Abstract
This is an observational study with the primary objective to measure donor-specific immune responses by pediatric liver transplant (LT) recipients, using cell surface expression of lymphocyte activation markers and cytokine secretion in mixed lymphocyte reactions. The secondary objective was to demonstrate possible mechanism(s) involved in those who demonstrated donor-specific hyporesponsiveness. Study participants included 17 recipients, their respective parental donors, the non-donor parent, as well as unrelated third party individuals. Within the CD4+ population, two distinct patterns of CD69 and CD71 expressions were observed: recipients who had a lower percentage of CD4+CD69+ and CD4+CD71+ cells after donor versus non-donor stimulation (therefore a donor/non-donor ratio <1); and recipients who had a higher percentage of CD4+CD69+ and CD4+CD71+ cells after donor versus non-donor stimulation (therefore a donor/non-donor ratio ≥1). Eight recipients had the above defined ratio of <1, with significantly decreased interferon-γ secretion after donor versus non-donor stimulation. CD4+CD25hi˙CD127– regulatory T cells from these eight recipients suppressed donor and non-donor cell induced proliferation. Suppression of proliferation was partially abrogated by interleukin-2. In conclusion, CD69 and CD71 cell surface expression with interferon-γ secretion can be used to identify two distinct populations in pediatric LT recipients. Both active regulation and anergy underlie donor specific hyporesponsiveness.
Keywords: Lymphocyte activation, Immune reactivity, Liver transplantation
1. Introduction
Given the undesirable consequences of long-term immunosuppression, the ability to adjust immunosuppression based on a measure of immune reactivity (or lack thereof) to donor antigens would be a major step forward in the management of immunosuppression by transplant physicians. This is an observational study with the primary objective to measure donor specific alloreactivity in a group of pediatric living related liver transplant (LRLT) recipients using cell surface expression of lymphocyte activation markers and cytokine secretion in mixed lymphocyte reactions (MLR). The secondary objective was to demonstrate possible mechanism(s) involved in those who demonstrated donor-specific hyporesponsiveness.
CD69 is a C type lectin expressed on T-lymphocytes and natural killer cells and is one of the earliest surface molecules expressed upon T-cell activation [1]. Activated, proliferating lymphocytes are also known to express the molecules CD25 and CD71 on their surface and are thus termed “activation antigens” [2]. Several groups have reported heightened peripheral blood lymphocyte expression of CD69 in acute renal and cardiac allograft rejection [3,4]. In addition, infiltrating CD8+69+ lymphocytes have been shown to play a role in renal and cardiac allograft rejection with abundant CD69+ lymphocytes demonstrated in allograft infiltrates in both subclinical and clinical acute rejection [5–7].
More recently, Brouard et al. looked at blood gene expression profiles in a cohort of renal transplant recipients and identified a “tolerant footprint” of 49 genes, 33 of which correctly segregated tolerant and chronic rejection phenotypes with 99% and 86% spec-ificity. Their report indicates that classical markers of early and late T-cell activation (CD69, TACTILE, LAG3, SLAM) were consistently reduced in tolerant patients [8].
We hypothesized that cell surface expression of lymphocyte activation markers and cytokine secretion in MLR could be used to predict the presence of donor specific alloreactivity in pediatric liver transplant (LT) recipients. We sought to test this hypothesis in a cohort of 17 LT recipients followed at Children's Memorial Hospital, Chicago.
2. Subjects and methods
2.1. Subjects
The study participants included 17 pediatric LRLT recipients, on a single-agent calcineurin inhibitor (tacrolimus), followed at The Siragusa Transplant Center at Children's Memorial Hospital (Chicago, IL). Their respective donors, the non-donor parents, and unrelated third party individuals were enrolled in this study. The study protocol was approved by the Institutional Review Board of Children's Memorial Hospital, Chicago, and all participants gave written informed consent.
2.2. Collection and storage of peripheral blood mononuclear cells in liquid nitrogen
Citrated peripheral blood samples were collected from all participants from whom informed consent had been obtained, according to the guidelines of our institution's Institutional Review Board. Peripheral blood mononuclear cells (PBMC) were isolated by density centrifugation as previously described [9], and cryopreserved in liquid nitrogen for cell cultures at a later date. Viability of all cell preparations, as estimated by Trypan blue exclusion, was in excess of 98%.
2.3. HLA typing
DNA from whole blood obtained from the recipient, donor parents, non-donor parents, and unrelated third party individuals was isolated and sequenced for specific HLA genes (HLA typing) as described below.
2.4. DNA isolation
DNA from 350 μl of whole blood was purified by using the Qiagen BioRobot EZ1 System (following EZ1 DNA blood 350-μl protocol), according to the manufacturer's instructions. The extracted DNA was eluted with 200 μl of elution buffer.
2.5. HLA typing
High-resolution molecular typing of class I and II alleles (HLA-A, -B, -C, DQB1 and DRB1) was performed using the AlleleSEQR Sequencing-Based Typing kit from Atria Genetics (South San Francisco, distributed by Abbott Laboratories) and data analyzed with ASSIGN 3.5+ software (Conexio Genomics, Australia).
2.6. Cell sorting by magnetic separation and CFSE labeling
Cryopreserved PBMC from the donor parent, non-donor parent and unrelated third party (who represent the stimulators), were thawed. Of note, the donor parent and non-donor parent were matched for 1 HLA-DR locus (i.e., the recipient shares 1 allele at the HLA-DR locus with each parent), while the unrelated third party was HLA-DR mismatched. Antigen presenting cells (consisting of B cells, monocytes and dendritic cells) were positively selected from cryopreserved PBMC from the donor parent, non-donor parent and unrelated third party using magnetic bead separation (Miltenyi Biotech, Auburn, CA) according to the manufacturer's instructions, for mixed lymphocyte culture as described below.
The antigen presenting cells were then labeled with 2.5 μmol/L carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) according to the manufacturer's instruction, irradiated at 2500 Gy, washed before culture with recipient PBMC as described below. We labeled the APCs with CFSE to enable us to gate them out, ensuring that only events from the recipient PBMCs were captured during analysis.
2.7. Mixed lymphocyte culture
Cryopreserved PBMC were set up in 1 ml cultures containing 1.5 × 106 cells in 5 ml polystyrene round-bottom tubes (BD Biosciences, Bedford, MA). Recipient PBMC were at 1 × 106 cells and irradiated, CFSE-labeled stimulator antigen presenting cells of the donor parent, non-donor parent, and unrelated third party were at 0.5 × 106 cells. In all experiments, the donor and non-donor parent were matched for 1 HLA-DR locus (i.e., the recipient shares 1 allele at the HLA-DR locus with each parent), and the third party was HLA-DR mismatched. Importantly, the non-donor parent and unrelated third party individual therefore served as effective controls to exclude any effect from both recipient and donor being haploidentical, as well as any effect from immunosuppression as the recipients mounted demonstrable responses to stimulation by third party antigens.
Recipient PBMCs and stimulator APCs were cultured for 48 hours. A 48-hour culture period was chosen as a time-course experiment had demonstrated maximum CD69 and CD71 expression at 48 hours. Negative control tubes contained no stimulators, and recipient PBMC with phytohemaglutinin served as the positive control.
2.8. Immunophenotyping studies
The following human mAbs were purchased from (BD Biosciences, San Jose, CA): anti-CD4-PerCP, anti-CD69-APC, anti-CD25-APC, and anti-CD71-APC and immunophenotyping was performed using four-color flow cytometry after the 48-hour culture period. Isotype-matched controls were used to ascertain fluorescence.
2.9. Flow cytometry
Cells were acquired and analyzed on a FACSCalibur flow cytometer (BD Immunocytometry Systems, San Jose, CA). At least 50,000 gated events were collected for cell-surface studies. Data analysis was performed with CellQuest Pro software (BD Immunocytometry Systems, San Jose, CA). Cells were routinely gated on forward scatter/side scatter plots followed by selection of CD4+ and CD4− cells. CD69, CD25 and CD71 expression within activated CD4+ (CFSE-negative) and CD4− (CFSE-negative) recipient PBMC, following phytohemaglutinin, donor parent, non-donor parent, and unrelated third party stimulation was subsequently analyzed.
Within the CD4+ population, we compared CD69, CD71, CD25 expression following donor versus non-donor stimulation: a lower percentage of CD4+CD69+, CD4+CD71+, CD4+CD25+ cells after donor versus non-donor stimulation resulted in a donor/non-donor ratio <1; a higher percentage of CD4+CD69+, CD4+CD71+, CD4+CD25+ cells after donor versus non-donor stimulation resulted in a donor/non-donor ratio ≥1.
The same was also done for the CD4− population.
2.10. Measurement of secreted cytokines with electrochemiluminescence
After the 48-hour culture, tubes were centrifuged and the supernatants were harvested and stored at −70°C. Interferon (IFN)–γ, tumor necrosis factor (TNF)–α, interleukin (IL)–1β, IL-6, IL-17, IL-4, and IL-10 levels in culture supernatants were determined using electrochemiluminescence (MSD, Gaithersburg, MD) according to the manufacturer's instructions.
2.11. Sorting of regulatory T cells by flow cytometry
Cryopreserved recipient PBMCs were thawed and stained with mouse anti-human CD4 PerCP, CD25 APC and CD127 PE (BD Biosciences, San Jose, CA) and subsequently sorted on a flow cytometer (MoFlo, Cytomation, Boston, MA) for CD4+CD25hi˙CD127− cells and CD4+CD25− cells. The CD4+CD25hi˙CD127− cells (defined as regulatory T cells) and the CD4+CD25− cells were subsequently used in the [3H] thymidine incorporation experiments described below.
2.12. T-regulatory functional suppression assay
Cryopreserved PBMCs were prepared in 200 μl cultures containing 3 × 104 cells in 96-well cell culture plates (CoStar Corning, Corning, NY). Recipient CD4+CD25− cells were at 1 × 104cells/well and irradiated donor parent, non-donor parent and unrelated third party PBMCs were at 2 × 104 cells/well. [3H] thymidine was added in the last 18 hours of culture. After 7 days of culture, proliferation of recipient CD4+CD25− cells in response to stimulation by phytohemaglutinin (positive control), irradiated donor parent, non-donor parent, and unrelated third party PBMC, in the absence of and presence of increasing concentration of regulatory T cells, was measured by [3H] thymidine incorporation. The ratio of effector cells to regulatory T cells ranged from 1:1 to 10:1. Background [3H] thymidine uptake observed in cultures containing no stimulators was subtracted from the cultures containing stimulators and results were expressed as CPM.
2.13. FoxP3+ staining
Citrated peripheral blood samples were collected from recipients of a living related donor liver transplant, PBMC was isolated by density centrifugation as described above, and stained for CD4+CD25+FoxP3+ according to manufacturer's instructions (Bio-legend, San Diego, CA). Cells that were CD4+CD25hi˙FoxP3+ were identified as regulatory T cells.
2.14. Statistical analysis
Demographic variables are presented as median (interquartile range).
Fisher exact test was used for analysis of the difference in the above-defined ratios of lymphocyte activation markers following donor and non-donor stimulation in the patients. Student's t test for unpaired samples was used for statistical comparison of means. Wilcoxon rank-sum test was used to test for comparisons of means among non-parametric variables. Analysis of variance (ANOVA) was used to compare lymphoproliferation after donor parent, non-donor parent, and unrelated third party stimulation, and the p values were adjusted using Dunnett method. Statistical calculations were performed using SAS for Windows, version 9.1 (SAS Institute, Cary, NC). Differences with p values <0.05 were considered significant.
3. Results
Early and late T-cell activation marker cell surface expression was assessed after mixed leukocyte cultures in a cohort of pediatric living donor liver transplant recipients whose baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of the 17 transplant recipients
| Pretransplantation diagnosis | Donor gender | Recipient gender |
|---|---|---|
| Biliary atresia | F | M |
| Progressive familial intrahepatic cholestasis | M | M |
| Biliary atresia | F | F |
| Biliary atresia | F | F |
| Neonatal sclerosing cholangitis | F | M |
| Biliary atresia | F | F |
| Biliary atresia | F | F |
| Biliary atresia | F | F |
| Biliary atresia | F | M |
| Primary sclerosing cholangitis | M | M |
| Biliary atresia | M | M |
| Biliary atresia | M | M |
| Biliary atresia | M | F |
| Biliary atresia | F | M |
| Biliary atresia | M | F |
| Biliary atresia | F | F |
| Biliary atresia | F | F |
F, female; M, male.
3.1. Decreased lymphocyte activation after donor stimulation but not non-donor stimulation
Within the CD4+ population, 2 distinct patterns of CD69 and CD71 expressions were observed in these patients: those who had a lower percentage of CD4+CD69+ and CD4+CD71+ cells after donor versus non-donor stimulation (therefore a donor/non-donor ratio <1); and those who had a higher percentage of CD4+CD69+ and CD4+CD71+ cells after donor versus non-donor stimulation (therefore a donor/non-donor ratio ≥1). We identified a total of 8 patients with the above defined ratio of <1(donor hyporesponsiveness) and 9 patients with a ratio ≥1 (immune reactivity to donor) (Figs. 1A, 1B). This difference in ratios was significant for both CD69 and CD71 (p = 0.05 and p = 0.01, respectively), but not for CD25.
Fig. 1.
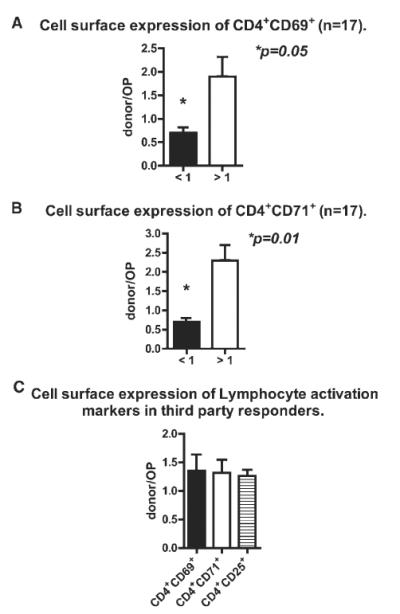
Cell surface CD69, CD71, and CD25 expression within the CD4−population was not significantly different following donor and non-donor stimulation.
Patients who demonstrated a donor hyporesponsiveness pattern for CD69 also had donor hyporesponsiveness pattern for CD71.
To address concerns that the donor hyporesponsiveness could be a result of the donor cells being poor stimulators, third party responders were cultured with both donor and non-donor cells and cell surface expression of CD69, CD71and CD25 by the third party responders was assessed. As can be seen in Fig. 1C, stimulation of the third party responders resulted in a higher percentage of CD4+CD69+, CD4+CD71+, and CD4+CD25+ cells after donor versus non-donor stimulation (i.e., donor/non-donor ratio ≥1).
3.2. Comparison of cytokine production after donor and non-donor stimulation
We next looked to see whether secretion of pro and anti-inflammatory cytokines would be different after donor and non-donor stimulation in our study population.
The recipients with a ratio <1 (i.e., donor hyporesponsiveness) secreted significantly less IFN-γ following donor stimulation compared with IFN-γ production after non-donor stimulation (Fig. 2A) (p = 0.04).
Fig. 2.
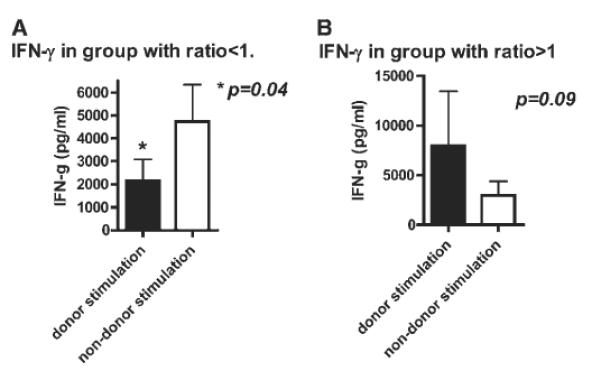
(A) IFN-γ production after donor compared with non-donor stimulation in the patient cohort with donor hyporesponsiveness (n = 8). Recipient PBMCs were cultured with donor and non-donor APCs for 48-hours, and cytokine secretion measured at the end of the culture period. The eight recipients who demonstrated donor hyporesponsiveness (as evidenced by ratio <1 of cell surface expression of lymphocyte activation markers) secreted significantly less IFN-γ after donor stimulation compared with non-donor stimulation (p = 0.04). (B) IFN-γ production after donor compared with non-donor stimulation in the patient cohort with immune reactivity to donor (n = 9). Recipient PBMCs were cultured with donor and non-donor APCs for 48 hours, and cytokine secretion measured at the end of the culture period. The nine recipients with immune reactivity to donor (as evidenced by ratio ≥1 of cell surface expression of lymphocyte activation markers) had increased IFN-γ secretion after donor stimulation compared with non-donor stimulation (p = 0.09).
In contrast, the recipients with a ratio ≥1 (i.e., immune reactivity to donor) secreted more IFN-γ after donor stimulation compared with IFN-γ production after non-donor stimulation (Fig. 2B); although there was a trend of difference, it did not achieve statistical significance (p = 0.09). Other cytokines measured included IL-1β, IL-6, IL-17, TNF-α, IL-4, and IL-10; however, the difference following donor and non-donor stimulation in the two groups did not achieve statistical significance (data not shown).
3.3. Comparison of regulatory T-cell percentage and number in the peripheral blood
To investigate the role of regulation in those recipients with donor hyporesponsiveness, we measured regulatory T-cell percentages and numbers in all study participants.
Recipients with a ratio <1 (donor hyporesponsiveness) tended to have a higher percentage of regulatory T cells compared with the recipients with a ratio ≥1 (immune reactivity to donor); this difference did not achieve statistical significance (data not shown). Regulatory T-cell numbers followed a similar pattern.
3.4. Regulatory T cells inhibit antigen-specific and non–antigen-specific proliferation of recipient T cells
Although regulatory T-cell numbers and percentages were not significantly different between the two groups of patients, we investigated whether they were functionally different. Recipient PBMC were sorted by Mo/Flo (as described above with purities consistently <90%) for CD4+CD25hi˙CD127− regulatory T cells and CD4+CD25− cells and the CD4+CD25− cells were cultured with irradiated donor and non-donor PBMC. CD4+CD25hi˙CD127− regulatory T cells from the eight patients with donor hyporesponsiveness suppressed donor cell induced proliferation, and to a lesser extent, non–donor cell–induced proliferation (Fig. 3A). In contrast, proliferation was minimally suppressed in the presence of regulatory T cells in the nine recipients with immune reactivity to donor (Fig. 3B).
Fig. 3.
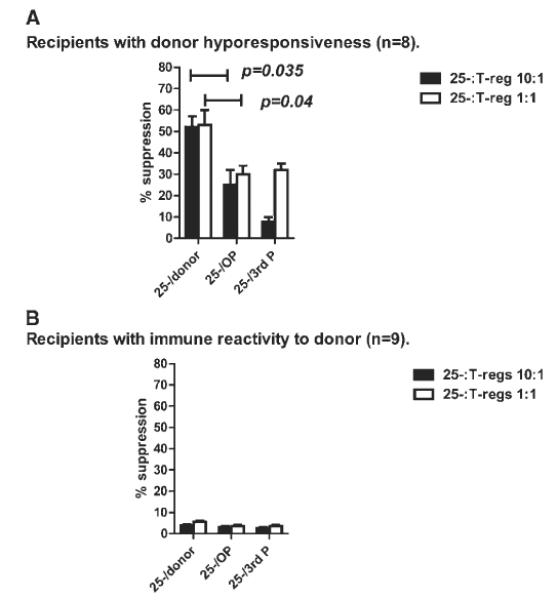
(A) Regulatory T-cells inhibit antigen-specific and antigen non-specific proliferation of recipient T-cells (n = 8). Recipient PBMC were sorted by Mo/Flo for CD4+CD25hi˙CD127− T-regulatory cells and CD4+CD25− cells. The CD4+CD25− cells were cultured for 7 days with irradiated donor and non-donor PBMC and proliferation measured by tritiated thymidine incorporation. Recipient CD4+CD25− cells proliferated when cultured with irradiated donor and non-donor PBMC, however, in the presence of T-regulatory cells, proliferation was suppressed by over 50% after donor stimulation compared with 25 to 30% after non-donor stimulation in the 8 recipients with donor hyporesponsiveness (p = 0.04). Key: OP = DR matched non-donor; third P = DR mismatched non-donor. (B) Regulatory T-cells fail to suppress antigen-specific proliferation of recipient T-cells (n = 9). Recipient PBMC were sorted by Mo/Flo for CD4+CD25hi˙CD127− T-regulatory cells and CD4+CD25−cells. The CD4+CD25− cells were cultured for 7 days with irradiated donor and non-donor PBMC and proliferation measured by tritiated thymidine incorporation. Recipient CD4+CD25− cells proliferated when cultured with irradiated donor and non-donor PBMC; however, proliferation was minimally suppressed (<10%) in the presence of T-regulatory cells in the nine recipients with immune reactivity to donor. Key: OP = DR matched non-donor; third P = DR mismatched non-donor.
3.5. Partial abrogation of donor hyporesponsiveness by rIL-2
We next investigated whether anergy was contributing to the donor hyporesponsiveness observed. Recipient CD4+CD25− cells (n = 8) were set up in culture for 7 days with irradiated donor and non-donor PBMCs. Recombinant IL-2 was added to the donor cultures and proliferation measured by tritiated thymidine incorporation.
Proliferation in response to non-donor stimulation significantly exceeded that following donor stimulation, however, in the presence of rIL-2, anergy was partially overcome as demonstrated by the marked increase in proliferation seen after donor stimulation (Fig. 4).
Fig. 4.
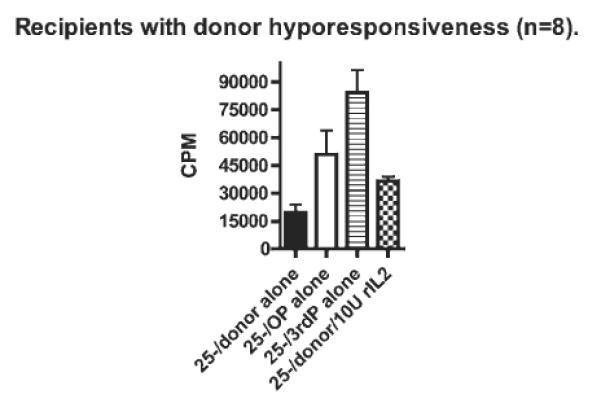
Partial abrogation of donor hyporesponsiveness by rIL-2 (n = 8). Recipient CD4+CD25− cells were set up in culture for 7 days with irradiated donor and non-donor PBMCs. Recombinant IL-2 was added to the donor cultures and proliferation measured by tritiated thymidine incorporation. In the eight recipients who demonstrate donor hyporesponsiveness, culture in the presence of rIL-2 partially abrogates donor hyporesponsiveness with proliferation approaching that seen after non-donor stimulation. Key: OP = DR-matched non-donor; third P = DR mismatched non-donor.
3.6. Effect of calcineurin inhibitor, age at transplantation, and interval from transplantation on lymphocyte activation
To address concerns that the two distinct phenotypes, i.e., donor hyporesponsiveness and immune reactivity to donor, described in our study participants could be due to potential confounding factors such as significantly different immunosuppression levels at the time of blood draw between the recipients in the two groups, age at transplantation and duration from transplantation at the time of blood draw, we compared these variables in the two groups. We also collected data on donor gender.
The median age at transplantation was 0.7 years (IQR 0.6–1.4) in recipients with donor hyporesponsiveness, and 0.5 years (IQR 0.5–0.6) in recipients with immune reactivity to donor (p = 0.1). The median interval from transplantation at the time of blood draw was 5.7 years (IQR 4.7–7.3) in those recipients with donor hyporesponsiveness and 5.0 years (IQR 3.4–10.5) in those recipients with immune reactivity to donor (p = 0.5). As Tacrolimus was the predominant calcineurin inhibitor used, the median Tacrolimus level at the time of blood draw was 3.3 ng/ml (IQR 2.8–3.9) in those recipients with donor hyoresponsiveness and 4.8 ng/ml (IQR 1.8–5.4) in those recipients with immune reactivity to donor p = 0.4 (Table 2). Among the recipients with donor hyporesponsiveness, six of the donors were maternal donors whereas two of the donors were maternal donors in those recipients with immune reactivity to donor.
Table 2.
Median age at transplantation, interval from transplantation at time of blood draw, Tacrolimus level at time of blood draw, and donor characteristics
| Characteristic | Ratio ≥1 (n = 9) | Ratio <1 (n = 8) | p Value |
|---|---|---|---|
| Median age at transplantation (y) (25% and 75% interquartile range) | 0.5 (0.5–0.6) | 0.7 (0.6–1.4) | 0.1 |
| Median interval from transplantation at blood draw (y) (25% and 75% interquartile range) | 5.0 (3.4–10.5) | 5.7 (4.7–7.3) | 0.5 |
| Median tacrolimus level at time of blood draw (ng/ml) (25% and 75% interquartile range) | 4.8 (1.8–5.4) | 3.3 (2.8–3.9) | 0.4 |
| Subjects with a maternal donor | 2 | 6 | NA |
NA, not applicable (too small for any meaningful statistics).
4. Discussion
Our results suggest that cell surface expression of lymphocyte activation markers following donor versus non-donor stimulation can identify pediatric LRLT recipients with phenotypes suggestive of immunologic quiescence and reactivity to their donor. We also demonstrated significantly diminished donor-induced proliferation (in the presence of regulatory cells) in those recipients with a phenotype suggestive of immunologic quiescence to their donor. Furthermore, we observed partial abrogation of donor-hyporesponsiveness after addition of rIL-2. Our findings support our hypothesis that cell surface expression of lymphocyte activation markers and cytokine secretion in MLR can be used to predict donor specific alloreactivity in pediatric LT. To our knowledge, this is the first time cell surface expression of lymphocyte activation markers have been applied in this manner to distinguish differing phenotypes within pediatric LRLT recipients.
Published reports on the use of lymphocyte activation markers in human solid organ transplantation have mostly been limited to cell surface expression of these markers on resting lymphocytes [3,4], and have described a strong association between biopsy proven rejection and CD 69 expression on CD4+ and CD8+ T cells in peripheral blood. Similarly, immunohistochemical analysis of rejection biopsy specimens has shown that a high proportion of graft-infiltrating T cells express CD 69 [5,6,7]. However, not all reports have shown an association between lymphocyte activation markers and rejection [10], perhaps because of differences in the duration from transplantation between the groups studied, as well as differences in the severity of rejection episodes.
Although these studies did not look at activation marker expression in response to donor stimulation as our study did, they do suggest that differences in lymphocyte activation marker expression may be able to distinguish transplant recipients who differ biologically and clinically.
The use of an immunoreactivity index, IR (defined as a ratio of donor:third party–induced proliferation of T cells in a mixed lymphocyte reaction), has been previously described in liver and intestinal transplant recipients, with ratios >1 indicating enhanced donor-specific alloreactivity with an increased risk of rejection and ratios <1 indicating a decreased rejection risk [11–14]. The subjects described in our manuscript did not undergo immunosuppression minimization or weaning to assess whether the immuno-reactive recipients were more prone to rejection compared with the hyporesponsive recipients.
Along similar lines, the recipients whose lymphocyte activation marker expression was suggestive of immunologic quiescence to donor, secreted significantly less IFN-γ after donor stimulation in contrast to recipients whose lymphocyte activation marker expression had suggested immune reactivity to donor, who secreted markedly more IFN-γ after donor stimulation. Data from clinical studies have highlighted the relationship of IFN-γ and the transcription factor T-bet with the process of rejection [15,16] as well as the relationship of low IFN-γ levels with the absence of rejection in solid organ transplant recipients [17]. Interestingly, allospecific CD154+ T cells have been shown to be associated with rejection risk following pediatric liver transplantation [18] (CD 154 is thought to be an excellent surrogate for IFN-γ antigen specific helper T cells). However it is important to note that IFN-γ does not appear to be a positive indicator of rejection in all cohorts [19,20].
Our finding of regulatory T cells in the recipients with a phenotype suggestive of immunologic quiescence to donor significantly suppressed donor-induced proliferation (in contrast to the absence of suppression of donor-induced proliferation seen in the recipients with immune reactivity to donor) is consistent with previous reports [21,22].
We went a step further and demonstrated that donor hyporesponsiveness in MLR is partially overcome by the addition of recombinant IL-2; suggesting that those recipients with donor hyporesponsiveness have potentially reactive T cells which are suppressed by regulatory T cells.
The differences in the two distinct phenotypes observed among the recipients studied, i.e., donor hyporesponsiveness and immune reactivity, were not due to confounding factors such as different immunosuppression levels at the time of blood draw, difference in the age at transplantation, and duration from time of transplant to study enrollment, as we did not find any significant difference in these factors between the two recipient groups (Table 2).
We therefore postulate that recipients with a ratio <1 of cell surface expression of lymphocyte activation markers following donor compared with non-donor stimulation are more likely to be hyporesponsive to their donor as opposed to recipients with a ratio ≥1.
As with all observational and single center studies, our study has some inherent limitations. We could not sort out the mechanism by which recipient regulatory T cells induce suppression of donor-induced proliferation, as we found no significant difference in IL-10 production after donor and non-donor stimulation in the two groups of patients. We also had no access to pretransplantation blood, so we cannot comment on whether donor specific hyporesponsiveness was present pretransplantation. Donor-specific antibodies and panel reactive antibodies were not measured in our cohort of patients, as their routine measurement is not a standard part of our practice and thus we cannot determine how their presence or absence contributes to our results. Furthermore, it is difficult to know the influence of noninherited parental antigens on our results as reports of the influence of noninherited and inherited parental antigens on transplantation describe both immunizing (especially inherited paternal antigen [IPA]) and tolerizing (the noninherited maternal antigen [NIMA] effect) effects [23]. Although the mechanism responsible for the induction of the NIMA effect is still not clear, mechanisms proposed to play a role in the induction of NIMA-specific tolerance include; microchimerism, transfer of soluble HLA from child to mother and vice versa, the concept of privileged sites with modulation of APC, immune deviation, and induction of regulatory T cells because of shared HLA-DR. In our recipients with a phenotype suggestive of immunologic quiescence to donor, six of eight donors were maternal donors; whereas two of nine donors were maternal donors in the recipients with a phenotype suggestive of immune reactivity to donor.
However, the consistency of our findings with previous reports and the potential clinical implication, particularly the use of an assay to predict donor specific alloreactivity is compelling.
In conclusion, using cell surface expression of CD69 and CD71, as well as IFN-γ secretion, we have been able to demonstrate two immunologically distinct phenotypes within a cohort of pediatric LRLT recipients. Whether these findings can be used to tailor immunosuppression in LT recipients remains to be studied in a trial of immunosuppression withdrawal or minimization. Validation of our findings in a larger patient cohort, including deceased donor LT recipients is also required.
Acknowledgments
This work was supported by a Child Health Research Career Development Award from the Department of Pediatrics, Feinberg School of Medicine to UDE.
References
- [1].Testi R, D'Ambrosio D, De Maria R, Santoni A. The CD69 receptor: A multipurpose cell-surface trigger for hematopoietic cells. Immunol Today. 1994;15:479–83. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- [2].Caruso A, D'Ambrosio D, De Maria R, Santoni A. Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation. Cytometry. 1997;27:71–6. doi: 10.1002/(sici)1097-0320(19970101)27:1<71::aid-cyto9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- [3].Posselt AM, D'Ambrosio D, De Maria R, Santoni A. CD69 expression on peripheral CD8 T cells correlates with acute rejection in renal transplant recipients. Transplantation. 2003;76:190–5. doi: 10.1097/01.TP.0000073614.29680.A8. [DOI] [PubMed] [Google Scholar]
- [4].Schowengerdt KO, D'Ambrosio D, De Maria R, Santoni A. Increased expression of the lymphocyte early activation marker CD69 in peripheral blood correlates with histologic evidence of cardiac allograft rejection. Transplantation. 2000;69:2102–7. doi: 10.1097/00007890-200005270-00023. [DOI] [PubMed] [Google Scholar]
- [5].Santamaria M, D'Ambrosio D, De Maria R, Santoni A. The activation antigen CD69 is selectively expressed on CD8+ endomyocardium infiltrating T lymphocytes in human rejecting heart allografts. Hum Immunol. 1992;33:1–4. doi: 10.1016/0198-8859(92)90044-n. [DOI] [PubMed] [Google Scholar]
- [6].Copin MC, D'Ambrosio D, De Maria R, Santoni A. Diagnostic and predictive value of an immunohistochemical profile in asymptomatic acute rejection of renal allografts. Transpl Immunol. 1995;3:229–39. doi: 10.1016/0966-3274(95)80029-8. [DOI] [PubMed] [Google Scholar]
- [7].Grimm PC, D'Ambrosio D, De Maria R, Santoni A. Clinical rejection is distinguished from subclinical rejection by increased infiltration by a population of activated macrophages. J Am Soc Nephrol. 1999;10:1582–9. doi: 10.1681/ASN.V1071582. [DOI] [PubMed] [Google Scholar]
- [8].Brouard S, D'Ambrosio D, De Maria R, Santoni A. Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc Natl Acad Sci U S A. 2007;104:15448–53. doi: 10.1073/pnas.0705834104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].James SP, et al. Current Protocols in Immunology. Strober; 2004. Measurement of basic immunologic characteristics of human mononuclear cells. In Barbara Biereo JEC. [Google Scholar]
- [10].Karpinski M, D'Ambrosio D, De Maria R, Santoni A. Heightened peripheral blood lymphocyte CD69 expression is neither sensitive nor specific as a non-invasive diagnostic test for renal allograft rejection. J Am Soc Nephrol. 2003;14:226–33. doi: 10.1097/01.asn.0000039543.97369.4e. [DOI] [PubMed] [Google Scholar]
- [11].Khera N, D'Ambrosio D, De Maria R, Santoni A. Persistent donor-specific alloreactivity may portend delayed liver rejection during drug minimization in children. Front Biosci. 2007;12:660–3. doi: 10.2741/2090. [DOI] [PubMed] [Google Scholar]
- [12].Ashokkumar C, D'Ambrosio D, De Maria R, Santoni A. Proliferative alloresponse of T-cytotoxic cells identifies rejection-prone children with steroid-free liver transplantation. Liver Transplant. 2009;15:978–85. doi: 10.1002/lt.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ashokkumar C, D'Ambrosio D, De Maria R, Santoni A. Allospecific CD154+ B cells associate with intestine allograft rejection in children. Transplantation. 1994;90:1226–31. doi: 10.1097/TP.0b013e3181f995ce. [DOI] [PubMed] [Google Scholar]
- [14].Ashokkumar C, D'Ambrosio D, De Maria R, Santoni A. Allospecific CD154+ T cells identify rejection-prone recipients after pediatric small-bowel transplantation. Surgery. 2009;146:166–73. doi: 10.1016/j.surg.2009.04.006. [DOI] [PubMed] [Google Scholar]
- [15].Hauser IA, D'Ambrosio D, De Maria R, Santoni A. Prediction of acute renal allograft rejection by urinary monokine induced by IFN-gamma (MIG) J Am Soc Nephrol. 2005;16:1849–58. doi: 10.1681/ASN.2004100836. [DOI] [PubMed] [Google Scholar]
- [16].Hoffmann SC, D'Ambrosio D, De Maria R, Santoni A. Functionally significant renal allograft rejection is defined by transcriptional criteria. Am J Transplant. 2005;5:573–81. doi: 10.1111/j.1600-6143.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- [17].Takatsuki M, D'Ambrosio D, De Maria R, Santoni A. Analysis of alloreactivity and intragraft cytokine profiles in living donor liver transplant recipients with graft acceptance. Transpl Immunol. 2001;8:279–86. doi: 10.1016/s0966-3274(01)00027-2. [DOI] [PubMed] [Google Scholar]
- [18].Ashokkumar C, D'Ambrosio D, De Maria R, Santoni A. Allospecific CD154+ T cells associate with rejection risk after pediatric liver transplantation. Am J Transplant. 2009;9:179–91. doi: 10.1111/j.1600-6143.2008.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sadeghi M, D'Ambrosio D, De Maria R, Santoni A. Evidence for IFN-gamma upand IL-4 downregulation late post-transplant in patients with good kidney graft outcome. Clin Transpl. 2007;21:449–59. doi: 10.1111/j.1399-0012.2007.00665.x. [DOI] [PubMed] [Google Scholar]
- [20].Ghafari A, D'Ambrosio D, De Maria R, Santoni A. Serum T-lymphocyte cytokines cannot predict early acute rejection in renal transplantation. Transplant Proc. 2007;39:958–61. doi: 10.1016/j.transproceed.2007.03.015. [DOI] [PubMed] [Google Scholar]
- [21].Koshiba T, D'Ambrosio D, De Maria R, Santoni A. Clinical, immunological, and pathological aspects of operational tolerance after pediatric living-donor liver transplantation. Transpl Immunol. 2007;17:94–7. doi: 10.1016/j.trim.2006.10.004. [DOI] [PubMed] [Google Scholar]
- [22].Yoshizawa A, Ito A, Li Y, Koshiba T, Sakaguchi S, Wood KJ. The roles of CD25+CD4+ regulatory T cells in operational tolerance after living donor liver transplantation. Transplant Proc. 2005;37:37–9. doi: 10.1016/j.transproceed.2004.12.259. [DOI] [PubMed] [Google Scholar]
- [23].van den Boogaardt DE, D'Ambrosio D, De Maria R, Santoni A. The influence of inherited and noninherited parental antigens on outcome after transplantation. Transpl Int. 2006;19:360–71. doi: 10.1111/j.1432-2277.2006.00304.x. [DOI] [PubMed] [Google Scholar]


