Abstract
Purpose
To determine the clinical and biologic effects of bevacizumab, an anti–vascular endothelial growth factor (VEGF) monoclonal antibody, in unresectable hepatocellular carcinoma (HCC).
Patients and Methods
Adults with organ-confined HCC, Eastern Cooperative Oncology Group performance status of 0 to 2, and compensated liver disease were eligible. Patients received bevacizumab 5 mg/kg (n = 12) or 10 mg/kg (n = 34) every 2 weeks until disease progression or treatment-limiting toxicity. The primary objective was to determine whether bevacizumab improved the 6-month progression-free survival (PFS) rate from 40% to 60%. Secondary end points included determining the effects of bevacizumab on arterial enhancement and on plasma cytokine levels and the capacity of patients’ plasma to support angiogenesis via an in vitro assay.
Results
The study included 46 patients, of whom six had objective responses (13%; 95% CI, 3% to 23%), and 65% were progression free at 6 months. Median PFS time was 6.9 months (95% CI, 6.5 to 9.1 months); overall survival rate was 53% at 1 year, 28% at 2 years, and 23% at 3 years. Grade 3 to 4 adverse events included hypertension (15%) and thrombosis (6%, including 4% with arterial thrombosis). Grade 3 or higher hemorrhage occurred in 11% of patients, including one fatal variceal bleed. Bevacizumab was associated with significant reductions in tumor enhancement by dynamic contrast-enhanced magnetic resonance imaging and reductions in circulating VEGF-A and stromal-derived factor-1 levels. Functional angiogenic activity was associated with VEGF-A levels in patient plasma.
Conclusion
We observed significant clinical and biologic activity for bevacizumab in nonmetastatic HCC and achieved the primary study end point. Serious bleeding complications occurred in 11% of patients. Further evaluation is warranted in carefully selected patients.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer deaths worldwide and the most rapidly increasing cause of cancer death in the United States.1 Hepatitis B virus, alcohol abuse, hepatitis C virus, and fatty liver disease are major risk factors for HCC.2,3
Current treatment modalities for localized HCC include percutaneous ethanol injection, transarterial chemoembolization, hepatic resection, and liver transplantation. Recently, sorafenib, an oral multitargeted tyrosine kinase inhibitor against vascular endothelial growth factor (VEGF) and raf kinase was shown to prolong survival in advanced HCC in a phase III trial.4
HCCs are hypervascular tumors in which angiogenesis contributes to growth and metastasis.5–7 HCC neovascularization is mediated by growth factors, particularly VEGF.8,9 Bevacizumab is a recombinant humanized version of a murine antihuman VEGF monoclonal antibody. Because of the role of angiogenesis in HCC progression, we sought to determine the clinical and biologic effects of bevacizumab in patients with advanced HCC. Clinical effects were assessed via tumor regression and progression-free survival (PFS). Biologic effects were evaluated in a subset of patients in three ways. First, we examined tumor vascularity using dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) imaging.10 Second, we measured the effects of bevacizumab on circulating VEGF-A and stromal-derived factor-1 (SDF-1), which are biomarkers that have been used to evaluate effectiveness of antiangiogenic treatment.11 Third, we evaluated the capacity of patients’ plasma to support angiogenesis via an in vitro assay.
PATIENTS AND METHODS
The protocol was approved by the Cancer Therapy Evaluation Program of the National Cancer Institute (P5611) and each site’s institutional review board. All patients provided written informed consent. Patients were required to have HCC confirmed by biopsy or diagnosed by clinical criteria. These criteria included cirrhosis or chronic hepatitis B or hepatitis C infection, hypervascular liver masses more than 2 cm, and either serum α-fetoprotein (AFP) more than 400 ng/mL or AFP greater than three times normal and doubling in value in the antecedent 3 months. Other eligibility requirements included surgically unresectable disease; age 18 years or greater; Eastern Cooperative Oncology Group performance status of 0 to 2; no more than one prior antineoplastic chemotherapy; and adequate organ function including hepatic (total bilirubin ≤ 3 mg/dL, albumin ≥ 2.5 mg/dL, and Child-Pugh Class A or compensated Class B liver dysfunction [no refractory ascites or encephalopathy]), hematologic (international normalized ratio < 1.5, absolute neutrophil count > 1,500/μL, and platelets ≥ 75,000/μL), and renal function (creatinine < 1.5 g/dL and urinary protein < 500 mg/24 h). Exclusion criteria included extrahepatic disease; tumor invasion of the main portal vein or inferior vena cava; greater than 50% tumor involvement in the liver; thromboembolic event within the last 12 months; full-dose anticoagulation therapy and/or aspirin at 325 mg/d or more or other platelet-inhibitory agents; clinically significant cardiovascular disease (uncontrolled hypertension, unstable angina, congestive heart failure, or New York Heart Association grade 2 or greater), serious cardiac arrhythmia, or clinically significant (grade 2 or greater) peripheral vascular disease within 1 year before study entry; and documented brain metastases, cerebrovascular disease, or seizures. Those on an active liver transplantation list and/or considered likely to receive a liver transplantation in the coming 6 months were not eligible.
Patients who had undergone needle biopsy (core or fine-needle aspiration) could not begin therapy for 14 days after procedure; those who had any other surgical procedure could not begin therapy for 28 days. After a fatal variceal bleed in the second enrolled patient, the protocol was amended so that patients with a history of prior varices or evidence of varices on prestudy computed tomography/MRI imaging were required to undergo endoscopy within 4 weeks of study entry. Those who had received banding and/or sclerotherapy and had not bled within the prior 6 months were eligible, provided that a re-endoscopy was performed and that varices remained obliterated, minimal, or grade 1.
Treatment Plan
Patients received intravenous infusion of bevacizumab (5 or 10 mg/kg) every 2 weeks until disease progression or development of treatment-limiting toxicity. We had initially planned to treat the first six patients at a 5 mg/kg dose level, with escalation at that time to 10 mg/kg. However, given early toxicities, this cohort was expanded to include an additional six patients at the 5 mg/kg dose as described in Results.
Toxicity Evaluation and Dose Modification
Adverse events/toxicities were categorized and graded by the Common Terminology Criteria of Adverse Events (version 3.0). Patients developing treatment-limiting toxicity had bevacizumab withheld until resolution to grade 1 or less and were then restarted on treatment at half dose. Hepatic or neutrophil toxicity had to resolve to grade 2 or better and the platelet count had to improve to 75,000/μL before retreatment. Patients who developed more than 2 g/24 h of proteinuria during therapy did not receive additional bevacizumab until proteinuria resolved to less than 2 g/24 h. Criteria for discontinuation included more than one dose reduction; grade 3 or 4 (or other treatment-limiting) toxicity developing after a dose reduction; grade 3 toxicity persisting for more than 3 weeks or recurring after resumption of treatment; platelet counts less than 20,000/μL; grade 3 or 4 hemorrhage or thrombosis; grade 4 hypertension; disease progression, and patient request or refusal to undergo therapy or assessments.
Statistical Considerations
The primary end point was PFS; a 6-month PFS of 40% was considered likely in the absence of effective therapy or minimally effective therapy.12 To distinguish between a 60% 6-month PFS expected for bevacizumab and a 40% 6-month PFS expected for supportive care, assuming α and β error rates of 0.10, 46 patients were required with an initial assessment for futility after enrolling 18 patients. For the final analysis, a 6-month PFS in 23 or more patients was considered promising. Survival and PFS were analyzed using Kaplan-Meier product-limit estimating techniques. All data were analyzed using SAS system software (SAS Institute, Cary, NC). Comparisons of baseline measurements with 8-week measurements for patients (and 8-week measurements with measurements at time of progression) were made using the paired t test and the Wilcoxon signed rank test. Correlations of arterial enhancement and angiogenic scale variables were made using Spearman’s rank order correlation.
Correlative Studies
Eight patients from one site (Mt. Sinai, New York, NY) receiving bevacizumab 5 or 10 mg/kg underwent pretreatment DCE-MRI evaluation and a subsequent MRI after 8 weeks of therapy. These same patients also underwent biomarker analyses and evaluation for angiogenic scale. The MRI protocol used a routine liver sequence performed on either 1.5-tesla Signa (GE Healthcare, London, United Kingdom) or Sonata (Siemens, Munich, Germany) machines. Data were collected from the three-dimensional, spoiled gradient echo, fat-saturated, T1-weighted sequence. Signal intensity was assessed from non-necrotic portions of tumors, and a second region of interest was assessed in adjacent non-neoplastic liver parenchyma.13 All pretreatment MRI assessments were performed between 3 and 13 days before initial bevacizumab dose; all post-treatment assessments were performed within 13 days of receiving the fourth bevacizumab dose.
Assessment of Circulating VEGF-A, SDF-1, and Human Umbilical Vein Endothelial Cell–Based Angiogenic Scale
Plasma was isolated from patients before bevacizumab therapy (between 1 and 14 days before treatment), at 8 weeks, and at time of HCC progression on therapy (no greater than 21 days after a final dose of bevacizumab). Plasma was collected in sodium citrate containers, placed on ice, centrifuged within 45 minutes of collection at 1,000 ×g for 15 minutes, and stored at −80°C. Plasma levels of VEGF-A and SDF-1 were determined by commercially available enzyme-linked immunosorbent assay kits according to the manufacturer’s recommendation (Quantikine; R&D Systems, Minneapolis, MN).
A functional bioassay (angiogenic scale) was used to evaluate plasma angiogenic activity. Early passaged human umbilical vein endothelial cells (HUVECs) were maintained in enriched endothelial cell culture medium until approximately 70% confluent in six-well plates precoated with gelatin and then changed to serum-free medium. Patient plasma was then added to the cells to a final concentration of 2.5% (in triplicate). After 24 hours of incubation at 37°C and 5% carbon dioxide, angiogenic morphology was examined under light microscopy and scored by two independent observers according to the following arbitrary angiogenic scale: 0 = well-separated individual cells; 1 =cells demonstrating migration and alignment; 2 =visible capillary tubes; 3 =sprouting of new capillary tubes; 4 =formation of polygonal structures; and 5 = presence of complex mesh-like structures. Plasma samples from age-matched healthy volunteers were tested concurrently with the patient samples for each batch of HUVECs to ensure a baseline angiogenic score of 0.
RESULTS
Forty-six patents were enrolled between February 2003 and September 2006, including 12 patients who received bevacizumab 5 mg/kg and 34 patients who received bevacizumab 10 mg/kg every 2 weeks. Patient characteristics are listed in Table 1. The median age was 58 years. Eighty-three percent of patients were male, and 95% had an Eastern Cooperative Oncology Group performance status of 0 or 1. One third of patients had received no prior therapy.
Table 1.
Baseline Patient Characteristics
| Characteristic | No. of Patients | %* |
|---|---|---|
| Age, years | ||
| Median | 58 | |
| Mean | 58.8 | |
| Range | 21–81 | |
|
| ||
| Sex | ||
| Male | 38 | 83 |
| Female | 8 | 17 |
|
| ||
| Race | ||
| White | 25 | 54 |
| Black | 2 | 4 |
| Hispanic | 5 | 11 |
| Asian | 14 | 31 |
|
| ||
| ECOG status | ||
| 0 | 19 | 43 |
| 1 | 23 | 52 |
| 2 | 2 | 5 |
|
| ||
| CLIP score | ||
| 0 | 2 | 4 |
| 1 | 19 | 42 |
| 2 | 15 | 33 |
| 3 | 8 | 18 |
| 4 | 1 | 2 |
| Median score | 2 | |
|
| ||
| Prior treatment | ||
| None | 15 | 33 |
| TACE | 19 | 41 |
| Resection | 12 | 26 |
| Chemotherapy/radiation | 2 | 4 |
| Transplantation | 1 | 2 |
| Ablation | 7 | 15 |
|
| ||
| Etiology | ||
| HCV | 20 | 43 |
| Alcohol | 14 | 30 |
| HBV | 11 | 24 |
| Other | 6 | 13 |
|
| ||
| Maximum tumor size, cm | ||
| < 5 | 21 | 46 |
| 5–10 | 18 | 39 |
| > 10 | 7 | 15 |
|
| ||
| AFP, ng/mL | ||
| < 400 | 28 | 62 |
| 400–1,000 | 7 | 16 |
| > 1,000 | 10 | 22 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; CLIP score, Cancer of the Liver Italian Program score (includes Child-Pugh stage, tumor morphology and extension, serum AFP levels, and evidence of portal vein thrombosis); TACE, transarterial chemoembolization; HCV, hepatitis C virus; HBV, hepatitis B virus; AFP, α-fetoprotein.
Percentages may not add up to exactly 100% because of missing data or more than one prior treatment or etiology per patient.
Treatment Administered and Safety
We had initially planned to treat the first six patients at 5 mg/kg and then escalate to 10 mg/kg in subsequent patients. However, one patient died within the first 5 days after initial dose (attributed to spontaneous bacterial peritonitis), and another patient died within 30 days of treatment from a variceal bleed. As a result, we extended the safety evaluation period at the 5 mg/kg dose to another six patients before escalating the dose. No additional fatal treatment-associated events were seen. After the variceal bleed, the protocol was amended to more readily identify and exclude patients with esophageal varices as detailed in Patients and Methods. There were no subsequent variceal bleeding episodes after this amendment.
For the 12 patients treated at the 5 mg/kg dose level, the mean and median numbers of bevacizumab cycles administered were 15 and 15 (range, one to 48 cycles), respectively. For the 34 patients treated at the 10 mg/kg dose level, the mean and median numbers of treatment cycles administered were 12 and nine cycles (range, one to 45 cycles), respectively.
Adverse Events
Major adverse events are listed in Table 2. The most common grade 3 or 4 toxicities included hypertension (15%), thrombosis (6%; 4% arterial), and major bleeding (11%). There was one incidence of fatal variceal bleeding in the second patient enrolled at the 5 mg/kg dose (as noted earlier), one grade 4 hemoperitoneum, and one grade 3 intratumoral hemorrhage. No further variceal bleeding was seen after protocol modification. The arterial thrombotic events were a grade 3 transient ischemic attack and grade 4 hepatic arterial thrombosis. Two patients were removed from the trial for uncontrolled hypertension, whereas the rest improved with treatment. The increased bilirubin levels typically occurred in the setting of other adverse events or disease progression. For instance, the patients with hepatic arterial thrombosis and hemoperitoneum both had grade 3 bilirubin levels.
Table 2.
Major Toxicities/Adverse Effects Possibly Attributed to Bevacizumab
| Toxicity | All Grades
|
Grades 3 and 4
|
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Hypertension | 15 | 33 | 7 | 15 |
|
| ||||
| Proteinuria | 19 | 41 | 2 | 4 |
|
| ||||
| Epistaxis | 5 | 11 | 0 | 0 |
|
| ||||
| Hemorrhage | 12 | 26 | 5 | 11 |
|
| ||||
| Arterial thrombosis | 2 | 4 | 2 | 4 |
|
| ||||
| Venous thrombosis | 1 | 2 | 1 | 2 |
|
| ||||
| Rash | 6 | 13 | 0 | 0 |
|
| ||||
| Thrombocytopenia | 6 | 13 | 0 | 0 |
|
| ||||
| Increased AST | 10 | 22 | 1 | 2 |
|
| ||||
| Increased ALT | 9 | 20 | 1 | 2 |
|
| ||||
| Increased alkaline phosphatase | 5 | 11 | 1 | 2 |
|
| ||||
| Increased bilirubin | 12 | 26 | 5 | 11 |
|
| ||||
| Ascites | 5 | 11 | 2 | 4 |
|
| ||||
| Fatigue | 15 | 33 | 0 | 0 |
|
| ||||
| Vomiting | 5 | 11 | 0 | 0 |
|
| ||||
| Anorexia | 5 | 11 | 1 | 2 |
|
| ||||
| Nausea | 5 | 11 | 0 | 0 |
Objective Responses
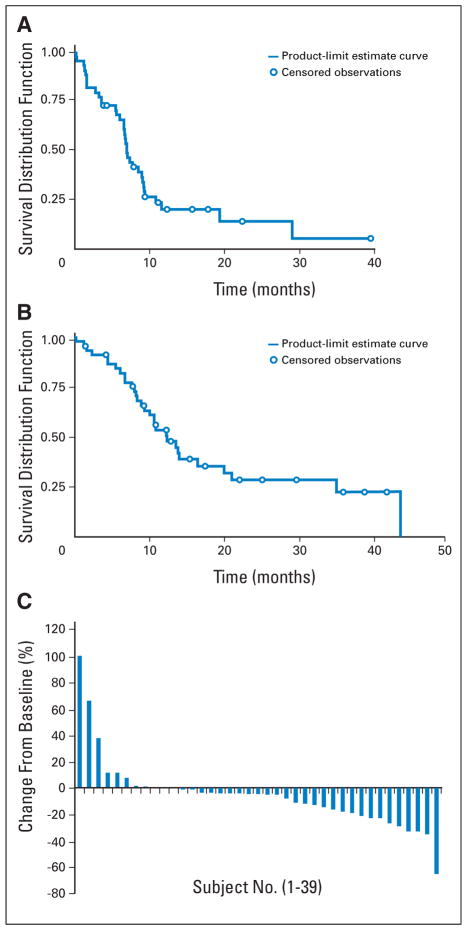
There were six objective responses (13%; 95% CI, 3% to 23%), including one complete response and five partial responses. A waterfall plot is shown in Figure 1 depicting changes in maximum tumor diameter at 8 weeks.
Fig 1.
(A) Kaplan-Meier estimates of progression-free survival; time is in months (number of patients at risk: 0 months, n = 46; 6 months, n = 29; 12 months, n = 7; 18 months, n = 4; 24 months, n = 2; 30 months, n = 1; and 36 months, n = 1). (B) Kaplan-Meier estimates of overall survival; time is in months (number of patients at risk: 0 months, n = 46; 6 months, n = 37; 12 months, n = 20; 18 months, n = 10; 24 months, n = 7; 30 months, n = 5; 36 months, n = 3; 42 months, n = 1). (C) Waterfall plot of change in tumor diameter at 8 weeks.
PFS and Overall Survival
The median PFS time was 6.9 months (95% CI, 6.5 to 9.1 months). Sixty-five percent of patients (95% CI, 51% to 79%) were progression free at 6 months, 23% were progression free at 1 year, and 17% were progression free at 2 years (Fig 2). Overall survival rates were 53% at 1 year, 28% at 2 years, and 23% at 3 years, and the median survival time was 12.4 months (95% CI, 9.4 to 19.9 months; Fig 3). Four patients ultimately underwent orthotopic or living donor liver transplantation, and none had perioperative toxicities attributed to bevacizumab. No significant changes were seen with respect to dose and outcome. The response rates for the 5 and 10 mg/kg groups were 8.3% and 14.7%, respectively (P =.99 by Fisher’s exact test). Median survival times for patients receiving 5 mg/kg (n = 12) and 10 mg/kg (n = 34) were 15.1 and 12.2 months, respectively (P = .64 by the log-rank test).
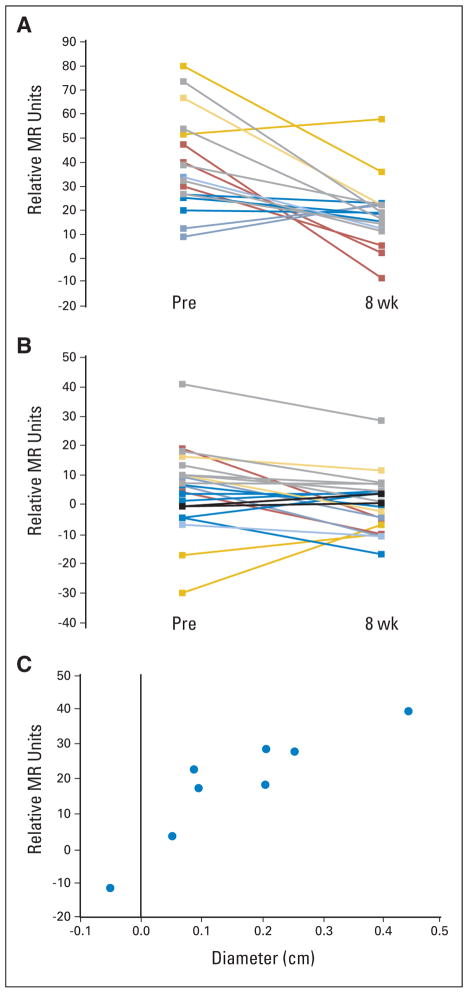
Fig 2.
Enhancement patterns of a subset of patients after 8 weeks of bevacizumab treatment. Individual lesion enhancement changes in the (A) arterial and (B) portal venous phases. The lesions are color matched from the same patients. (C) Sum of the longest diameter plotted against arterial enhancement change after 8 weeks of therapy for each of the eight patients. MR, magnetic resonance; Pre, baseline.
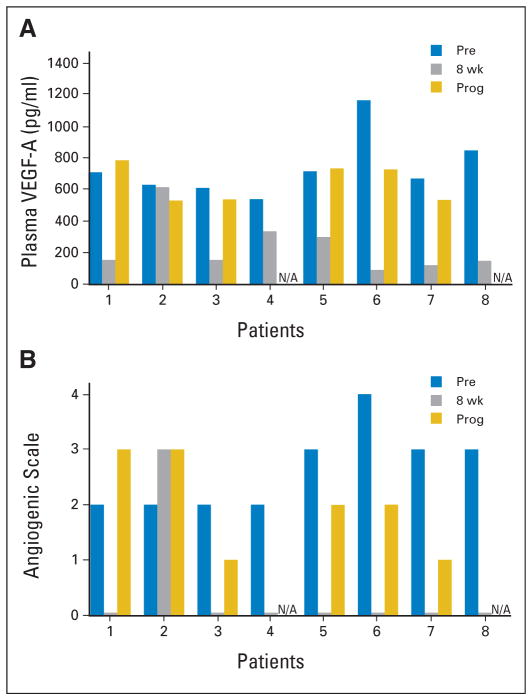
Fig 3.
Bevacizumab effects on (A) plasma vascular endothelial growth factor (VEGF) and (B) angiogenic scale. Angiogenic scale is a measure of functional angiogenic activity using patient plasma to measure morphologic features of angiogenesis in a cell culture model. N/A, not applicable; Pre, baseline; Prog, progression.
DCE-MRI Assessment of Arterial Enhancement
Contrast enhancement patterns on DCE-MRI are correlated with angiogenesis in HCC.14 Eight consecutive patients enrolled at one site (Mt. Sinai) were evaluated before and at 8 weeks after bevacizumab therapy with DCE-MRI, of whom four received bevacizumab 5 mg/kg and four received bevacizumab 10 mg/kg. There was a significant decrease in arterial enhancement after bevacizumab therapy in seven of eight patients (P =.023; Wilcoxon signed rank test). Nineteen of 22 lesions demonstrated decreased arterial enhancement on dynamic imaging. Mean initial arterial enhancement (per lesion) was 37.3 relative MR units (standard deviation [SD], 18.4 relative MR units). After 8 weeks of therapy, the mean enhancement decreased to 18.1 relative MR units (SD, 12.4 relative MR units), representing a mean decrease of 19.2 relative MR units (Fig 2).
Mean tumor diameter (of the lesions evaluated in this subset) before treatment was 1.72 cm (SD, 0.52 cm). The post-treatment average diameter was 1.43 cm (SD, 0.50 cm; Fig 2). We also observed a significant correlation between reduction in arterial enhancement and reduction in tumor diameter (Spearman’s correlation coefficient = 0.90; P <.01; Fig 2). At time of progression, arterial enhancement was increased in two of 18 assessable lesions, remained decreased in 13 of 18 assessable lesions, and could not be calculated in three lesions (that had completely resolved during therapy). Mean tumor diameter was 1.50 cm (SD, 0.9 cm), and the relative arterial enhancement remained low with a mean enhancement of 16.1 relative MR units (SD, 10.2 relative MR units).
Assessment of Circulating VEGF-A, SDF-1, and HUVEC-Based Angiogenic Scale
Plasma was available for assessment in these same eight HCC patients receiving bevacizumab both before therapy and after an initial 8-week treatment period. Plasma was also available at time of disease progression for six of these eight patients. VEGF-A levels decreased from baseline in all patients after 8 weeks of bevacizumab therapy (baseline: mean VEGF-A, 746 pg/mL; SD, 196 pg/mL; n =8; 8 weeks: mean VEGF-A, 249 pg/mL; SD, 175 pg/mL; n =8; P =.003, paired t test). Half of the patients received the lower dose of bevacizumab. At time of disease progression, VEGF-A increased from 8-week values to near-baseline levels in five of six patients for whom plasma was available (mean VEGF-A at progression, 653 pg/mL; SD, 119 pg/mL; n =6; P =.013, paired t test; Fig 3).
SDF-1 levels decreased from baseline in all patients after 8 weeks of bevacizumab with an increase noted in five of six patients at time of progression (baseline: mean SDF-1, 4,389 pg/mL; SD, 474 pg/mL; 8 weeks: mean SDF-1, 3,057 pg/mL; SD, 461 pg/mL; P =.0002, paired t test; at progression: mean SDF-1, 3,865 pg/mL; SD, 425 pg/mL; P =.035; paired t test).
Angiogenic scale score was also diminished after therapy, with concomitant increase at time of HCC progression (median score at baseline: 2.5; range, 2 to 4; median score at 8 weeks: 0; range 0 to 3; median score at progression: 2; range, 1 to 3). The decrease in angiogenic scale score between baseline and 8 weeks was significant (P =.016, Wilcoxon signed rank test; Fig 3). We observed significant correlations between pretreatment VEGF-A levels and angiogenic scale score (r = 0.82, n = 8, P = .012, Spearman correlation) and between SDF-1 levels and angiogenic scale score at time of progression (r = 0.84, n = 6, P = .038, Spearman correlation). Interestingly, patient 2 did not show decreased VEGF-A level or angiogenic scale score at 8 weeks. He had hepatitis B as his underlying etiology of liver disease and had an increased CA19-9, so he may have had a mixed cholangiohepatoma. It is unclear whether these factors affected his results.
DISCUSSION
We report the results of a phase II trial evaluating the safety and clinical and biologic effects of bevacizumab in patients with nonmetastatic, unresectable HCC. The 6-month PFS rate of 65% exceeded the 60% 6-month PFS rate we had defined a priori as being worthy of future evaluation. In addition, 13% of patients demonstrated an objective response, and a substantial proportion of patients showed evidence of minor tumor regression. The recently reported phase III trial of sorafenib (Sorafenib HCC Assessment Randomized Protocol [SHARP] trial) showed a 2% partial response rate and median PFS time of 6 months.4 It is difficult to directly compare this population with ours. Patients in the SHARP trial were older than our patients (median age of 65 years compared with 58 years in our study), and 70% had vascular invasion or extrahepatic spread. We excluded patients with extrahepatic disease because of concerns of poor outcome and bleeding. Thus, it is possible that our patients had more favorable tumor biology. Four of our patients had involvement of a branch of the portal vein (allowed in our inclusion criteria), 17 had an AFP of more than 400 ng/mL, and 12 were Child-Pugh Class B, in contrast to SHARP, which excluded Child-Pugh Class B patients. Although formal comparison with transarterial chemoembolization was not a primary study objective, it is worth noting that this median PFS time is comparable to that observed in a similarly sized cohort of patients undergoing transarterial chemoembolization at one of our institutions.15 We did observe episodes of serious bleeding (four GI, one intratumoral, and one hemoperitoneum), although more rigorous screening seemed to reduce the incidence of these complications.
Bevacizumab was associated with a significant reduction in tumor arterial enhancement as measured by DCE-MRI. We also observed that bevacizumab conferred significant reductions in both circulating VEGF-A and SDF-1; levels of both factors approached baseline values at time of disease progression.
The angiogenic assay was developed to assess the angiogenic milieu of plasma. We observed a strong correlation between VEGF-A levels and functional angiogenic activity at all time points. The functional orientation of the HUVEC-based assay makes it an attractive biomarker for assessing the efficacy of antiangiogenic therapy. Future studies will investigate whether baseline or early treatment levels of these entities are associated with disease control or other clinical outcomes.
We observed an increase in circulating VEGF-A at time of disease progression, despite ongoing bevacizumab therapy. These increases were seen in patients with early disease progression and in those with more substantial periods of disease control. A mechanism of eventual resistance in HCC patients with bevacizumab-mediated disease control may be VEGF-A upregulation, and a future direction for study may involve increasing bevacizumab dose at time of progression. Combinations of antiangiogenic therapies with different mechanisms, VEGF blockade combined with other targeted agents (such as erlotinib), and combinations of antiangiogenics with chemotherapy all merit continued investigation.16–19
In summary, we observed significant clinical and biologic activity for bevacizumab in patients with nonmetastatic HCC. Our results provide substantial rationale for additional investigations of bevacizumab in unresectable HCC.
Acknowledgments
We thank Joseph Sparano, MD, and Dawn Hershman, MD, for careful critical readings of the manuscript and many helpful suggestions; David Jin, MD, for help with the correlative studies; Janice Gabrilove, MD (supported by a National Institutes of Health K24 award, Grant No. CA-100287-05) for her mentorship and guidance; and Barry Siegel, DMD, and Jane Siegel, DMD, for their courage and encouragement.
Supported in part by a contract from the Department of Health and Human Services and the National Institutes of Health (NIH) to the New York Cancer Consortium an NIH Grant No. UL1 RR024156, a Pardes Scholarship and the Family and Friends of Steven Levinson (A.B.S.), and an NIH K23 award (Grant No. CA-90584; J.D.S.).
Footnotes
Presented in part at the 42nd Annual Meeting of the American Society of Clinical Oncology, June 2–6, 2006, Atlanta, GA.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
This article is dedicated to the memory of Dr. Scott Wadler, physician, scientist, mentor, friend, and founder of the New York Phase II Consortium.
Employment or Leadership Position: None Consultant or Advisory Role: Jonathan D. Schwartz, Genentech (C) Stock Ownership: None Honoraria: Allyson Ocean, Genentech; Jonathan D. Schwartz, Genentech Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Helen Chen, Jonathan D. Schwartz
Financial support: Helen Chen, Robert S. Brown Jr
Administrative support: Abby B. Siegel, Deborah Lehrer, Helen Chen, Sean Clark-Garvey, Robert S. Brown Jr, Jonathan D. Schwartz
Provision of study materials or patients: Abby B. Siegel, Emil I. Cohen, Allyson Ocean, Deborah Lehrer, Alec Goldenberg, Jennifer J. Knox, Elizabeta Popa, Robert S. Brown, Shahin Rafii, Jonathan D. Schwartz
Collection and assembly of data: Abby B. Siegel, Emil I. Cohen, Allyson Ocean, Deborah Lehrer, Alec Goldenberg, Jennifer J. Knox, Sean Clark-Garvey, Alan Weinberg, John Mandeli, Paul Christos, Madhu Mazumdar, Shahin Rafii, Jonathan D. Schwartz
Data analysis and interpretation: Abby B. Siegel, Emil I. Cohen, Jennifer J. Knox, Helen Chen, Sean Clark-Garvey, Alan Weinberg, John Mandeli, Paul Christos, Madhu Mazumdar, Robert S. Brown Jr, Shahin Rafii, Jonathan D. Schwartz
Manuscript writing: Abby B. Siegel
Final approval of manuscript: Abby B. Siegel, Emil I. Cohen, Allyson Ocean, Deborah Lehrer, Alec Goldenberg, Jennifer J. Knox, Helen Chen, Sean Clark-Garvey, Alan Weinberg, John Mandeli, Paul Christos, Madhu Mazumdar, Elizabeta Popa, Robert S. Brown Jr, Shahin Rafii, Jonathan D. Schwartz
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Davila JA, Petersen NJ, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: An update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 4.Llovet J, Ricci S, Mazzaferro V, et al. Sorafenib improves survival in advanced hepatocellular carcinoma (HCC): Results of a phase III randomized placebo-controlled trial (SHARP trial) J Clin Oncol. 2007;25:1s. (suppl; abstr LBA1) [Google Scholar]
- 5.Yamaguchi R, Yano H, Iemura A, et al. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998;28:68–77. doi: 10.1002/hep.510280111. [DOI] [PubMed] [Google Scholar]
- 6.Park YN, Kim YB, Yang KM, et al. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061–1065. doi: 10.5858/2000-124-1061-IEOVEG. [DOI] [PubMed] [Google Scholar]
- 7.Pang R, Poon RT. Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett. 2006;242:151–167. doi: 10.1016/j.canlet.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Thomas MB, Abbruzzese JL. Opportunities for targeted therapies in hepatocellular carcinoma. J Clin Oncol. 2005;23:8093–8108. doi: 10.1200/JCO.2004.00.1537. [DOI] [PubMed] [Google Scholar]
- 9.Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41:864–880. doi: 10.1016/j.jhep.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Morgan B, Thomas AL, Drevs J, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: Results from two phase I studies. J Clin Oncol. 2003;21:3955–3964. doi: 10.1200/JCO.2003.08.092. [DOI] [PubMed] [Google Scholar]
- 11.Jubb AM, Hurwitz HI, Bai W, et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2006;24:217–227. doi: 10.1200/JCO.2005.01.5388. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz JD, Sung M, Schwartz M, et al. Thalidomide in advanced hepatocellular carcinoma with optional low-dose interferon-alpha2a upon progression. Oncologist. 2005;10:718–727. doi: 10.1634/theoncologist.10-9-718. [DOI] [PubMed] [Google Scholar]
- 13.Pijl ME, Doornbos J, Wasser MN, et al. Quantitative analysis of focal masses at MR imaging: A plea for standardization. Radiology. 2004;231:737–744. doi: 10.1148/radiol.2313030173. [DOI] [PubMed] [Google Scholar]
- 14.Wang B, Gao ZQ, Yan X. Correlative study of angiogenesis and dynamic contrast-enhanced magnetic resonance imaging features of hepatocellular carcinoma. Acta Radiol. 2005;46:353–358. doi: 10.1080/02841850510021247. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz J, Gabrilove J, Madeli J, et al. Circulating vascular endothelial growth factor (VEGF) and related cytokines in unresectable hepatocellular carcinoma (HCC). 1st Annual Proceedings of the International Liver Cancer Association; Barcelona, Spain. October 5–7, 2007; p. (abstr). [Google Scholar]
- 16.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 17.Thomas MB, Chadha R, Iwasaki K, et al. The combination of bevacizumab and erlotinib shows significant biological activity in patients with advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2007;25:214s. doi: 10.1200/JCO.2008.18.3301. (suppl; abstr 4567) [DOI] [PubMed] [Google Scholar]
- 18.Abou-Alfa G, Johnson J, Knox JJ, et al. Final results from a phase II randomized, double-blind study of sorafenib plus doxorubicin versus placebo plus doxorubicin in patients with advanced hepatocellular carcinoma. 5th Annual Gastrointestinal Cancers Symposium; Orlando, FL. January 25–27, 2008; p. (abstr). [Google Scholar]
- 19.Zhu AX, Blaszkowsky LS, Ryan DP, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:1898–1903. doi: 10.1200/JCO.2005.04.9130. [DOI] [PubMed] [Google Scholar]