Abstract
Dietary supplements containing black cohosh are alternatives to conventional hormone replacement therapy. This study investigates the maximum tolerated dose of a 75% ethanol extract of black cohosh and determines the pharmacokinetics of one of its most abundant triterpene glycosides, 23-epi-26-deoxyactein. Single doses of black cohosh extract containing 1.4, 2.8, or 5.6 mg of 23-epi-26-deoxyactein were administered to three groups of five women each. Serial blood draws and 24 h urine samples were obtained; blood chemistries, hormonal levels, and 23-epi-26-deoxyactein levels were determined. No acute toxicity or estrogenic hormone effects were observed. Pharmacokinetic analysis of 23-epi-26-deoxyactein in serum indicated that the maximum concentration and area under the curve increased proportionately with dosage, and the half-life was approximately 2 h for all dosages. Less than 0.01% of the 23-epi-26-deoxyactein was recovered in urine 24 h after administration. No phase I or phase II metabolites were observed in clinical specimens or in vitro.
Keywords: black cohosh, Cimicifuga racemosa L. (Nutt.), 23-epi-26-deoxyactein, phase I clinical trial, pharmacokinetics
INTRODUCTION
Cimicifuga racemosa L. (Nutt.) (syn. Actaea racemosa L.), commonly known as black cohosh, is a perennial herb native to North America. The roots/rhizomes of black cohosh have been used traditionally by Native Americans for the relief of menopausal symptoms such as hot flashes (1–3), and preparations have been used in Germany for over 50 years (4). Several small, short-term clinical investigations suggest black cohosh extracts are effective in alleviating hot flashes among menopausal women (5–7). In addition, black cohosh constituents and/or extracts are reported to have antioxidant, anti-AIDS, collagenolytic, and anti-proliferative activities toward human breast cancer cell lines, and to antagonize the human aryl hydrocarbon receptor (8–14).
Since black cohosh roots/rhizomes are not estrogenic (15–17), alternative mechanisms of action have been proposed for the management of menopausal symptoms such as serotonergic activity for the relief of hot flashes (18, 19) and positive effects on bone loss/resorption by black cohosh triterpenes (20). Investigations of the mechanisms of action of black cohosh continue, and active principles remain to be discovered. Accordingly, it is common practice to chemically standardize black cohosh dietary supplements to the characteristic spiroketal triterpene glycosides, e.g., with reference to one of the most abundant congeners, 23-epi-26-deoxyactein previously also known as 27-deoxyactein (Figure 1) (21–23). In this study, an ethanolic black cohosh extract prepared from botanically authenticated plant material was standardized to total triterpene glycosides and used in a phase I clinical trial in women to evaluate the maximum tolerated dose and pharmacokinetics in preparation for a phase II trial of efficacy and safety.
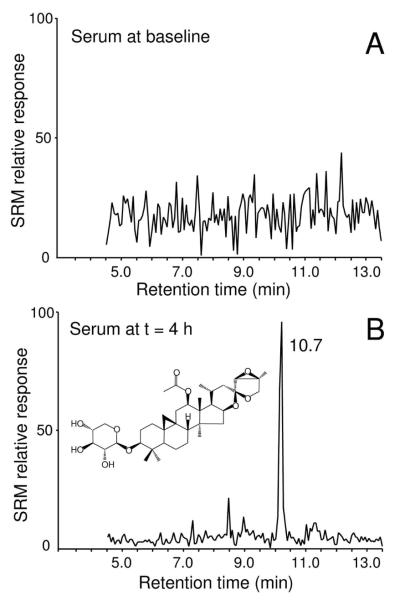
Figure 1.
Positive ion electrospray LC-MS-MS chromatograms of 23-epi-26-deoxyactein in serum at A) 0 h (baseline); and at B) 4 h after administration of a single dose of a black cohosh extract containing 5.6 mg (8.5 μmol) of 23-epi-26-deoxyactein.
RESULTS
Assay validation
Because of the need for quantitative analysis of low levels of 23-epi-26-deoxyactein in serum and urine, a new method based on liquid chromatography-tandem mass spectrometry (LC-MS-MS) was developed for this study. The standard curve for 23-epi-26-deoxyactein measurement was linear over the range of 5.0 to 33.0 pg injected on-column with a coefficient of determination of R2 > 0.999. The limit of detection (signal-to-noise ratio of 3:1) was 2 pg (3 fmol), and the limit of quantitation (signal-to-noise ratio of 10:1) was 5 pg (7.6 fmol). The reproducibility of the assay, evaluated by analyzing three sets of quality controls (23-epi-26-deoxyactein spiked into serum or plasma at 0.5, 2.0 or 8.0 ng/mL) on three consecutive days, showed a relative standard deviation of <10.2%. The recoveries of 0.5 ng/mL (0.76 nM) and 5.0 ng/mL (7.6 nM) 23-epi-26-deoxyactein from serum were 105 ± 14% and 88 ± 9%, respectively, and the recoveries of 23-epi-26-deoxyactein from urine were 86 ± 6% and 90 ± 9% at the 0.5 and 5.0 ng/mL levels, respectively.
Pharmacokinetics of 23-epi-26-deoxyactein in women
Following oral administration of a standardized ethanolic extract of black cohosh roots/rhizomes (32, 64 or 128 mg/dose containing 1.57, 3.14 or 7.27 mg/dose total triterpene glycosides) to 15 women (5 women per group; three dosage groups), serial serum samples were collected and urine was collected for the first 24 h. All serum and urine samples were assayed for 23-epi-26-deoxyactein. In addition, analysis of the black cohosh extract indicated that it contained 1.4, 2.8 or 5.6 mg (2.1, 4.2, or 8.5 μmol) 23-epi-26-deoxyactein per dose. At baseline, no 23-epi-26-deoxactein was detected in serum or urine of any subject (Figures 1 and 2). However, 23-epi-26-deoxyactein was detected during the first 24 h in all subsequent serum and urine samples from every subject regardless of dosage (see examples in Figure 1).
Figure 2.
LC-MS-MS chromatograms of 23-epi-26-deoxyactein in urine obtained using positive ion electrospray at A) 0 h (baseline); and B) in an aliquot of pooled 24-h urine collected after a single dose of a black cohosh dietary supplement containing 5.6 mg (8.5 μmol) of 23-epi-26-deoxyactein.
The serum concentration-time profiles for each dosage group are shown in Figure 3. A non-compartmental analysis was used to calculate the pharmacokinetic parameters which are summarized in Table 1. During the first hour after oral administration of the extract, the serum concentration of 23-epi-26-deoxyactein increased rapidly; then, the concentration increased more slowly during the second hour until a maximum was reached between 2.0 h and 2.9 h for all three dosages (Figure 3 and Table 1). The peak serum concentration of 23-epi-26-deoxyactein was dose-dependent and ranged from 2.2 ng/mL (3.3 nM) to 12.4 ng/mL (18.8 nM). The half-life of 23-epi-26-deoxyactein was nearly constant across all dosages and was 2.1 ± 0.4 h, 2.7 ± 0.4 h and 3.0 ± 1.0 h following administration of 1.4, 2.8 and 5.6 mg, respectively.
Figure 3.

Concentration-time curves of 23-epi-26-deoxyactein in human serum after oral administration of a single dose of a black cohosh extract containing 1.4, 2.8 or 5.6 mg (2.1, 4.2 or 8.5 μmol, respectively) of 23-epi-26-deoxyactein to healthy women (5 women per dosage group). Each data point represents the mean ± S.D. (N = 5 subjects). The inset shows a semi-logarithmic plot of the same data.
Table 1.
Pharmacokinetics of 23-epi-26-deoxyactein in women following oral administration of a single dose of a standardized black cohosh extract.a
| Dosage 23-epi-26-deoxyactein | |||
|---|---|---|---|
| 5.6 mg | 2.8 mg | 1.4 mg | |
| AUC0-t (h*ng/mL)b | 39.2 ± 20.2 | 15.8 ± 6.9 | 5.7 ± 2.1 |
| AUC0-inf (h*ng/mL)c | 44.7 ± 20.1 | 16.9 ± 7.5 | 6.1 ± 2.0 |
| T1/2(h)d | 3.0 ± 1.0 | 2.7 ± 0.4 | 2.1 ± 0.4 |
| Tmax(h)e | 2.4 ± 1.8 | 2.0 ± 0.9 | 1.6 ± 0.6 |
| Cmax(ng/mL)f | 12.4 ± 7.2 | 5.5 ± 3.0 | 2.2 ± 0.4 |
| Vd/F (L)g | 535 ± 302 | 639 ± 352 | 705 ± 265 |
| CL/F (L/h)h | 151 ± 80 | 212 ± 139 | 267 ± 113 |
Results are shown as average values for five women ± S.D. for each dosage group.
AUC0-t = area under the concentration-time curve from 0 h to the last value measured above the limit of quantitation (10 h for the 5.6 mg and 2.8 mg dosages, and 7 h for the 1.4 mg dosage).
AUC0-inf = area under the concentration-time curve from 0 h with extrapolation to infinite time.
T1/2 = elimination half-life
Tmax = time to reach peak serum concentration
Cmax = peak serum concentration
Vd/F = apparent volume of distribution
CL/F = apparent clearance
The area under each concentration-time curve (Figure 3) was determined using the log-linear trapezoidal rule over the range at which 23-epi-26-deoxyactein could be measured in serum following oral administration of each dosage of black cohosh extract (AUC0-t). Alternatively, AUC was calculated from time zero to infinite time (AUC0-inf), and the results of both AUC determinations are shown in Table 1. The AUC for each of the three dosages increased linearly with dosage as indicated by the linear regression coefficients of determination (R2) for these values of 0.999 (AUC0-t) and 0.996 (AUC0-inf).
Aliquots of the 24 h urine collections were analyzed for 23-epi-26-deoxyactein. Women receiving single doses of black cohosh extract containing 1.4, 2.8 or 5.6 mg of 23-epi-26-deoxyactein excreted 58.1 ± 3.1 ng (87.9 ± 4.7 pmol), 238 ± 38 ng (360 ± 57 pmol) or 339 ± 46 ng (514 ± 70 pmol), respectively, of 23-epi-26-deoxyactein in urine during the first 24 h,. Based on these values, less than 0.01% of the dose was recovered in urine during the first 24 h.
Safety of black cohosh
Because there have been reports linking the consumption of dietary supplements containing black cohosh to liver damage (24), serum samples taken at baseline, 1 h, and 12 h after administration of each dose of black cohosh extract were analyzed for markers of hepatotoxicity. The data for markers of liver damage such as liver enzymes in serum are shown in Table 2 for the highest dosage of the black cohosh extract, 128 mg. Regardless of dosage, none of the markers of liver function changed after administration of the black cohosh extract (Table 2).
Table 2.
Liver function assays of serum from women before and after administration of a single 128 mg dose of an ethanolic extract of black cohosh.a
| Analyte | Hours since oral administration of 128 mg black cohosh extract | P-valuea | ||
|---|---|---|---|---|
| Baseline | 1 h | 12 h | ||
| Alkaline phosphatase (IU/L) | 80.0 ± 27.3b | 79.0 ± 27.3 | 81.6 ± 26.3 | 0.93 |
| Alanine transaminase (IU/L) | 31.4 ± 24.0 | 30.2 ± 21.8 | 31.2 ± 24.7 | 0.99 |
| Aspartate transaminase (IU/L) | 30.6 ± 10.7 | 31.4 ± 7.0 | 5.2 ± 16.3 | 0.61 |
| gamma-Glutamyl transpeptidase (IU/L) | 49.0 ± 47.6 | 50.6 ± 49.5 | 47.6 ± 47.1 | 0.96 |
| Direct bilirubin (μmol/L) | 0.70 ± 0.51 | 0.74 ± 0.19 | 0.50 ± 0.28 | 0.47 |
| Glucose (mg/dL) | 100.8 ± 16.8 | 125.2 ± 34.2 | 102.6 ± 10.6 | 0.84 |
P-value, baseline compared to 12 h; p < 0.05 is considered significant
Results are shown as mean values for five women ± S.E. at each time point.
Although black cohosh is not estrogenic (15–17), possible effects on sex hormone levels were measured in serum samples obtained at baseline, and at 1 h and 12 h following the administration of 32, 64 or 128 mg of the standardized black cohosh extract. The data for the 128 mg black cohosh dosage (the highest dosage used) are shown in Table 3. Compared to baseline, no hormone levels were altered as a result of black cohosh consumption. In addition, hematology tests indicated no changes, and physical examinations showed no alterations in blood pressure or other side effects (data not shown).
Table 3.
Hormone levels in serum of women before and after administration of a single 128 mg dose of an ethanolic extract of black cohosh.
| Hormone | Sampling time | P-valuea | ||
|---|---|---|---|---|
| baseline | 1 h | 12 h | ||
| Estrone (pg/mL) | 20.2 ± 5.4b | 19.2 ± 3.8 | 14.4 ± 0.5 | 0.07 |
| Estradiol (pg/mL) | 20.6 ± 2.1 | 22.3 ± 3.2 | 24.1 ± 7.2 | 0.33 |
| FSHc (U/L) | 87.0 ± 29.6 | 79.7 ± 29.6 | 81.3 ± 29.6 | 0.77 |
| LHd (U/L) | 32.8 ± 11.6 | 28.4 ± 10.1 | 29.0 ± 7.4 | 0.55 |
| SHBGe (nmol/L) | 42.2 ± 15.6 | 41.6 ± 16.6 | 40.8 ± 12.9 | 0.88 |
| TSHf (mU/L) | 2.57 ± 0.96 | 1.76 ± 0.82 | 1.73 ± 0.87 | 0.18 |
| Testosterone (ng/dL) | 24.4 ± 10.4 | 22.2 ± 10.1 | 17.8 ± 6.9 | 0.27 |
P-value, baseline compared to 12 h; p < 0.05 is considered significant
Results are shown as mean values for five women ± S.E. at each time point.
Follicle stimulating hormone
Luteinizing hormone
Sex hormone binding globulin
Thyroid stimulating hormone
Metabolism and stability of 23-epi-26-deoxyactein
Although 23-epi-26-deoxyactein was detected and measured in serum and urine, no metabolites of this triterpenoid glycoside were detected. To determine if any metabolites should have been expected in vivo, metabolism experiments using human hepatocytes were carried out in vitro. Following incubation of 23-epi-26-deoxyactein with human hepatocytes, no phase I or phase II metabolism such as oxidation, conjugation or deglycosidation was detected using LC-MS (data not shown). Therefore, no structurally related metabolites of 23-epi-26-deoxyactein could be detected either in vivo or in vitro.
Despite the evident lack of metabolism, only small amounts of intact 23-epi-26-deoxyactein could be detected in both blood and urine. Therefore, it is likely that this spiroketal glycoside undergoes degradation in the stomach. This possibility was investigated by incubating 23-epi-26-deoxyactein with simulated human gastric fluid. The degradation curve and semi-logarithmic plot of concentration versus time are shown in Figure 4. The degradation followed pseudo first-order kinetics (R2 > 0.96), and the degradation rate constant k was 0.0086 min−1. The half-life in gastric fluid was calculated (based on 0.693/k) to be 80.6 min.
Figure 4.

Degradation curve and semi-logarithmic plot of 23-epi-26-deoxyactein concentration versus time in simulated gastric fluid. The half-life of 23-epi-26-deoxyactein was determined to be 80.6 min.
DISCUSSION
23-Epi-26-deoxyactein was absorbed into the blood stream following oral administration of a 75% ethanol extract of black cohosh and showed a half-life of approximately 2 h. No metabolites of 23-epi-26-deoxyactein were detected, and the intact compound was excreted in the urine. However, urinary excretion of 23-epi-26-deoxyactein during the first 24 h represented only 0.006, 0.008, and 0.004% of the three doses administered, respectively. The dose-dependent increase in urinary excretion of 23-epi-26-deoxyactein indicated that there was no saturation of the urinary excretion pathway. Since also no metabolic transformation was observed in vivo, or could be reasonably predicted from the in vitro experiments, the small amount of 23-epi-26-deoxyactein excreted in urine suggested that renal clearance is probably not the primary route of clearance.
Instead of urinary excretion, most 23-epi-26-deoxyactein was probably excreted intact in bile and degraded in the gastrointestinal tract. Biliary and fecal elimination could not be verified as no bile or feces were collected for analysis. However, the graphs of 23-epi-26-deoxyactein levels in serum over time (Figure 3) suggest secondary absorption peaks that would support biliary excretion and reabsorption. Based on studies of stability in simulated human gastric fluid, 23-epi-26-deoxyactein has a gastric half-life of 80.6 min. Since the capsules containing the black cohosh extract disintegrated rapidly in the stomach and released 23-epi-26-deoxyactein in approximately 5 min, some degradation of 23-epi-26-deoxyactein can be expected to occur in the stomach prior to absorption.
Case reports suggesting a connection between the use of dietary supplements containing black cohosh and liver damage were reviewed recently by the U. S. Pharmacopeia (24). Despite acknowledging that many factors such as contamination of the supplements or alcohol consumption might have been responsible for the toxicity in these case reports, the U. S. Pharmacopeia panel still recommended warning labels for black cohosh supplements (24). However, our study found no evidence of hepatotoxicity or any other form of toxicity caused by black cohosh. It is important to note that the supplements/preparations reported to have caused hepatotoxicity had not been analyzed for black cohosh content, purity, contaminants, or other ingredients such as synthetic adulterants. In contrast, the black cohosh extract used in the present study was chemically standardized and a chemically and botanically authenticated preparation (25).
Since 23-epi-26-deoxyactein was absorbed following oral administration of a black cohosh extract, there is the potential for this or perhaps other black cohosh triterpene glycosides to inhibit drug metabolizing enzymes. This would create the potential for drug-black cohosh interactions that might be a source of toxicity for some prescription medications or might affect efficacy by impeding the activation of some prodrugs. Such interactions would be most likely to occur in menopausal women seeking relief from hot flashes while avoiding hormone replacement therapy. Since 23-epi-26-deoxyactein is not metabolized by cytochrome P450 enzymes or conjugating enzymes such as glucuronyltransferases, this abundant black cohosh compound is unlikely to be a source of drug-botanical interactions caused by competition for these drug metabolizing enzymes.
Acute toxicity that has been documented to occur after the consumption of botanical dietary supplements has usually been caused by adulteration of the supplements with other (toxic) plants and/or by contamination with pharmaceutical agents (26). These problems may be avoided through the use of good laboratory and manufacturing practices. Chronic toxicities attributed to botanical dietary supplements may also be caused by contamination by heavy metals, pesticides or microbes, which may be prevented by the application of good agricultural practices as well as good manufacturing practices (26). By botanically authenticating the black cohosh used in dietary supplements, using good manufacturing practices to prevent contamination, and by chemically standardizing the product, black cohosh dietary supplements should be safe for human consumption. Additional studies of efficacy are needed to evaluate the potential benefits of consuming black cohosh products, and based on the lack of toxicity observed in this phase I study, we have undertaken a phase II study of the chronic safety of black cohosh and its potential efficacy in the relief of menopausal hot flashes (27).
MATERIALS AND METHODS
Materials
The triterpene glycoside 23-epi-26-deoxyactein (structure in Figure 1) was isolated from Cimicifuga racemosa L. (Nutt.) as described previously (21), and its structure and purity were confirmed using NMR, mass spectrometry, and X-ray crystallography. The internal standard, astragaloside IV, was purchased from ChromaDex (Santa Ana, CA). Blank human serum and pepsin from porcine gastric mucosa (800–2,500 units/mg protein) were purchased from Sigma-Aldrich (St. Louis, MO). Cryopreserved human hepatocytes, thawing media, and incubation media were purchased from In Vitro Technologies (Baltimore, MD). All solvents were HPLC grade or better and were purchased from Thermo Fisher Scientific (Hanover Park, IL).
The 75% ethanol extract of black cohosh roots/rhizomes (Lot no. CR-01-49-10) was prepared and spray dried by PureWorld Botanicals (now Naturex; South Hackensack, NJ), and capsules containing 32 mg of the dry extract plus rice powder and highly dispersed silicon dioxide as excipients were prepared by Pharmavite (Northbridge, CA), both using good manufacturing practices. The black cohosh raw material used to prepare the extract had been botanically authenticated, and the extract was chemically standardized such that each capsule filled with 32 mg of the extract contained 2.26 mg (7.0%) of the major triterpenoid glycosides 23-epi-26-deoxyactein (1.41. mg), S-actein (0.73 mg) and R-actein (0.12 mg) (25). The dissolution profile of the capsules was determined as recommended by the U.S. Pharmacopoeia (28). The mean dissolution time of the capsules (150 tested) was 5 min. As indicated using LC-MS-MS (see method below), the concentration of 23-epi-26-deoxyactein released from the capsules reached a plateau by 5 min indicating good dissolution characteristics.
In vitro stability and metabolism
Cryopreserved hepatocytes were used according to the manufacturer's instructions, and approximately 1 × 106 cells in a 1 mL suspension were incubated with 10 μM 23-epi-26-deoxyactein at 37 °C for 4 h. The enzymatic incubation was terminated by adding 1.5 mL ice-cold acetonitrile/ethanol. Immediately before analysis using high performance liquid chromatography-tandem mass spectrometry (LC-MS-MS), the suspension was centrifuged, evaporated to dryness in vacuo, and reconstituted in 100 μL methanol/water (1:1, v/v). Control incubations were carried out that were identical except for the omission of either the hepatocytes or 23-epi-26-deoxyactein.
Simulated human gastric fluid was prepared containing sodium chloride, pepsin and hydrochloric acid with a pH of 1.2 (29). In 4 mL of the simulated gastric fluid, 23-epi-26-deoxyactein (5 μM) was incubated at 37 °C in 0, 2, 10, 20, 30, 45, 60, or 90 min. At each time point, a 150 μL aliquot was removed, neutralized with 100 μL 0.1 M aqueous sodium carbonate, and extracted three times using 500 μL portions of ethyl acetate. After centrifugation, the ethyl acetate supernatants were pooled, evaporated to dryness in vacuo, and reconstituted in 100 μL methanol/water (80:20, v/v) containing 1.0 μg/mL astragaloside IV as an internal standard. Aliquots of 20 μL were analyzed for 23-epi-26-deoxyactein using LC-MS-MS as described below.
Clinical design
The human subject protocol for the phase I-type study was approved by the Institutional Review Board and the General Clinical Research Center of the University of Illinois at Chicago and explained to all volunteers giving informed consent. The study was registered on clinicaltrials.gov as NCT00066144. Fifteen healthy menopausal women aged 45–59 y were recruited. Subjects were excluded if they smoked, used any prescription medicine within the previous 2 weeks, had chronic diseases such as diabetes or high blood pressure, or took other dietary supplements. All subjects tested negative for pregnancy. Subjects were randomly assigned to one of three dosage groups. Each subject fasted overnight before consuming a single dose of the black cohosh extract in capsule form the next morning at the General Clinical Research Center. Five subjects received one capsule each of the black cohosh extract (each capsule was chemically standardized to contain 32 mg total triterpene glycosides from black cohosh and 1.4 mg of 23-epi-26-deoxyactein), another five subjects received two capsules each, and the remaining group of five women each received four capsules, which corresponded to black cohosh extracts containing 1.4, 2.8 or 5.6 mg of 23-epi-26-deoxyactein, respectively.
Three weeks prior to the study, the subjects were instructed to restrict certain foods containing phytoestrogens as well as other over-the-counter supplements. In addition, each subject maintained a dietary intake diary beginning three weeks prior to the dosing to identify any potential compounding factors. After dosing, the subjects remained at General Clinical Research Center for 24 h, where they received identical meals prepared by the University of Illinois Medical Center hospital food service. Blood was drawn at the following intervals: 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 24, 36, 48, 72, 96, 120, and 144 h. Blood samples were centrifuged, and the serum was prepared and frozen at −80 °C until analysis. Urine was collected from each subject until 24 h after the dosing and then frozen at −80 °C until analysis.
Statistical analysis was carried out using SPSS 16.0 (Chicago, IL). All data are summarized as means (± standard deviation) with 5 subjects per group. The paired t-test was used to determine whether serum levels at 12-h differed from those at baseline (time 0) in a significant way under the assumptions that the paired differences are independent and identically normally distributed. P-values of less than 0.05 were considered statistically significant.
Mass spectrometry
Due to the need for sensitive and selective quantitative analysis of 23-epi-26-deoxyactein in human serum and urine, a new assay using LC-MS-MS was developed. Protein was precipitated and 23-epi-26-deoxyactein extracted by adding 1.5 mL of ice-cold acetonitrile/ethanol (50:50, v/v) to 500 μL serum. After mixing and centrifugation, the supernatant was removed and evaporated to dryness in vacuo. For the measurement of 23-epi-26-deoxyactein in urine, a 500 μL aliquot was loaded onto a Waters (Milford, MA) Oasis HLB solid-phase extraction cartridge, rinsed with 0.5 mL water, eluted with 2 mL methanol, and then evaporated to dryness in vacuo.
The residues of each serum and urine extract were reconstituted in 100 μL methanol/water (80:20, v/v) containing 1.0 μg/mL astragaloside IV as an internal standard, and 30 μL aliquots were analyzed using LC-MS-MS with a Micromass (Manchester, UK) Quattro II triple quadrupole mass spectrometer and Waters 2690 Alliance HPLC system. If the amount of 23-epi-26-deoxyactein in a sample exceeded the upper limit of the standard curve, the sample was diluted and reanalyzed. A Waters Xterra MS C18 column (2.1 × 100 mm, 3.5 μm) was used for the HPLC separations with a solvent system consisting of a 17 min linear gradient from 20–90% aqueous acetonitrile containing 0.1% formic acid at a flow rate of 120 μL/min. Positive ion electrospray and selected reaction monitoring of the transitions of m/z 661 to 451 and m/z 785 to 419 were used for 23-epi-26-deoxyactein and the internal standard astragaloside IV, respectively, with a dwell time of 20 ms/transition.
Incubation mixtures containing possible metabolites of 23-epi-26-deoxyactein were analyzed using LC-MS with a Micromass Q-TOF2 quadrupole-time-of-flight hybrid mass spectrometer equipped with a Waters 2690 Alliance HPLC system. Separations were carried out using a Waters YMC ODS-AQ C18 column (5.5 μm, 2.0 × 250 mm) with a 60 min linear gradient from 20–60% methanol in water (containing 0.05% acetic acid) at a flow rate of 200 μL/min. Positive ion electrospray mass spectra were acquired over the range m/z 250–900 at 2 s/spectrum. Micromass MetaboLynx software was used to facilitate the identification of possible metabolites. The internal standard astragaloside IV and 23-epi-26-deoxyactein eluted at 9.4 (not shown) and 10.7 min, respectively (Figure 1).
Pharmacokinetic assessment
The pharmacokinetic parameters were calculated using WinNonlin 3.0 (standard edition; Pharsight Cooperation, Mountain View, CA) for each dosage group (five individual subjects per group) and expressed as the mean ± SD. The kinetics, determined using noncompartmental analysis, were characterized by the area under the concentration-time curve (AUC), the peak serum concentration (Cmax), time to reach peak concentration (Tmax), the volume of distribution normalized to the bioavailability fraction (Vd/F), the elimination half-life (T1/2), and the apparent clearance (CL/F). The AUC was calculated using the log-linear trapezoidal rule either from time zero until the serum concentration of 23-epi-26-deoxyactein dropped below the limit of quantitation or from time zero to infinite time. The half-life was calculated using the equation T1/2 = 0.693/λz, where λz was the terminal elimination constant determined by linear regression analysis of the serum concentrations of 23-epi-26-deoxyactein that were measured after the absorption phase and above the limit of quantitation.
ACKNOWLEDGMENTS
We thank Emily Haak for assistance in editing and preparing this article and Kevin Krock for measuring the capsule dissolution profile. This research was supported by grant P50 AT00155 from the Office of Dietary Supplements (ODS), the National Center for Complementary and Alternative Medicine (NCCAM), and the Office for Research on Women's Health (ORWH) of the National Institutes of Health (NIH). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the ODS, NCCAM, ORWH, or the NIH.
Footnotes
CONFLICT OF INTEREST/DISCLOSURE During the course of these studies, Norman R. Farnsworth was a paid consultant to Pharmavite.
REFERENCES
- (1).Lieberman S. A review of the effectiveness of Cimicifuga racemosa (black cohosh) for the symptoms of menopause. J. Womens Health. 1998;7:525–529. doi: 10.1089/jwh.1998.7.525. [DOI] [PubMed] [Google Scholar]
- (2).Compton JA, Culham A, Jury SL. Reclassification of Actaea to include Cimicifuga and Souliea (Ranunculaceae): Phylogeny inferred from morphology, nrDNA ITS, and epDNA trnL-F sequence variation. Taxon. 1998;47:593–634. [Google Scholar]
- (3).Foster S. Black cohosh, Cimicifuga racemosa, a literature review. Herbalgram. 1999;45:35–50. [Google Scholar]
- (4).Liske E. Therapeutic efficacy and safety of Cimicifuga racemosa for gynecologic disorders. Adv. Ther. 1998;15:45–53. [PubMed] [Google Scholar]
- (5).Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann. Intern. Med. 2002;137:805–813. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- (6).Mahady GB, Parrot J, Lee C, Yun GS, Dan A. Botanical dietary supplement use in peri- and postmenopausal women. Menopause. 2003;10:65–72. doi: 10.1097/00042192-200310010-00011. [DOI] [PubMed] [Google Scholar]
- (7).McKenna DJ, Jones K, Humphrey S, Hughes K. Black cohosh: efficacy, safety, and use in clinical and preclinical applications. Altern. Ther. Health Med. 2001;7:93–100. [PubMed] [Google Scholar]
- (8).Burdette JE, et al. Black cohosh (Cimicifuga racemosa L.) protects against menadione-induced DNA damage through scavenging of reactive oxygen species: bioassay-directed isolation and characterization of active principles. J. Agric. Food Chem. 2002;50:7022–7028. doi: 10.1021/jf020725h. [DOI] [PubMed] [Google Scholar]
- (9).Borrelli F, Izzo AA, Ernst E. Pharmacological effects of Cimicifuga racemosa. Life Sci. 2003;73:1215–1229. doi: 10.1016/s0024-3205(03)00378-3. [DOI] [PubMed] [Google Scholar]
- (10).Hostanska K, Nisslein T, Freudenstein J, Reichling J, Saller R. Cimicifuga racemosa extract inhibits proliferation of estrogen receptor-positive and negative human breast carcinoma cell lines by induction of apoptosis. Breast Cancer Res. Treat. 2004;84:151–160. doi: 10.1023/B:BREA.0000018413.98636.80. [DOI] [PubMed] [Google Scholar]
- (11).Jeuken A, Keser BJ, Khan E, Brouwer A, Koeman J, Denison MS. Activation of the Ah receptor by extracts of dietary herbal supplements, vegetables, and fruits. J. Agric. Food Chem. 2003;51:5478–5487. doi: 10.1021/jf030252u. [DOI] [PubMed] [Google Scholar]
- (12).Sakurai N, et al. Anti-AIDS agents. Part 57: Actein, an anti-HIV principle from the rhizome of Cimicifuga racemosa (black cohosh), and the anti-HIV activity of related saponins. Bioorg. Med. Chem. Lett. 2004;14:1329–1332. doi: 10.1016/j.bmcl.2003.12.035. [DOI] [PubMed] [Google Scholar]
- (13).Kusano A, Seyama Y, Nagai M, Shibano M, Kusano G. Effects of fukinolic acid and cimicifugic acids from Cimicifuga species on collagenolytic activity. Biol. Pharm. Bull. 2001;24:1198–1201. doi: 10.1248/bpb.24.1198. [DOI] [PubMed] [Google Scholar]
- (14).Einbond LS, et al. Growth inhibitory activity of extracts and purified components of black cohosh on human breast cancer cells. Breast Cancer Res. Treat. 2004;83:221–231. doi: 10.1023/B:BREA.0000014043.56230.a3. [DOI] [PubMed] [Google Scholar]
- (15).Liu J, et al. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J. Agri. Food Chem. 2001;49:2472–2479. doi: 10.1021/jf0014157. [DOI] [PubMed] [Google Scholar]
- (16).Einer-Jensen N, Zhao J, Andersen KP, Kristoffersen K. Cimicifuga and Melbrosia lack oestrogenic effects in mice and rats. Maturitas. 1996;25:149–153. doi: 10.1016/0378-5122(96)01052-3. [DOI] [PubMed] [Google Scholar]
- (17).Mahady GB. Is black cohosh estrogenic? Nutr. Rev. 2003;61:183–186. doi: 10.1301/nr.2003.may.183-186. [DOI] [PubMed] [Google Scholar]
- (18).Burdette JE, et al. Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J. Agric. Food Chem. 2003;51:5661–5670. doi: 10.1021/jf034264r. [DOI] [PubMed] [Google Scholar]
- (19).Powell SL, et al. In vitro serotonergic activity of black cohosh and identification of Nω-methylserotonin as active constituent. J. Agric. Food Chem. 2008;56:11718–11726. doi: 10.1021/jf803298z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Qiu SX, et al. A triterpene glycoside from black cohosh that inhibits osteoclastogenesis by modulating RANKL and TNFalpha signaling pathways. Chem. Biol. 2007;14:860–869. doi: 10.1016/j.chembiol.2007.06.010. [DOI] [PubMed] [Google Scholar]
- (21).Chen SN, et al. Isolation, structure elucidation, and absolute configuration of 26-deoxyactein from Cimicifuga racemosa and clarification of nomenclature associated with 27-deoxyactein. J. Nat. Prod. 2002;65:601–605. doi: 10.1021/np010494t. [DOI] [PubMed] [Google Scholar]
- (22).He K, Zheng B, Kim CH, Rogers L, Zheng Q. Direct analysis and identification of triterpenes glycosides by LC/MS in black cohosh, Cimicifuga racemosa, and in several commercial available black cohosh products. Planta Med. 2000;66:635–640. doi: 10.1055/s-2000-8619. [DOI] [PubMed] [Google Scholar]
- (23).Li W, et al. High-performance liquid chromatographic analysis of black cohosh (Cimicifuga racemosa) constituents with in-line evaporative light scattering and photodiode array detection. Anal. Chim. Acta. 2002;471:61–75. [Google Scholar]
- (24).Mahady GB, et al. United States Pharmacopeia review of the black cohosh reports of hepatotoxicity. Menopause. 2008;15:1–11. doi: 10.1097/gme.0b013e31816054bf. [DOI] [PubMed] [Google Scholar]
- (25).Fabricant DS. Ph.D. Dissertation: Pharmacognostic Investigation of Black Cohosh (Cimicifuga racemosa (L.) Nutt.) University of Illinois at Chicago: Department of Medicinal Chemistry and Pharmacognosy; Chicago: 2006. [Google Scholar]
- (26).Breemen RB, Fong HHS, Farnsworth NR. Ensuring the safety of botanical dietary supplements. Am. J. Clin. Nutr. 2008;87(Suppl 1):509S–513S. doi: 10.1093/ajcn/87.2.509S. [DOI] [PubMed] [Google Scholar]
- (27).Farnsworth NR, Krause EC, Bolton JL, Pauli GF, van Breemen RB, Graham JG. The University of Illinois at Chicago/National Institutes of Health Center for Botanical Dietary Supplements Research for Women's Health: from plant to clinical use. Am. J. Clin. Nutr. 2008;87(Suppl 1):504S–508S. doi: 10.1093/ajcn/87.2.504S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).U.S. Pharmacopoeia 28 . <711> Dissolution test. U.S. Pharmacopoeial Convention, Inc.; Rockville, MD: 2005. [Google Scholar]
- (29).U.S. Pharmacopoeia 30 . Simulated gastric fluid. U.S. Pharmacopoeial Convention, Inc; Rockville, MD: 2007. [Google Scholar]