2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin JH, Han BK, Ko EY, Kim SJ, Mun SH, Oh YL, et al. Imaging-pathology discordant thyroid nodules: analysis of causes on ultrasonography. J Korean Thyroid Assoc 2008;1:112–16 [Google Scholar]
- 6.Lo Gerfo P, Colacchio T, Caushaj F, Weber C, Feind C. Comparison of fine-needle and coarse-needle biopsies in evaluating thyroid nodules. Surgery 1982;92:835–8 [PubMed] [Google Scholar]
- 7.Renshaw AA, Pinnar N. Comparison of thyroid fine-needle aspiration and core needle biopsy. Am J Clin Pathol 2007;128:370–4 doi: 10.1309/07TL3V58337TXHMC [DOI] [PubMed] [Google Scholar]
- 8.Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid 2009;19:1159–65 doi: 10.1089/thy.2009.0274 [DOI] [PubMed] [Google Scholar]
- 9.Baloch ZW, Cibas ES, Clark DP, Layfield LJ, Ljung BM, Pitman MB, et al. The National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference: a summation. Cytojournal 2008;5:6 doi: 10.1186/1742-6413-5-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park KT, Ahn SH, Mo JH, Park YJ, Park do J, Choi SI, et al. Role of core needle biopsy and ultrasonographic finding in management of indeterminate thyroid nodules. Head Neck 2011;33:160–5 doi: 10.1002/hed.21414 [DOI] [PubMed] [Google Scholar]
- 11.Na DG, Kim JH, Sung JY, Baek JH, Jung KC, Lee H, et al. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid 2012;22:468–75 doi: 10.1089/thy.2011.0185 [DOI] [PubMed] [Google Scholar]
- 12.Samir AE, Vij A, Seale MK, Desai G, Halpern E, Faquin WC, et al. Ultrasound-guided percutaneous thyroid nodule core biopsy: clinical utility in patients with prior nondiagnostic fine-needle aspirate. Thyroid 2012;22:461–7 doi: 10.1089/thy.2011.0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol 2011;12:1–14 doi: 10.3348/kjr.2011.12.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam M, Shin JH, Han BK, Ko EY, Ko ES, Hahn SY, et al. Thyroid lymphoma: correlation of radiologic and pathologic features. J Ultrasound Med 2012;31:589–94 [DOI] [PubMed] [Google Scholar]
- 15.Alexander EK, Heering JP, Benson CB, Frates MC, Doubilet PM, Cibas ES, et al. Assessment of nondiagnostic ultrasound-guided fine needle aspirations of thyroid nodules. J Clin Endocrinol Metab 2002;87:4924–7 [DOI] [PubMed] [Google Scholar]
- 16.Gharib H. Fine-needle aspiration biopsy of thyroid nodules: advantages, limitations, and effect. Mayo Clin Proc 1994;69:44–9 [DOI] [PubMed] [Google Scholar]
- 17.Sung JY, Na DG, Kim KS, Yoo H, Lee H, Kim JH, et al. Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol 2012;22:1564–72 doi: 10.1007/s00330-012-2405-6 [DOI] [PubMed] [Google Scholar]
- 18.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–214 doi: 10.1089/thy.2009.0110 [DOI] [PubMed] [Google Scholar]
- 19.Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. J Endocrinol Invest 2010;33:51–6 [PubMed] [Google Scholar]
- 20.Chow LS, Gharib H, Goellner JR, van Heerden JA. Nondiagnostic thyroid fine-needle aspiration cytology: management dilemmas. Thyroid 2001;11:1147–51 doi: 10.1089/10507250152740993 [DOI] [PubMed] [Google Scholar]
- 21.Baloch Z, LiVolsi VA, Jain P, Jain R, Aljada I, Mandel S, et al. Role of repeat fine-needle aspiration biopsy (FNAB) in the management of thyroid nodules. Diagn Cytopathol 2003;29:203–6 doi: 10.1002/dc.10361 [DOI] [PubMed] [Google Scholar]
- 22.McHenry CR, Walfish PG, Rosen IB. Non-diagnostic fine needle aspiration biopsy: a dilemma in management of nodular thyroid disease. Am Surg 1993;59:415–19 [PubMed] [Google Scholar]
- 23.Moon HJ, Kwak JY, Choi YS, Kim EK. How to manage thyroid nodules with two consecutive non-diagnostic results on ultrasonography-guided fine-needle aspiration. World J Surg 2012;36:586–92 doi: 10.1007/s00268-011-1397-8 [DOI] [PubMed] [Google Scholar]
- 24.Taki S, Kakuda K, Kakuma K, Annen Y, Katada S, Yamashita R, et al. Thyroid nodules: evaluation with US-guided core biopsy with an automated biopsy gun. Radiology 1997;202:874–7 [DOI] [PubMed] [Google Scholar]
- 25.Quinn SF, Nelson HA, Demlow TA. Thyroid biopsies: fine-needle aspiration biopsy versus spring-activated core biopsy needle in 102 patients. J Vasc Interv Radiol 1994;5:619–23 [DOI] [PubMed] [Google Scholar]
- 26.Screaton NJ, Berman LH, Grant JW. US-guided core-needle biopsy of the thyroid gland. Radiology 2003;226:827–32 doi: 10.1148/radiol.2263012073 [DOI] [PubMed] [Google Scholar]
- 27.Titton RL, Gervais DA, Boland GW, Maher MM, Mueller PR. Sonography and sonographically guided fine-needle aspiration biopsy of the thyroid gland: indications and techniques, pearls and pitfalls. AJR Am J Roentgenol 2003;181:267–71 doi: 10.2214/ajr.181.1.1810267 [DOI] [PubMed] [Google Scholar]
- 28.Schlinkert RT, van Heerden JA, Goellner JR, Gharib H, Smith SL, Rosales RF, et al. Factors that predict malignant thyroid lesions when fine-needle aspiration is “suspicious for follicular neoplasm”. Mayo Clin Proc 1997;72:913–16 doi: 10.1016/S0025-6196(11)63360-0 [DOI] [PubMed] [Google Scholar]
- 29.Choi YJ, Yun JS, Kim DH. Clinical and ultrasound features of cytology diagnosed follicular neoplasm. Endocr J 2009;56:383–9 [DOI] [PubMed] [Google Scholar]
- 30.Sahin M, Gursoy A, Tutuncu NB, Guvener DN. Prevalence and prediction of malignancy in cytologically indeterminate thyroid nodules. Clin Endocrinol (Oxf) 2006;65:514–18 doi: 10.1111/j.1365-2265.2006.02625.x [DOI] [PubMed] [Google Scholar]
- 31.Koike E, Noguchi S, Yamashita H, Murakami T, Ohshima A, Kawamoto H, et al. Ultrasonographic characteristics of thyroid nodules: prediction of malignancy. Arch Surg 2001;136:334–7 [DOI] [PubMed] [Google Scholar]
- 32.Jung SL, Jung CK, Kim SH, Kang BJ, Ahn KJ, Kim BS, et al. Histopathologic findings related to the indeterminate or inadequate results of fine-needle aspiration biopsy and correlation with ultrasonographic findings in papillary thyroid carcinomas. Korean J Radiol 2010;11:141–8 doi: 10.3348/kjr.2010.11.2.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crile G, Jr, Hawk WA., Jr Aspiration biopsy of thyroid nodules. Surg Gynecol Obstet 1973;136:241–5 [PubMed] [Google Scholar]
- 34.Wang C, Vickery AL, Jr, Maloof F. Needle biopsy of the thyroid. Surg Gynecol Obstet 1976;143:365–8 [PubMed] [Google Scholar]


