Abstract
Background
We report the outcome of early donor lymphocyte infusions (DLI) following T-cell depleted non-myeloablative transplantation using stem cells from human leukocyte antigen (HLA) matched or mismatched donors.
Patients and Methods
Sixty-nine patients with high risk hematologic malignancies received DLI following fludarabine, cyclophosphamide, and alemtuzumab with infusion of stem cells from a matched sibling (52) or partially matched family member donor (17).
Patients received the first infusion a median of 50 days following transplant and doses ranged from 1×104 CD3+ cells/kg to 3.27 ×108 CD3+ cells/kg, depending on clinical status and physician’s discretion. A median cell dose of 1× 105 CD3+ cells/kg in the mismatched setting and 1×106 CD3+ cells/kg in the matched sibling setting appears safe with only 1/7 (14%) and 4/31 (13%) respectively experiencing severe aGVHD at these doses. Importantly, 38% of patients with persistent disease prior to DLI attained a remission following infusion. Nine of the 69 remain alive and disease free 32–71 months following the first DLI infusion.
Conclusion
Low doses of DLI can be safely provided soon after T-cell depleted non-myeloablative therapy and provide a chance of remission. However, long term survival still remains poor due primarily to relapse in these patients however.
Keywords: DLI, haploidentical donors, Non-myeloablative Allogeneic Transplantation, Leukemia/Lymphoma
Introduction
The effectiveness of non-myeloablative allogeneic hematopoietic stem cell transplantation has been reported in advanced hematologic malignancies using matched and mismatched donors. 1,2,3,4 However, significant limitations continue due to relapse, graft vs host disease (GVHD), infections, and treatment related mortality.5,6,7 Host and graft T cell depletion during the conditioning regimen often results in lower rejection and GVHD rates, however, most methods employed curtail the graft vs tumor effect and may allow an increase in infections.8,9,10,11,12 Alemtuzumab, a humanized monoclonal IgG antibody against CD52, has been suggested as a means to effectively T cell deplete a graft while maintaining natural killer cells, felt to be crucial to the anti-cancer effect of allogeneic transplantation.13,14 In the ablative setting, use of alemtuzumab for T cell depletion combined with a donor lymphocyte boost following recovery has been associated with an improved toxicity profile without an increase in relapse compared to historical controls with matched donors, making its use attractive in the nonmyeloablative setting.15,16,17,18 For improvements in immune recovery and response duration, there is mounting evidence supporting the efficacy of donor lymphocytes infusions (DLIs) in patients with hematopoietic malignancies.19,20,21,22 However, there is limited data concerning the timing and dose of lymphocyte infusions that can be safely given shortly after T-cell depleted non-myeloablative therapy. We present a case series of 69 consecutive patients who underwent T cell depleted non-myeloablative therapy using a 3–6/6 HLA matched related donor graft and subsequently received donor lymphocyte infusions shortly after transplantation. Response, development of acute GVHD, and survival are reported.
Patients and Methods
Patients
Patients in this cohort study are a subset of our center’s larger, prospective, non-myeloablative trial and were included if they had a hematologic malignancy or marrow failure syndrome and were consented and treated on our IRB approved protocols for T cell depleted nonmyeloablative allogeneic hematopoietic stem cell transplantation and long term follow up studies as described elsewhere.4,18
Preparative Regimen
Briefly, the preparative regimen included 5 days of intravenous alemtuzumab 20 mg/day on days −4 to 0 and four days of fludarabine 30 mg/m2 and cyclophosphamide 500 mg/m2 per day on days −5 to −2. Recipients of matched sibling stem cells did not receive any other therapy for post transplant prophylaxis of aGVHD while patients who received a family member 3–5/6 HLA matched graft received mycophenolate 1 gram orally twice daily for 60 days following transplantation. Starting day +1 patients received filgrastim 5 mcg/kg (rounded to nearest vial) till absolute neutrophil count was > 1 × 109 /L for 2 days. None of the patients was able to undergo ablative therapy due to advanced age, history of aspergillus or other fungal infection, no available matched donor, and/or other co-morbidities. Chimerism was defined using short tandem repeat analysis as we have described4.
Donor lymphocyte infusions (DLI) Infusions
DLI were planned for all patients within the first few months of transplantation if they had at least 2.5% donor chimerism and did not have severe GVHD, but had relapse or persistence of disease (by conventional or molecular testing), high risk of relapse (patients in second or greater remission), or decreasing (at least a 15% decrease) or very low donor chimerism (under 20%). Deviations from this timing were primarily from restaging evaluations, assuring patients had co-morbidities stabilized, delays in chimerism assessments, donor availability, and insurance reviews. Lymphocytes were collected without growth factor mobilization and were a mixture of fresh or frozen products. Though we planned at least one boost for all patients following this T cell depleted transplant procedure, the optimal dosage of lymphocytes in this setting is unknown and thus was chosen at the physician’s discretion considering the patient’s disease and clinical status. Thus, this report is a retrospective review of the historical cohort of patients on the study who actually received at least 1 DLI. DLI dosages thus ranged from 1×104 CD3+ cells/kg to 3.27 ×108 CD3+ cells/kg. Patients were considered for a second and third DLI 8 weeks apart if they did not have >grade 2 toxicity from the initial DLI, donor availability, and insurance approved the infusion. In those who had more than 1 infusion, patients did not have doses escalated from the original dose unless there was persistence or relapse of disease at the time of the next planned DLI.
Toxicity and Response
For evaluation of toxicity and response, patients were grouped into 4 categories within the range of cell doses delivered (Table 1) and were considered evaluable from the day of first DLI infusion. Acute GVHD was graded according to the consensus criteria and CTC v3 was used for all other toxicities. Recognizing that acute GVHD pathology in the non-ablative and DLI setting may occur late, we tabulated skin, gut and liver toxicity consistent with aGVHD at anytime in the year following the infusion as aGVHD. Toxicities were formally recorded for all patients twice weekly for the first 100 days, at each follow up visit, and as needed intercurrently. DLI response was assessed by examining the underlying disease status 4–6 weeks following infusion and thereafter at 3 month intervals for the first year and then as clinically indicated, using recently updated standardized disease response criterion.23,24,25
Table 1.
DLI Dose categories for the 69 patients:
| DLI Dose (CD3+ cells/kg) | N | HLA Match | |||
|---|---|---|---|---|---|
| 3/6 | 4/6 | 5/6 non-sibling | 5–6/6 sibling | ||
| <5×105 (median 1× 105) | 10 | 5 | 2 | - | 3 |
| 5×105−4.9×106 (median 1× 106) | 40 | 4 | 3 | 2 | 31 |
| 5×106−5×107 (median 1× 107) | 14 | - | - | - | 14 |
| >5×107 (median 5× 107) | 5 | - | 1 | - | 4 |
| Total | 69 | 9 | 6 | 2 | 52 |
(Min= 1×10 cells/kg, Max=3.27×10 cells/kg)
Results
Patient Characteristics
Lymphocyte boosts of some type were planned for all patients in this feasibility study for T cell depleted non-myeloablative therapy treated between December 1999 and September 2006, though the doses were not proscribed prospectively as little data was available during the conduct of the study on optimal dosing strategies. Of seventy five 5–6/6 human leukocyte antigen (HLA) matched sibling transplanted patients, there were 52 (70%) who received at least one DLI. The 23 subjects of these 75 that did not receive a DLI had at least grade 2 GVHD (18), secondary graft failure (2), or early treatment related mortality (3).
In the other cohort of patients on the feasibility studies of 3–5/6 HLA matched family member non-myeloablative transplantation, 17 of the 24 treated patients received at least 1 DLI (71%). Of the 7 that did not receive a DLI, 2 had early treatment related death, 2 graft rejection, and 3 severe GVHD.
Thus, this is a report of sixty nine of the 99 total patients transplanted with a median age of 57 in the matched and 46 in the mismatched group (range18–70 years). Forty-one (59%) patients had leukemia or myelodysplasia (MDS), 17 (25%) had lymphoma or myeloma, and 11 (16%) had a myeloproliferative disorders (MPD) or hemoglobinopathy. At the time of DLI, 48 patients had persistent disease while 2 were given DLI for a decreasing donor percentage not related to relapse, and 19 had high risk disease including multiple relapses (≥ CR2), refractory disease, or induction failure prior to transplantation (Table 2). The interval between transplant and DLI administration ranged from 10 to 630 days with a median of 50 days. Four patients had their first DLI within 4 weeks (all had very low donor chimerism and 3 of 4 had persistent marrow disease as well) while 10 patients had their first beginning ≥ 4 months from transplant. Twenty-two received at least two donor lymphocyte infusions, 18 in the matched group and 4 in the mis-matched group.
Table 2.
Primary Reason for DLI by Disease Category:
| Disease Category | Reason for DLI (N) | |||
|---|---|---|---|---|
| Relapse or Persistent Disease | High Risk Disease | Decreasing Donor Percentage | Total | |
| Leukemia, Myelodysplasia | 22 | 17 | 2 | 41 |
| Lymphoma, Myeloma, CLL | 16 | 1 | - | 17 |
| Myeloproliferative Diseases and marrow failure | 10 | 1 | - | 11 |
| Total | 48 | 19 | 2 | 69 |
Toxicity of early delivery of DLI
Neutropenia was encountered in 12 patients (23%) in the matched group and in 3 (18%) in the mis-matched group. Nine reactivated CMV and CMV disease occurred in two of these patients (both were neutropenic). A total of 21 of the 52 patients in the matched and 5 of the 17 in the mis-matched group experienced some degree of aGVHD (Table 3). In the nonmyeloablative and DLI setting, the occurrence of pathologic and clinical changes consistent with aGVHD are noted to occur beyond day 100 and therefore we agree with prior suggestions to code these occurrences as aGVHD 26 beyond this time frame (we have seen this up to 12 months following infusion) to more accurately reflect the clinical syndrome rather than provide a potentially false sense of lower risk for this severe complication. Skin was the most commonly involved organ and was involved in 20 patients while 15 patients had GI disease and 8 had liver disease. However, only 9 of 52 patients (17%) in the matched group and 3 of 17 (18%) patients in the mis-matched group had severe (grade 3–4) aGVHD. Seven of the 18 patients in the matched group who had more than 1 DLI developed aGVHD and 3 of these were severe. One of the 4 in the mis-matched group who had more than 1 DLI developed severe aGVHD. Five patients died from the direct effect of acute graft vs host disease (Table 4). None of the patients in the matched or mismatched group who did not have aGVHD with the first DLI experienced severe aGVHD with subsequent infusions at the same dose level. In the matched setting, 4/14 (29%) receiving a median dose of 1×107 CD3+ cells/kg developed severe aGVHD while only 4/31 (13%) receiving a median dose of 1×106 CD3+ cells/kg developed severe aGVHD. In the mismatched setting, 2/9 (22%) receiving a median dose of 1×106 CD3+ cells/kg developed severe aGVHD (1 patient both of gut and liver) and only 1/7 (14%) receiving a median dose of 1×105 CD3+ cells/kg did so. Only 1 of the long term surviving patients is noted to have significant chronic GVHD.
Table 3.
Number of patients at each dose level with documented aGVHD from any of their DLI infusions:
| Match Group | Median Dose Level | N | Overall | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Matched | 1×105 | 3 | - | - | - | - |
| 1×106 | 31 | 4 | 6 | 4 | - | |
| 1×107 | 14 | 1 | 1 | 2 | 2 | |
| 5×107 | 4 | - | - | - | 1 | |
| SubTotal | 52 | 5 | 7 | 6 | 3 | |
| Mismatched | 1×105 | 7 | - | 1 | - | 1 |
| 1×106 | 9 | - | - | - | 2 | |
| 1×107 | - | - | - | - | - | |
| 5×107 | 1 | - | 1 | - | - | |
| SubTotal | 17 | - | 2 | - | 3 | |
Table 4.
Cause of Death and Current Status
| Disease | Status pre DLI | Total Patients | MultiOrgan failure | GVHD | Infections | Progressive Disease | Alive |
|---|---|---|---|---|---|---|---|
| Leukemia | CR | 19 | 2 | 1 | 3 | 12 | 1 |
| PR | 7 | - | - | - | 5 | 2 | |
| PD | 15 | 3 | 1 | 2 | 9 | 0 | |
| Total | 41 | 5 | 2 | 5 | 26 | 3 | |
| Lymphoma | CR | 1 | - | - | - | 1 | 0 |
| PR | 4 | - | - | 1 | 1 | 2 | |
| PD | 12 | 2* | 3 | 2 | 4 | 1 | |
| Total | 17 | 2 | 3 | 3 | 6 | 3 | |
| MPD/marrow failure | CR | 1 | - | - | 1 | - | 0 |
| PR | 2 | - | - | - | - | 2 | |
| PD | 8 | 1 | - | 1 | 5 | 1 | |
| Total | 11 | 1 | - | 2 | 5 | 3 | |
| Grand Totals | 69 | 8 | 5 | 10 | 37 | 9 | |
Both from post transplant lymphoproliferative disorder
Response and survival
At the time of DLI, 48 patients had relapse or persistent disease as the main determinant leading to DLI, while 2 patients were given DLI for decreasing donor percentage (due to non relapse rejection or viral disease). The remaining 19 patients had high risk disease including multiple relapses (≥ CR2) or previous induction failure. Following administration of DLI, fourteen patients had a decrease in donor hematopoiesis following DLI. Eleven were coincident with progressive disease, 3 with CMV disease and/or antiviral therapy. Importantly, 7 of 22 (32%) leukemia patients, 7 of 16 (44%) lymphoma patients, and 4 of 10 (40%) patients with MPD / hemoglobinopathy with measurable disease prior to DLI attained a CR following infusion (Table 5).
Table 5.
Response to DLI by Disease Status prior to infusion:
| Disease | Status pre DLI | Total Patients | Best Response post DLI | ||
|---|---|---|---|---|---|
| CR | PR | SD/PD | |||
| Leukemia | CR | 19 | 19 | - | - |
| PR | 7 | 2 | 1 | 4 | |
| PD | 15 | 5 | 1 | 9 | |
| Total | 41 | 26 | 2 | 13 | |
| Lymphoma | CR | 1 | - | - | 1 |
| PR | 4 | 3 | - | 1 | |
| PD | 12 | 4 | 1 | 7 | |
| Total | 17 | 7 | 1 | 9 | |
| MPD/marrow failure | CR | 1 | 1 | - | - |
| PR | 2 | 2 | - | - | |
| PD | 8 | 2 | - | 6 | |
| Total | 11 | 5 | - | 6 | |
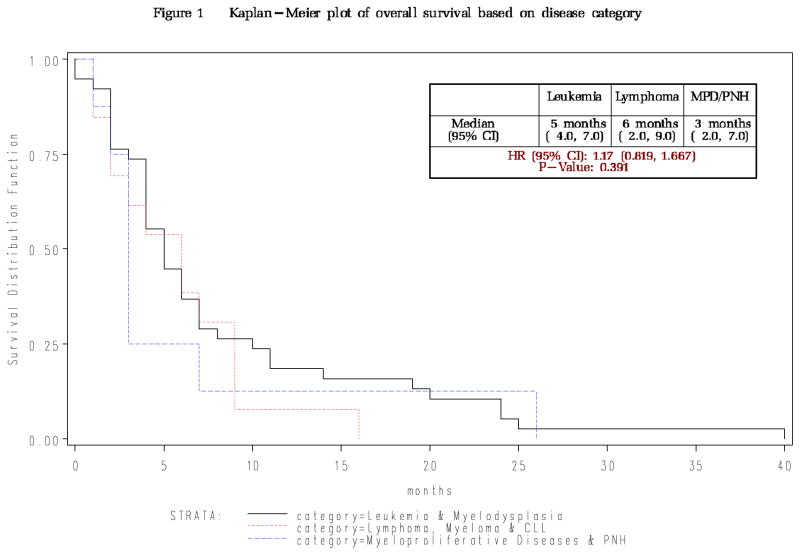
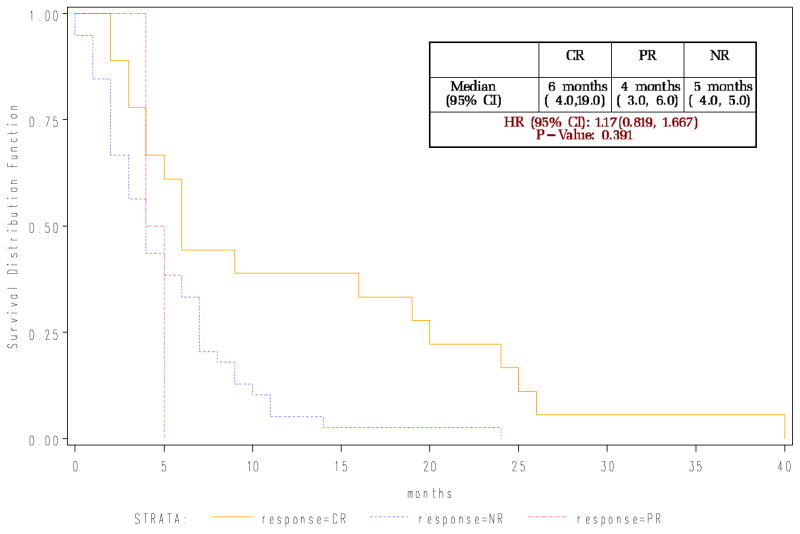
Relapse was by far the most common cause of death, accounting for 37 of the 60 deaths while five patients died primarily from GVHD and 10 primarily from infections. With median follow up of survivors of 57 months from transplant (range 34–72 months) and 55 months from first DLI (range 32–71 months), overall median post-DLI survival at time of publication is 6 months for those who attain remission, with 9 of 69 patients still alive and disease-free. Long term outcomes for each disease type are similar. While our survival curves indicate those who attained a complete response had a better survival than non-responders the first few years, the long follow up of this cohort of patients indicates that all the groups had significant death rates indicating great room for improvement (Figure 1A and B).
Figure 1.
Figure 1A: Overall Survival for all 69 patients based on disease category
Figure 1B: Survival for patients following DLI based upon best response achieved
Discussion
There is significant evidence that allogeneic immunotherapy may improve survival not based solely upon rescuing patients from very high doses of chemotherapy, but via the new immune system attacking the cancer cells. Evidence for this includes a temporal relationship between graft versus host disease and hematologic remission 21,27; reduced incidence of leukemic relapse after allogeneic transplantation compared to syngeneic transplantation 28; and a reduced incidence of leukemic relapse in allogeneic transplant recipients who do develop GVHD compared to those who do not.29 The promise of non-myeloablative therapy lies in exploiting this anti-tumor effect in a safe way.
Non-myeloablative hematopoietic transplantation is an effective approach to manage advanced hematologic malignancies, allowing treatment of older, more infirmed patients. However, failure rates due primarily to procedure toxicity, relapse or infections remain a concern. 1,5,6,18,4,30 While stringent T cell depletion has been utilized to improve the safety of allogeneic therapy, there are concerns of heightened infections and tumor relapse via interference with the anti-tumor effect discussed above. 8,9,10,31 The addition of alemtuzumab, a monoclonal antibody targeting B and T cells that express CD52, has been shown to minimize aGVHD while allowing reliable engraftment and maintenance of encouraging anti – tumor responses, possibly due to its less complete depletion of the T cell pool and relative sparing of the NK cell population. NK cells from a donor may attack cancers in a new host by recognizing the lack of antigens on the cancer cells due to HLA disparities between host and donor. Recent work by the Perugia group has provided further clinical support for the importance of this cell population by noting that patients with an NK alloreactive donor have a better outcome from transplantation compared to those with a matched non-alloreactive donor.32 However, immune recovery still leaves much to be desired as relapse rates and infection rates remain high with this and other approaches to non-myeloablative therapy. Therefore, post transplantation immunotherapy in the form of bulk dose or selected lymphocyte subset infusions remains a focus of current efforts. 15,17,16, 18,4,33
Recent reports using DLI for hematopoietic malignancies have noted efficacy for this population. The EBMT Acute Leukemia Working Party has recently reported use primarily for relapse following ablative therapy. In this population, the median time of relapse following initial transplant (and subsequent DLI with or without reinduction chemotherapy) was 5 months with a median of 107 CD3/kg in the matched donor setting used. Thirty five percent attained a remission, though forty three percent experienced aGVHD.22 Prior reports on DLI following alemtuzumab reduced intensity regimens have focused primarily on matched donors and lymphomatous disorders with infusions long removed from the time of transplantation. Peggs et al. 34 have noted that a dose of 106 CD3+ cells/kg was well tolerated in that setting but did not result in a significant rate of durable responses in their patients. They also noted that matched unrelated donor recipients experienced a high rate of severe aGVHD even when the DLI at this dose were delivered many months after transplantation. However, little is known about the tolerable doses, safety, or efficacy of lymphocyte infusions shortly after T cell depleted non-myeloablative therapy, particularly in mismatched recipients.
We report on 69 consecutive patients (3–6/6 HLA matched) who received a DLI shortly following non-myeloablative allogeneic transplantation using alemtuzumab, fludarabine and cyclophosphamide. While levels of alemtuzumab remain measurable in the body for weeks following delivery of these doses, the concentration is likely at sublytic levels within a few weeks of transplant.35 Most patients had their first infusion within 3 months of transplantation and the majority had high risk or active disease. Our cohort is biased though, in that while having a DLI was standardized within this cohort, the timing and dosage were varied according to the clinical status of the patient, disease status, and physician’s discretion and not in an objective pre-planned method. With this caveat, we agree with prior reports involving matched siblings transplantation and conclude that DLI doses in the range of 1×106 CD3+ cells/kg in the HLA matched sibling setting appear to be safe when delivered soon after T cell depleted non-myeloablative transplantation with only 3 of 31 (10%) experiencing severe aGVHD with the first or any subsequent DLI at this dose. Early infusion in the second month as in our study is tolerated similarly to the late infusions previously reported by Peggs and colleagues.
Recent interesting work has reported manipulating post transplant T cell infusions to minimize their toxicity in the haploidentical setting by depleting certain subsets or ‘allo reactive’ cells.36 To our knowledge, the current report is the first to include haploidentical donors receiving unmanipulated DLIs shortly after T cell depleted non-myeloablative transplantation in this manner. In this group, DLI soon after transplant in the range of 1× 105 CD3+ cells/kg appears to be safe with only 1 of 7 (14%) experiencing severe aGVHD with the first or any subsequent DLI at this dose. While there were responses at higher doses of DLI as well, over 20% of patients in each of the matched and mismatched groups had severe GVHD at the next higher dose range than that recommended above, making correlation of higher doses to better overall response non feasible in this report.
As a review of our experience with this historical cohort, our report is limited in that while a DLI was planned for all transplanted patients on study, the doses and numbers of DLI given were not predetermined, but related to patient’s clinical status, physician discretion, dose available from a donor, or insurance approval. This formal statistical comparisons between dose levels would not be appropriate. The trends reported herein, however, are still instructive in that responses were noted and when combined with the toxicities encountered it provides a framework for planned comparative studies.
Response to DLI as reported in the literature varies, particularly with myeloid diseases which often progress quickly. We document response to unmanipulated DLI for patients with high risk lymphoid or myeloid diseases. Eight-teen of 48 (38%) infused in the presence of measurable disease attained a complete remission. However, with only 13% remaining as long term disease free survivors, relapse remains the primary problem, followed by infectious related deaths. The exact contribution of DLIs to these outcomes remains unclear as most infusions were provided early in recovery, when the full effect of the initial infusion may not yet be evident. However, given reports of higher relapse and infections in the T cell depleted setting without post transplant DLI, a post transplant T cell boost of some sort is considered important to optimize outcomes, though the most advantageous way to do this remains unclear. Our data indicate DLI following both matched as well as mismatched T cell depleted nonmyeloablative therapy can be well tolerated even provided early following transplantation and serves as a basis to compare safety and efficacy of future studies. Efforts to improve post transplant immune therapies to provide more durable responses continue with selected lymphocyte infusions such as CD8+ cells, selected NK cell infusions, or vaccine strategies currently underway.
Acknowledgments
We acknowledge and thank our residents, fellows and nurses in the Bone Marrow Transplant Unit for the fine care of our patients, as well as the cooperation and support of our referring physicians. We also thank Ms. Rhonda Garrett for significant support in data management.
RESEARCH SUPPORT: This research was supported in part by the NIH, Grant #5K23RR16063-01 (DAR), Grant #2PO-1CA47741 (NJC), Grant #M01-RR30 (NCRR, Clinical Research), Bayer Laboratories, Inc., and the Leukemia and Lymphoma Society (DAR)
References
- 1.de Lima M, Anagnostopoulos A, Munsell M, Shahjahan M, Ueno N, Ippoliti C, et al. Nonablative versus reduced intensity conditioning regimens in the treatment of AML and high risk MDS: dose is relevant to long term disease control. Blood. 2004;104:865–72. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 2.Martino R, Iacobelli S, Brand R, Jansen T, van Biezen A, Finke J, et al. Myelodysplastic Syndrome subcommittee of the Chronic Leukemia Working Party of the European Blood and Marrow Transplantation Group. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108:836–46. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 3.Kottaridis PD, Milligan DW, Chopra R, Chakraverty RK, Chakrabarti S, Robinson S, et al. In vivo CAMPATH–1H prevents graft versus host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96:2419–2425. [PubMed] [Google Scholar]
- 4.Rizzieri DA, Koh LP, Long GD, Gasparetto C, Vredenburgh J, Smith C, et al. Clinical outcome and immune reconstitution following Alemtuzumab T cell depleted mismatched non-myeloablative allogoneic immunotherapy. J Clin Oncol. 2007;25:690–697. doi: 10.1200/JCO.2006.07.0953. [DOI] [PubMed] [Google Scholar]
- 5.Giralt S, Estey E, Albitar M, van Besien K, Rondón G, Anderlini P, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analogue-containing chemotherapy: Harness engraft vs leukemia without myeloablative therapy. Blood. 1997;89:4531–4536. [PubMed] [Google Scholar]
- 6.Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, et al. Non-myeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 7.Khouri IF, Keating M, Korbling M, Przepiorka D, Anderlini P, O’Brien S, et al. Transplant lite induction of graft vs malignancy using Fludarabine-based non-ablative chemotherapy and allogeneic blood progenitor cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;98:2817–2824. doi: 10.1200/JCO.1998.16.8.2817. [DOI] [PubMed] [Google Scholar]
- 8.Lacerda JF, Martins C, Carmo JA, Lourenço F, Juncal C, Rodrigues A, et al. Biol Blood and Marrow Transpl. 2003;9:633–642. doi: 10.1016/s1083-8791(03)00263-5. [DOI] [PubMed] [Google Scholar]
- 9.Passweg JR, Meyer-Monard S, Gregor M, Favre G, Heim D, Ebnoether M, et al. Non-myeloablative stem cell transplantation: High stem cell dose will not compensate for T cell depletion in allogeneic non-myeloablative stem cell transplantation. Bone Marrow Transplantation. 2002;30:267–271. doi: 10.1038/sj.bmt.1703671. [DOI] [PubMed] [Google Scholar]
- 10.Walker C, van Burik J, De For T, Weisdorf D, et al. Cytomegalovirus infection after allogeneic transplantation: Comparison of Cord Blood with Peripheral Blood and Marrow Graft Sources. Bio Blood and Marrow Trans. 2007;13:1106–1115. doi: 10.1016/j.bbmt.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Rowley S, Goldberg S, Pecora A, Hsu JS, Brecher BA, Butrin L, et al. Unrelated donor hematopoietic stem cell transplantation for patients with hematologic malignancies using a nonmyeloablative conditioning regimen of fludarabine, low dose total body irradiation, and rabbit anti-thymocyte globulin. Biol Blood and Marrow Transplant. 2004;10:784–793. doi: 10.1016/j.bbmt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Kreiter S, Winkelmann N, Schneider PM, Schuler M, Fischer T, Ullmann AJ, et al. Failure of sustained engraftment after non-myeloablative conditioning with low-dose TBI and T cell-reduced allogeneic peripheral stem cell transplantation. Bone Marrow Transplantation. 2001;28:157–161. doi: 10.1038/sj.bmt.1703107. [DOI] [PubMed] [Google Scholar]
- 13.Ruggeri L, Cappani M, Urbani E, Peruccio K, Schlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoetic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Barrett A, Goldman J, Mavroudis DA. Association of natural killer cell immune recovery with a graft-versus-leukemia effect independent of graft-versus-host disease following allogeneic bone marrow transplantation. Ann Hematology. 1997;74:1–6. doi: 10.1007/s002770050246. [DOI] [PubMed] [Google Scholar]
- 15.Hale G, Zhang M, Bunjes D, Prentice HG, Spence D, Horowitz MM, et al. Improving the outcome of bone marrow transplantation by using CD52 monoclonal antibodies to prevent GVHD and graft rejection. Blood. 1998;92:4581–4590. [PubMed] [Google Scholar]
- 16.Perez-Simon JA, Kottaridis PD, Martino R, Craddock C, Caballero D, Chopra R, et al. Nonmyeloablative transplantation with or without alemtuzumab: comparison between prospective studies in patients with lymphoproliferative disorders. Blood. 2002;100:3121–3127. doi: 10.1182/blood-2002-03-0701. [DOI] [PubMed] [Google Scholar]
- 17.Morris E, Thomson K, Craddock C, Mahendra P, Milligan D, Cook G, et al. Outcomes after alemtuzumab-containing reduced intensity allogeneic transplantation for relapsed and refractory NHL. Blood. 2004;104:3865–3871. doi: 10.1182/blood-2004-03-1105. [DOI] [PubMed] [Google Scholar]
- 18.Rizzieri DA, Long GD, Vredenburgh J, Gasparetto C, Smith CS, Morris AK, et al. Chimerism mediated immunotherapy using CAMPATH T cell depleted peripheral blood progenitor cells with nonablative therapy provides reliable, durable allogeneic engraftment. Blood. 2000;96(Suppl 1):2241 (Ab). [Google Scholar]
- 19.Keil F, Kalhs P, Haas O, Fritsch G, Reiter E, Mannhalter C, et al. Relapse of Philadelphia chromosome positive acute lymphoblastic leukemia after marrow transplantation: sustained molecular remission after early and dose- escalating infusion of donor leukocytes. Br J Haematol. 1997;97:161–164. doi: 10.1046/j.1365-2141.1997.262674.x. [DOI] [PubMed] [Google Scholar]
- 20.Gurman G, Arslan O, Koc H, Akan H. Donor leukocyte infusion for relapsed ANLL after allogeneic BMT and the use of interferon alpha to induce graft versus leukemia effect. BMT. 1996;18:825–826. [PubMed] [Google Scholar]
- 21.Odom L, August C, Githens J, Humbert JR, Morse H, Peakman D, et al. Remission of relapsed leukemia during a graft versus host reaction. Lancet. 1978;2:537–540. doi: 10.1016/s0140-6736(78)92879-9. [DOI] [PubMed] [Google Scholar]
- 22.Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Fassas A, et al. Donor Lymphocyte Infusion in the Treatment of First Hematological Relapse After Allogeneic Stem-Cell Transplantation in Adults With Acute Myeloid Leukemia: A Retrospective Risk Factors Analysis and Comparison With Other Strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25:4938–4945. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 23.Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–50. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 24.Cheson B, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 25.Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 26.Mielcarek M, Storb R. Graft-vs-host disease after non-myeloablative hematopoietic cell transplantation. Leuk Lymphoma. 2005;46:1251–60. doi: 10.1080/10428190500125754. [DOI] [PubMed] [Google Scholar]
- 27.Drobyski W, Keever C, Roth M, Koethe S, Hanson G, McFadden P, et al. Salvage immunotherapy using donor leukocyte infusions as treatment for relapsed chronic myelogenous leukemia after allogeneic bone marrow transplantation: effficacy and toxicity of a defined T cell dose. Blood. 1993;82:2310–2318. [PubMed] [Google Scholar]
- 28.Weiden P, Flournoy N, Thomas E, Prentice R, Fefer A, Buckner CD, et al. Antileukemic effect of graft versus host disease in human recipients of allogeneic marrow grafts. New Engl J Med. 1979;300:1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan K, Weiden P, Storb R, Witherspoon RP, Fefer A, Fisher L, et al. Influence of acute and chronic graft versus host disease on relapse and survival after bone marrow transplantation from HLA identical siblings as treatment of acute and chronic leukemia. Blood. 1989;73:1720–1728. [PubMed] [Google Scholar]
- 30.Martino R, Iacobelli S, Brand R, Jansen T, van Biezen A, Finke J, et al. Myelodysplastic Syndrome subcommittee of the Chronic Leukemia Working Party of the European Blood and Marrow Transplantation Group. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108:836–46. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 31.Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, et al. Treatment of high-risk acute leukemia with T cell depleted stem cells from related donors with one fully mismatched HLA haplotype. N Eng J Med. 1998;339:1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 32.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novitsky N, Thomas V. Allogeneic stem cell transplantation with T cell depleted grafts for lymphoproliferative malignancies. Biol Blood Mar Transpl. 2007;13:107–115. doi: 10.1016/j.bbmt.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peggs KS, Thomson K, Hart DP, Geary J, Morris EC, Yong K, et al. Dose-escalated donor lymphocyte infusions following reduced intensity transplantation: toxicity, chimerism, and disease responses. Blood. 2004;103:1548–56. doi: 10.1182/blood-2003-05-1513. [DOI] [PubMed] [Google Scholar]
- 35.Morris EC, Rebello P, Thomson KJ, Peggs KS, Kyriakou C, Goldstone AH, et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood. 2003;102:404–406. doi: 10.1182/blood-2002-09-2687. [DOI] [PubMed] [Google Scholar]
- 36.Amrolia P, Muccioli-Casadei G, Huls H, Adams S, Durett A, Gee A, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108:1797–808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]