To the editor
Multiple myeloma (MM) is a malignant proliferation of plasma cells (PCs) in the bone marrow (BM). Activation of the canonical WNT/β-catenin signaling pathway is a frequent event in MM (1–4); however, the molecular mechanisms underlying this phenomenon remain unknown. In contrast to most epithelial cancers, MM lacks mutations in critical components of this pathway, suggesting that deregulated post-translational mechanisms contribute to the aberrant expression of WNT pathway proteins (1, 5). We and others have documented the overexpression of the central WNT signaling molecule, β-catenin, in a majority of MM PCs as compared to normal PCs (1–3, 6, 7). Post-translational regulation of β-catenin is typically mediated through phosphorylation events that tag the protein for ubiquitination and degradation through the 26S proteasome. In most cellular contexts, the ubiquitin-proteasome system (UPS) is sufficient to handle normal protein synthesis, misfolding, and turnover (8); however, exceeding the capacity of the UPS causes polypeptides to accumulate in cytosolic aggregates near the perinuclear microtubule organizing center (MTOC). Continued stress on the UPS causes additional protein aggregates to amass, forming a dense structural assembly termed the aggresome (9). Aggresomes recruit additional protein catabolic machinery to aid in the unfolding of compacted proteins for proteasomal or autophagosomal/lysosomal removal (10). Thus, aggresome formation provides a reserve protein processing unit to the proteasome to eliminate unwanted proteins.
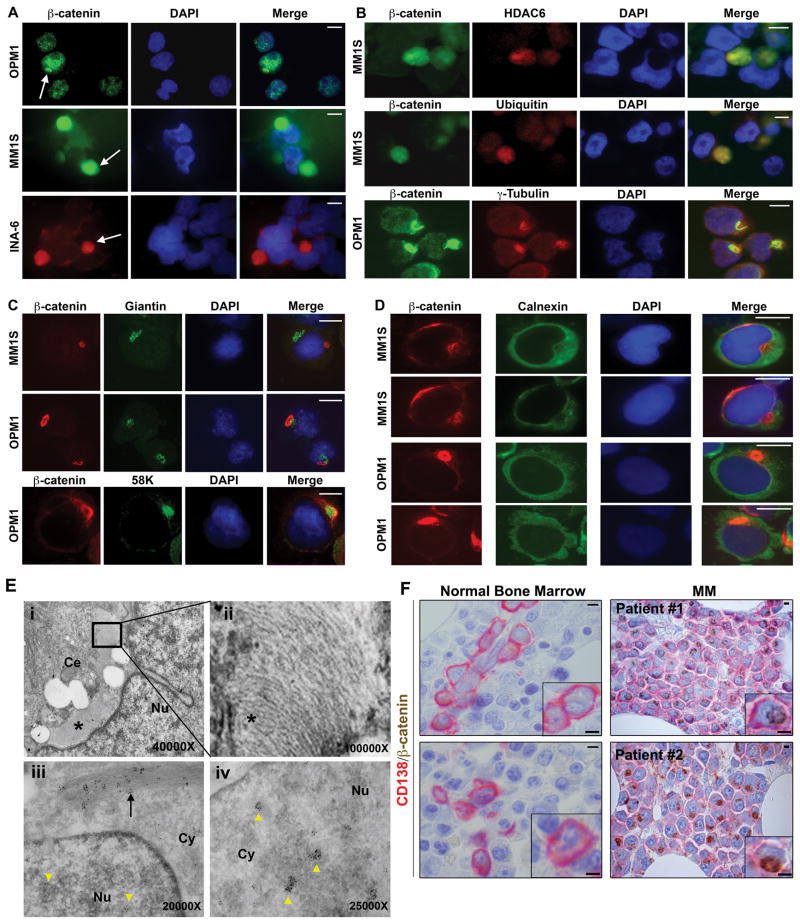
To determine the cellular localization of β-catenin, we first performed immunofluorescence (IFC) and immunohistochemical (IHC) analyses in MM cells using an antibody against this key signaling molecule. Consistent with prior reports, most cell lines showed diffuse distribution of β-catenin within the cytoplasm and punctuated signals within the nucleus (Figure 1A)(1, 2, 6). However, strong β-catenin signal was also observed in distinct perinuclear aggregates from a subpopulation of cultured MM cell lines (Figure 1A). In order to delineate the identity of these structures, we performed double IFC analysis that demonstrated colocalization of the β-catenin signal with known aggresomal markers such as HDAC6, ubiquitin, vimentin, and γ-tubulin (Figure 1B and data not shown)(9, 11). However, the expression of Golgi body markers Giantin and 58K as well as ER marker Calnexin was distinctly different from that of the aggresome (Figure 1C and 1D). Scanning electron microscopy (SEM) further confirmed these findings (Figure 1E and Supplemental Figure 1).
Figure 1. β-catenin accumulation in MM aggresomes.
(A) IFC analysis of β-catenin MM cell lines grown in vitro. Arrows: perinuclear β-catenin (Zymed, CAT5-H10). (B) Double IFC of β-catenin with HDAC6 (Santa Cruz, sc-28386), ubiquitin (Cell Signaling, P4D1) and γ-tubulin (Santa Cruz, sc-9133). Note that the shape, size and frequency of aggresomes vary. β-catenin is distinct from Golgi markers Giantin (Abcam, ab24586) and 58K (Sigma, 58K-9) (C) as well as ER marker Calnexin (Cell Signaling, C5C9) (D). (E)(i). SEM of an aggresome (asterisk) in an OMP1 cell. Boxed region is enlarged in (ii). (iii) Localization of gold-conjugated β-catenin antibodies (black arrow) in filamentous network and in transcriptionally active sites in nucleus (iv)(yellow arrowheads). (F) Representative examples of β-catenin IHC in NBM and MM patient samples. CD138 (BD Bioscience, 281-2). Scale bars: 5μm. Cy: cytoplasm; Nu: nucleus.
We suspected that β-catenin could be used as a marker of aggresomes to identify malignant PCs in patient samples. We therefore evaluated the staining pattern in MM paraffin-embedded biopsies using two different antibodies that recognize distinct epitopes of β-catenin. In accord with the IHC and SEM findings described above, use of either β-catenin antibody revealed a strong perinuclear signal (Supplemental Figure 2). The occurrence of aggresomes in MM cells was also verified with an aggresomal markers ubiquitin and vimentin (Supplemental Figure 3); however, we found β-catenin to be a more reliable marker for aggresomes in IHC. We expanded our analysis to include normal (n=20) and MM BM biopsies (n=90) using tissue microarrays; plasma cells were identified with an anti-CD138 antibody. In all normal samples, β-catenin expression in PCs was barely, if at all, detectable (Figure 1F). Strikingly, greater than 90% of MM cases showed aggresomal β-catenin and nearly all MM cells contained aggresomes (Figure 1F and Supplemental Figure 4). Thus, our data show that aggresomal β-catenin expression is nearly universal in MM but absent from normal PCs, which highlights its potential use as a biomarker for malignant plasma cells.
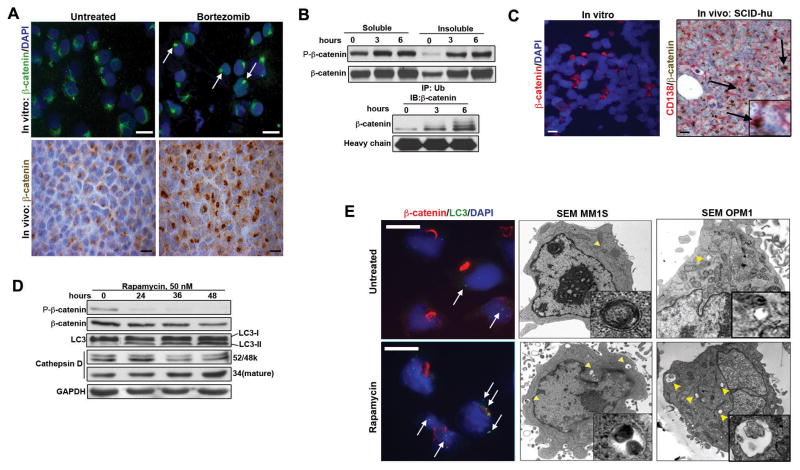
We then investigated the effect of the proteasome inhibitor bortezomib on β-catenin protein aggregates (11). We found the number of aggresomes containing β-catenin was significantly increased in MM cells treated with bortezomib compared to untreated cells in vitro (48.2%±5.5 vs 23.2%±2.9, p<0.0001) (Figure 2A, top). Moreover, additional β-catenin in aggresomes was also documented in MM xenograft mice (NOD/SCID) treated with bortezomib (Figure 2A, bottom). Immunoblot (IB) analysis that showed increased amounts of both total and phosphorylated β-catenin in the insoluble cellular fractions containing aggresomes, as well as increased ubiquitinated β-catenin associated with time of bortezomib treatment (Figure 2B). We then employed an in vivo SCID-hu model of myeloma that uses human fetal bone chips implanted into NOD/SCID mice to study the effect on β-catenin localization within the endogenous BM microenvironment compared to in vitro cell culture (12). IFC of the INA-6 cell line grown in vitro showed the presence of β-catenin in perinuclear aggregates in a small proportion of cells (Figure 2C, left). In contrast, a much larger population of INA-6 myeloma cells grown in SCID-hu mice showed intense staining of β-catenin within aggregates (Figure 2C, right). Collectively, these results indicate that β-catenin accumulation in these structures is an active process that is enhanced by disrupted proteasome function and in vivo conditions.
Figure 2. The proteasome-aggresome-phagosome system modulates β-catenin accumulation in MM cells.
(A) β-catenin accumulation in aggresomes after 6 hours of 5nM bortezomib treatment in MM1S cells (top, white arrows). Increased β-catenin in MM xenograft mice treated with bortezomib at 0.5mg/kg twice weekly i.p. for 3 weeks (bottom). (B) IB (top) and IP (bottom) of β-catenin following 2nM bortezomib treatment of MM1S. (C) β-catenin in INA-6 cells grown in vitro and in vivo, respectively. Note distinct perinuclear β-catenin localization (arrows and inset). (D) Enhanced LC3 activation and decreased phospho-β-catenin levels in rapamycin-treated MM1S cells. (E) IFC and SEM analysis demonstrating rapamycin treatment (50nM for 48 hours) of MM1S and OPM1 cells enhanced phagosome formation (white arrows and yellow arrowheads). Inserts: autophagasomes.
Aggresome clearance by autophagy is an important mechanism of removal and can be induced using rapamycin, an inhibitor of mTOR (13). Treatment of MM cells with rapamycin led to increased staining of the phagosomal marker LC3, an increased number of phagosomes per cell, as well as a significant decrease of β-catenin staining in aggresomes, as assessed by IB, IFC and SEM (Figure 2D and 2E). Moreover, rapamycin treatment led to decreased levels of total and phospho-β-catenin, while activating LC3 (Figure 2D), suggesting that the decrease in phospho-β-catenin is due in part to phagosomal clearance. Additionally, we observed an increased amount of the activated form of the lysosomal marker Cathepsin D following rapamycin treatment, indicating autophagosome-lysosome degradation of β-catenin (Figure 2D). These results demonstrate that the proteasome-autophagosome-lysosome pathways are all actively involved in modulating β-catenin accumulation in MM aggresomes.
Our findings are in accord with the fact that the UPS is inherently stressed in MM PCs due probably to the extremely high rate of immunoglobulin synthesis (resulting in the classic monoclonal Ig spike) and degree of somatic hypermutation that increases the probability of protein misfolding. Interestingly, aggresomes play a dynamic signaling role in the cell and can be involved in regulating protein levels by degradation of not only marked or misfolded proteins, but also their more physiologic counterparts (14). It is tempting to speculate that aggresomes in MM may play a similar role, acting as holding stations for β-catenin and thereby contributing to oncogenic signaling. This scenario is consistent with our observation of the coexistence of β-catenin both in aggresomes and in nuclei (Figure 1E). It is also possible that aggresome formation around the MTOC and centriole (Supplemental Figure 1B) may interfere with centrosome maturation and spindle assembly during mitosis and thereby contribute to chromosomal instability that is a hallmark of MM (15). The mechanisms underlying the formation of aggresomes in most, but not all, myeloma cells within the same tumor remains to be determined, but may reflect a degree of phenotypic and functional cellular heterogeneity. Prospective longitudinal studies of larger numbers of MM patients, its precursor monoclonal gammopathy of undetermined significance (MGUS), and normal individuals are needed to define the utility of β-catenin in aggresomes as a predictive biomarker or to identify residual disease in BM biopsies to refine assessment of treatment response. Lastly, future drug design could target the proteasome-autophagosome-lysosome system to take advantage of this important aspect of cancer biology for myeloma therapeutics.
Supplementary Material
Acknowledgments
D.R.C. is supported by R01CA151391-01, the Multiple Myeloma Research Foundation, and is a Sidney Kimmel Foundation Scholar.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu).
References
- 1.Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, van Oers MH, et al. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sukhdeo K, Mani M, Zhang Y, Dutta J, Yasui H, Rooney MD, et al. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci U S A. 2007 May 1;104(18):7516–7521. doi: 10.1073/pnas.0610299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrasco DR, Tonon G, Huang Y, Zhang Y, Sinha R, Feng B, et al. High-resolution genomic profiles define distinct clinico-pathogenetic subgroups of multiple myeloma patients. Cancer Cell. 2006 Apr;9(4):313–325. doi: 10.1016/j.ccr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Bjorklund CC, Ma W, Wang ZQ, Davis RE, Kuhn DJ, Kornblau SM, et al. Evidence of a role for activation of Wnt/beta-catenin signaling in the resistance of plasma cells to lenalidomide. J Biol Chem. 2011 Apr 1;286(13):11009–11020. doi: 10.1074/jbc.M110.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman MA, Lawrence MS, Keats JJ, Cibulskis K, Sougnez C, Schinzel AC, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011 Mar 24;471(7339):467–472. doi: 10.1038/nature09837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta-Simmons J, Zhang Y, Gorgun G, Gatt M, Mani M, Hideshima T, et al. Aurora kinase A is a target of Wnt/beta-catenin involved in multiple myeloma disease progression. Blood. 2009 Sep 24;114(13):2699–2708. doi: 10.1182/blood-2008-12-194290. [DOI] [PubMed] [Google Scholar]
- 7.Mani M, Carrasco DE, Zhang Y, Takada K, Gatt ME, Dutta-Simmons J, et al. BCL9 promotes tumor progression by conferring enhanced proliferative, metastatic, and angiogenic properties to cancer cells. Cancer Res. 2009 Oct 1;69( 19):7577–7586. doi: 10.1158/0008-5472.CAN-09-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays. 2000 May;22(5):442–451. doi: 10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 9.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998 Dec 28;143(7):1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabunmi RP, Wigley WC, Thomas PJ, DeMartino GN. Activity and regulation of the centrosome-associated proteasome. J Biol Chem. 2000 Jan 7;275(1):409–413. doi: 10.1074/jbc.275.1.409. [DOI] [PubMed] [Google Scholar]
- 11.Hideshima T, Bradner JE, Wong J, Chauhan D, Richardson P, Schreiber SL, et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci U S A. 2005 Jun 14;102(24):8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein J, Yaccoby S. The SCID-hu myeloma model. Methods Mol Med. 2005;113:183–190. doi: 10.1385/1-59259-916-8:183. [DOI] [PubMed] [Google Scholar]
- 13.Fortun J, Dunn WA, Jr, Joy S, Li J, Notterpek L. Emerging role for autophagy in the removal of aggresomes in Schwann cells. J Neurosci. 2003 Nov 19;23(33):10672–10680. doi: 10.1523/JNEUROSCI.23-33-10672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolodziejska KE, Burns AR, Moore RH, Stenoien DL, Eissa NT. Regulation of inducible nitric oxide synthase by aggresome formation. Proc Natl Acad Sci U S A. 2005 Mar 29;102(13):4854–4859. doi: 10.1073/pnas.0500485102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chng WJ, Ahmann GJ, Henderson K, Santana-Davila R, Greipp PR, Gertz MA, et al. Clinical implication of centrosome amplification in plasma cell neoplasm. Blood. 2006 May 1;107(9):3669–3675. doi: 10.1182/blood-2005-09-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.