Summary
The cardiac conduction system (CCS)-lacZ insertional mouse mutant strain genetically labels the developing and mature CCS. This pattern of expression is presumed to reflect the site of transgene integration rather than regulatory elements within the transgene proper. We sought to characterize the genomic structure of the integration locus and identify nearby gene(s) that might potentially confer the observed CCS-specific transcription. We found rearrangement of chromosome 7 between regions D1 and E1 with altered transcription of multiple genes in the D1 region. Several lines of evidence suggested that regulatory elements from at least one gene, Slco3A1, influenced CCS-restricted reporter gene expression. In embryonic hearts, Slco3A1 was expressed in a spatial pattern similar to the CCS-lacZ transgene and was similarly neuregulin-responsive. At later stages, however, expression patterns of the transgene and Slco3A1 diverged, suggesting that the Slco3A1 locus may be necessary, but not sufficient to confer CCS-specific transgene expression in the CCS-lacZ line.
Keywords: transgenics, mouse, conduction system, CCS-lacZ, Slco3A1
The cardiac conduction system (CCS)-lacZ mouse strain is an insertional mutant in which reporter gene expression is observed in the CCS of the developing and adult heart. These mice have been used to clarify mechanisms of CCS formation and function (Kitajima et al., 2006; Poelmann et al., 2004; Rentschler et al., 2001, 2002), visualize effects of various genetic mutations on CCS development (Morley et al., 2005; Yang et al., 2006), and identify the role of the CCS in cardiac rhythm disturbances (Gonzalez et al., 2004; Jongbloed et al., 2004, 2005). Our initial studies suggested that the expression of lacZ in the CCS was dependent upon the site of integration and the flanking genomic sequences rather than Engrailed-2 regulatory elements within the transgene proper (Rentschler et al., 2001, 2002). Accordingly, we sought to characterize the site of integration and identify nearby gene(s) that might confer CCS-specific transcription upon the integrated transgene.
Fluorescent in situ hybridization (FISH) on chromosomal DNA from a homozygous CCS-lacZ mouse revealed fluorescent signal on both copies of chromosome 7 (Fig. 1). The intensity of staining on one chromosome from each pair was greater than on the second copy, suggesting that during breeding to homozygosity the transgene locus had undergone a recombination event, resulting in unequal copies of transgene array on each chromosome of the mouse studied. Supporting this possibility, Southern blots performed with a lacZ probe revealed two distinct banding patterns in offspring from homozygous × wild-type matings (data not shown).
FIG. 1.
CCS-lacZ transgene is on chromosome 7. (a) Fluorescent in situ hybridization (FISH) performed on CCS-lacZ mouse chromatin with tagged nucleotide probe for lacZ. Arrows indicate probe binding at region of transgene integration. (b) DAPI staining with chromosome 7 labeled.
To identify sequences flanking the transgene, we prepared a phage library from genomic DNA from a homozygous CCS-lacZ mouse. The library was screened for clones including transgene elements using a radiolabeled lacZ DNA probe. Ten out of 12 independent phage clones contained portions of lacZ sequence and flanking genomic sequence from chromosome 7. However, given the size of the transgene array (over 14 kB per copy), none of the positive clones included flanking genomic sequence at both ends of insert. Surprisingly, analysis of genomic DNA sequences from the isolated phage clones implicated three distinct regions of chromosome 7 as flanking portions of the transgene. These regions (chromosomal regions D1, E1, and F2) span over 30 million bases. The discrete localization of the FISH signal at a single locus of chromosome 7 suggested that portions of these three disparate regions were brought into proximity during transgene integration through a complex recombination event. The presence of more than two unique flanking sequence elements also indicated a complex genomic structure and suggested that there must be (a) two different integration sites on at least one copy of chromosome 7, separated from one another at a distance below the level of resolution by FISH analysis, and/or (b) different integration sites on each chromosome. Because all hemizygous offspring have the CCS phenotype, the first possibility appeared more likely.
The size and complexity of the integration site led us to create a bacterial artificial chromosome (BAC) library from CCS-lacZ genomic DNA. Pooled samples of BACs were screened by PCR using primers for the Engrailed-2/lacZ junction of the transgene. Ten independent positive BAC clones were purified and end sequenced. Of these 10 clones, six had genomic sequence from chromosome 7 regions E1 or D1, including three with E1 at both ends and three with a combination of E1 and D1, confirming our hypothesis that the chromosome had undergone rearrangement during integration of the transgene. Raw sequence data from phage and BAC libraries are available in an online data supplement.
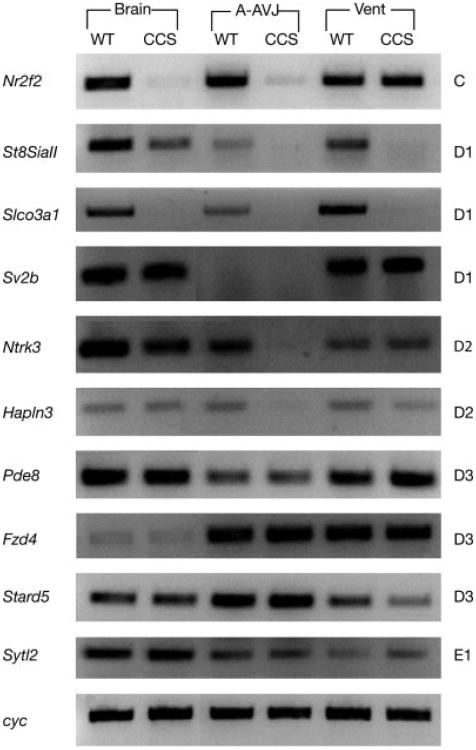
Using RT-PCR, we characterized the expression patterns of 10 genes in the C–E1 region in wild type and homozygous CCS-lacZ mice (Fig. 2). Three tissues were examined, including the brain, the superior portion of the heart (atria, appendages and AV junction), and the inferior portion (ventricle). Six of the 10 genes exhibited a reduction in transcript levels between wild type and mutant (Fig. 2). Furthermore, we saw tissue specific differences in the extent of altered expression (Fig. 2).
FIG. 2.
RT-PCR analysis of integration region. Ten genes (listed on left) from the C–E1 (position listed on right) region of chromosome 7 were tested for alterations in transcription. RNA from the brain, atria, appendages, and AV junction (A-AVJ), and ventricle (Vent) of wild type (WT) or homozygous CCS-lacZ (CCS) mice was used as template. Cyclophilin (cyc) was employed as a loading control for each reaction. A representative set of control reactions is shown.
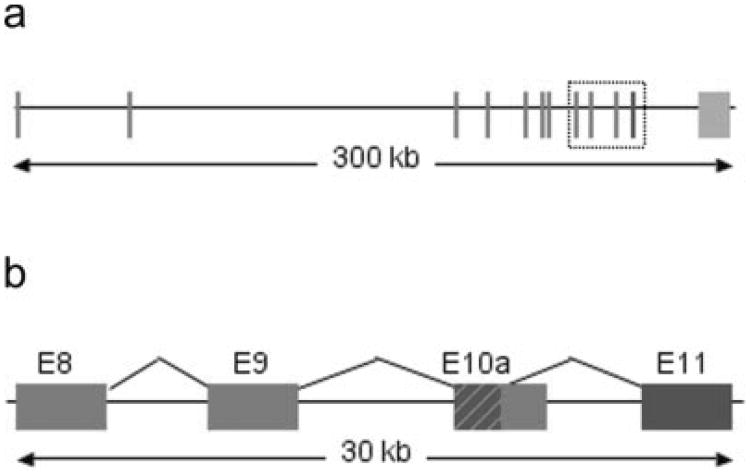
The RT-PCR data suggested the D1 region was the most influenced by the transgene integration. To narrow our search for candidate genes, we examined a list of sequence tags from a SAGE library generated from microdissected left bundle branch (LBB) tissue that met two criteria. First, they were enriched in LBB preparations compared to whole ventricular muscle, and second, they mapped to the D1 region of chromosome 7. A single transcript from the Slco3A1 gene met both these criteria. RT-PCR (Fig. 2) and Northern blot analysis (not shown) demonstrated marked downregulation of Slco3A1 expression in the hearts of homozygous CCS-lacZ mice compared to wild-type controls. Slco3a1 is a member of a large protein superfamily (Hagenbuch and Meier, 2003, 2004), and it has been cloned from mouse as well as rat and human (Adachi et al., 2003; Melia et al., 1998; Tamai et al., 2000). Members of the Slco family, each having 12 transmembrane domains, have been implicated in the transport of both natural substances (prostaglandins, bile salts, thyroid, and steroid hormones) and exogenous drugs (including but not limited to digoxin, angiotensin converting enzyme inhibitors, HMG-coenzyme A reductase inhibitors, methotrexate, and rifampin) across the cell membrane (Hagenbuch and Meier, 2003, 2004). To date, there are no data indicating an association of Slco3A1 with the CCS. Interestingly, our initial sequence analysis placed the SAGE library sequence tag ∼9 kB downstream of the last exon (exon 10) of Slco3A1, i.e., beyond the putative transcribed region. However, detailed examination of mouse EST databases and a comparison to ESTs from the human Slco3A1 orthologue predicted an alternatively spliced transcript joining a portion of exon 10 with a previously unrecognized mouse exon 11, within which the sequence tag resided (Fig. 3a,b). RT-PCR with isoform specific primers confirmed expression in the heart (Fig. 4a).
FIG. 3.
Slco3A1 locus (a) Diagram of Slco3A1 gene with previously described exons 1–10 in red. Exon 11 is shown in blue. (b) Detail of exons 8– 11 (box outline in A) showing the common region of exon10 (E10a) and the two splice variants, Slco3A1a (red) and Slco3A1b (blue). Primers to detect the different forms were made specifically to these areas.
FIG. 4.
RT-PCR analysis of Slco3A1 (a) Both Slco3A1b and Slco3A1a transcripts are expressed in the heart. (b) RT-PCR for Cx40, cyclophilin (cyc), and Slco3A1 using RNA from working ventricular muscle (M) and Purkinje-fiber (P) enriched tissue. Cyclophilin serves as a loading control.(c) Analysis of Slco3A1 and cyclophilin (cyc) expression in hearts from E10.5 and E12.5 embryos either untreated (−) or treated (+) with neuregulin for 48 h.
We next examined whether Slco3A1 was preferentially expressed in microdissected tissue enriched in Purkinje fibers. RNA was prepared from subendocardial Purkinje fibers isolated from adult murine hearts and working ventricular muscle from the epi- and mid-myocardium. RT-PCR was performed using primers that amplified both Slco3A1 transcripts. To confirm the specificity of the harvesting procedure, we also examined expression of the CCS marker Connexin 40 (Cx40). Both Cx40 and Slco3A1 are enriched in the Purkinje fiber preparations compared to working myocardium (Fig. 4b).
LacZ expression in cultured CCS-lacZ hearts is markedly induced by neuregulin only between E9.5 and E12.5 (Rentschler et al., 2002). To determine whether the endogenous Slco3A1 gene was similarly neuregulin-responsive, we treated pools of 10 isolated hearts from E10.5 or E12.5 mice with neuregulin or vehicle alone. After 48 h in culture, expression of the Slco3A1 transcript was detectable in control hearts and markedly induced in the neuregulin-treated E10.5 but not E12.5 samples (Fig. 4c), paralleling the behavior of the CCS-lacZ reporter gene.
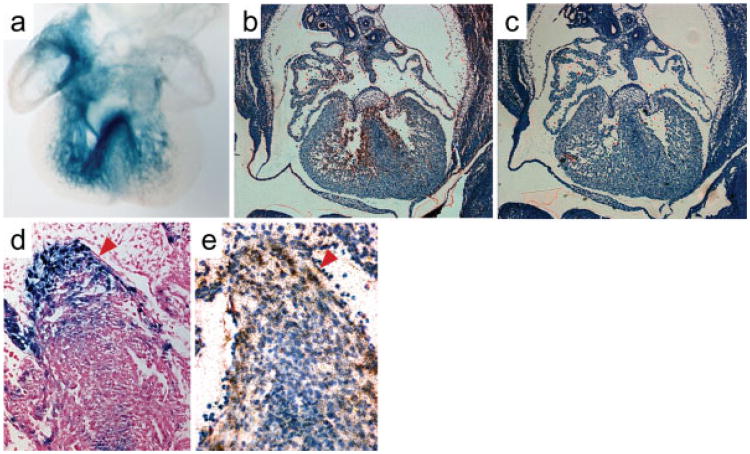
RNA in situ hybridization was performed on sections from E12.5 and neonatal hearts to determine the endogenous Slco3A1 expression pattern. Although weakly expressed, Slco3A1 was observed in the right atria, AV junction, along the interventricular septum, and ventricular trabeculae in a pattern quite similar to CCS-lacZ reporter gene expression at this stage (Fig. 5a-c). Higher magnification images revealed Slco3A1 expression to be primarily myocardial, rather than endocardial (Fig. 5d,e). In neonates, expression appeared modestly enriched in the AV node (Fig. 6a-d) and His bundle (Fig. 6e-h). However, at this latter stage, no specific expression was observed in the bundle branches or Purkinje fibers as suggested by SAGE and RT-PCR, conceivably due to a difference in the sensitivity of the assays and the very low abundance of the Slco3A1 transcript.
FIG. 5.
In situ hybridization of E12.5 embryos. (a) Whole-mount lacZ staining of an E12.5 CCS-lacZ heart. Reproduced with permission from Rentschler et al. (2001). (b) Radioactive RNA in situ hybridization of E12.5 embryonic heart performed with a cocktail of three Slco3A1 antisense probes. (c). Hybridization of serial section with control Slco3A1 sense probes.(d) LacZ staining of an E12.5 minK-lacZ heart shows primarily subendocardial staining in the septum where the forming His bundle and bundle branches are located.(e) Slco3A1 expression does not appear to be in the endocardium (arrowheads).
FIG. 6.
Central conduction system expression of Slco3A1 in neonatal hearts. (a) Section of the atrioventricular junction at the level of the AV node in a neonatal mouse heart hybridized with Slco3A1 sense control probes. (b) Serial section probed with antisenseSlco3A1 showing expression in the node. (c, d) Close up views of the AV node regions from panels a and b. (e) Control section at the level of the His bundle. (f) Slco3A1 expression can be clearly seen in the His bundle in this serial section. (g, h) High magnification views of panels e and f.
We examined this issue further by investigating the expression of other candidate genes. Nr2f2 (Coup-TFII) is required in the embryo for many processes including heart development and angiogenesis, but its expression is not associated with the CCS (Lee et al., 2004; Pereira et al., 1999). ST8SiaII (STX) is a polysialyic acid transferase whose target is the neural cell adhesion molecule (NCAM) (Angata and Fukuda, 2003). This was intriguing, as polysialyted-NCAM and NCAM itself have been used as markers of conduction system in chick and human respectively (Soler et al., 2001; Watanabe et al., 1992). Immunohistochemical studies with PSA-NCAM antibodies on E13.5 mice revealed neural but not cardiac expression (data not shown). Furthermore, ST8SiaII was neuregulin inducible at E12.5, a stage when we no longer see significant upregulation of CCS-lacZ transgene expression (data not shown). Neurotrophin 3, (TrkC; Ntrk3), is expressed in the developing CCS of the rat from E11 onwards (Hiltunen et al., 1996). There was no expression of Ntrk3 within or adjacent to the CCS in adult mouse hearts (data not shown). Hapln3 is expressed within smooth muscle cells but not cardiac myocytes (Ogawa et al., 2004). A bioinformatics analysis of all known microRNAs from chromosome 7 was performed, but none were associated with the D1 region. Therefore, of the various candidate genes in the D1 region, Slco3A1 most faithfully, albeit incompletely, reflected the expression pattern of the CCS-lacZ reporter gene.
Taken together, these data suggest that regulatory elements from the Slco3A1 gene may influence, but are not solely responsible for, conduction system specific transgene expression in the CCS-lacZ line. The extensive chromosomal rearrangement may have resulted in the serendipitous assembly of requisite cis-acting sequences. Alternatively, a yet uncharacterized gene within this locus exists, whose pattern of expression more closely parallels that of the CCS-lacZ transgene. Regardless, it appears unlikely that discrete regulatory elements from this strain that can be used to heterologously express other genes of interest specifically in the CCS will be identified. Nonetheless, the CCS-lacZ mouse remains an important tool for studies of conduction system biology. In addition to our initial studies visualizing CCS maturation and functional activity (Rentschler et al., 2001, 2002), the mice have recently been used in lineage tracing experiments, studies of the role of the neural crest in CCS formation, as well as studies of arrhythmogenic foci in CCS remnants (Jongbloed et al., 2005; Kitajima et al., 2006; Poelmann et al., 2004). Thus, the CCS-lacZ line has been and will continue to be a valuable resource for analysis of mutants with potential morphologic or functional conduction system abnormalities (Morley et al., 2005; Yang et al., 2006).
Methods
Mouse Strains
The CCS-lacZ line is a result of a fortuitous insertion of an Engrailed-2/lacZ fusion construct previously described (Logan et al., 1993; Rentschler et al., 2001). It is maintained in a CD-1 background. The minK-lacZ line was generated by knockin of lacZ into the endogenous minK locus (Kupershmidt et al., 1999).
Fluorescent In Situ Hybridization
Fluorescent in situ hybridization was performed by SeeDNA (Windsor, Ontario) according to standard techniques using spleen lymphocytes (Feng et al., 1994; Heng et al., 1992). Biotinylated lacZ DNA was hybridized with the chromosomes, and fluorescein isothiocyanate conjugated with avidin was used to detect adherent probe. Comparison with samples stained with 4,6-diamidino-2-phenylindole (DAPI) was used to identify each chromosome (Heng et al., 1992).
Construction and Screening of Genomic Libraries
Genomic DNA from homozygous CCS-lacZ mice was partially digested with Sau3A1 and cloned into λFIX II phage DNA (Stratagene, La Jolla, CA). Genomic DNA was also used to prepare a BAC library (Bio S&T, Montreal, Quebec). BACs were screened by PCR using oligonucleotide primers for the Engrailed-2/lacZ junction of the transgene (Logan et al., 1993). NCBI and the UCSC genome browser (http://genome.ucsc.edu/) were used for sequence analysis (Kent, 2002; Kent et al., 2002). See online data supplement for raw sequence data from both phage and BAC libraries.
RNA Preparation and RT-PCR from Adult and Embryonic Mouse Tissues
RNA was prepared from adult and embryonic mouse tissues using TRIZOL reagent (Invitrogen, Carlsbad, CA) or the PicoPure RNA isolation kit (Arcturus, Mountain View, CA) according to the manufacturer's instructions. “Purkinje fiber” fractions (P) were prepared by microdissection of the fiber network away from the exposed myocardial surface of the ventricle. This population will therefore contain both endocardial and subendocardial cells. “Working myocyte” fractions (M) were prepared from epicardial and mid-myocardial tissue, primarily from the left ventricle. Enrichment of the PF fraction for conduction tissue was analyzed by reverse transcriptase (RT)-PCR for the Connexin40 transcript relative to the working myocyte fraction. RT-PCR was performed using a PTC-100 (MJ Research, Waltham, MA).
Preparation and Analysis of SAGE Library
Comparisons were made between SAGE libraries constructed from left-bundle branch conduction tissue and entire left ventricles from 19-day-old SvEv mice. The SAGE library was constructed by the micro sage technique (St. Croix et al., 2000). The SAGE 3.0.1 program (courtesy of Victor Velculescu and Ken Kinzler, Johns Hopkins University School of Medicine, Baltimore, Maryland, United States) was used to extract SAGE tags and eliminate duplicate ditags. Identity of SAGE tags was obtained from the National Center for Biotechnology Information (NCBI) “reliable” tag map set for UniGene (available at http://www.ncbi.nlm.nih.gov/SAGE). The tag (CATGATATAAAGTA) corresponding to the Slco3A1 gene was present in six copies in the conduction system library and one copy from the left ventricular library, corresponding to a P-value <0.10 (P-values determined by the monte carlo method using SAGE 2000 version 4.12, available at www.sagenet.org). Although these tag counts do not demonstrate conduction system enrichment by statistical criteria due to low tag count, the presence of the tag corresponding to Slco3A1 in the conduction system library made it a candidate gene in the chromosomal region of interest.
RNA In Situ Hybridization
Three riboprobes were made using RT-PCR against a mouse cDNA heart library. The PCR primers had both T7 and T3 promoter sequences for transcription (T7 for antisense and T3 for sense probes). The riboprobes were transcribed using 35S-UTP for labeling. In situ hybridizations were carried out on hearts or embryos fixed with 4% paraformaldehyde. Tissues were paraffin embedded and cut into 7 μm sections. Slides were dewaxed and rehydrated with (30, 50, 70, 95, 100% × 3) EtOH and DEPC mixture. Slides were prepared for hybridization with 0.85% Tampon Saline Buffer, microwaved in citrate buffer, bleached with hydrogen peroxide, and proteinase K treated for 20 min. Probes were hybridized overnight at 50°C in a moist chamber. After washing the slides with several washes in 5×, 2× and 1 × SSC, the slides were emulsed in Kodak NTB2 autoradiography emulsion. Slides were exposed for 4 weeks and then developed using Kodak developer and fixer. The slides were counter-stained with toluidine blue and mounted. Images were viewed with Fiber-Lite red light illuminator.
LacZ Staining
Hearts were whole-mount lacZ stained, paraffin embedded and sectioned as previously described (Moskowitz et al., 2004).
Supplementary Material
Acknowledgments
minK-lacZ mice were generously provided by Sabina Kupershmidt and Dan M. Roden.
Footnotes
This article contains Supplementary Material available via the Internet at http://www.interscience.wiley.com/jpages/1526-954X/suppmat.
Literature Cited
- Adachi H, Suzuki T, Abe M, Asano N, Mizutamari H, Tanemoto M, Nishio T, Onogawa T, Toyohara T, Kasai S, Satoh F, Suzuki M, Tokui T, Unno M, Shimosegawa T, Matsuno S, Ito S, Abe T. Molecular characterization of human and rat organic anion transporter OATP-D. Am J Physiol Renal Physiol. 2003;285:F1188–F1197. doi: 10.1152/ajprenal.00402.2002. [DOI] [PubMed] [Google Scholar]
- Angata K, Fukuda M. Polysialyltransferases: Major players in polysialic acid synthesis on the neural cell adhesion molecule. Biochimie. 2003;85:195–206. doi: 10.1016/s0300-9084(03)00051-8. [DOI] [PubMed] [Google Scholar]
- Feng GS, Shen R, Heng HH, Tsui LC, Kazlauskas A, Pawson T. Receptor-binding, tyrosine phosphorylation and chromosome localization of the mouse SH2-containing phosphotyrosine phosphatase Syp. Oncogene. 1994;9:1545–1550. [PubMed] [Google Scholar]
- Gonzalez MD, Contreras LJ, Jongbloed MR, Rivera J, Donahue TP, Curtis AB, Bailey MS, Conti JB, Fishman GI, Schalij MJ, Gittenberger-de Groot AC. Left atrial tachycardia originating from the mitral annulus-aorta junction. Circulation. 2004;110:3187–3192. doi: 10.1161/01.CIR.0000147613.45259.D1. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609:1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: Phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- Heng HH, Squire J, Tsui LC. High-resolution mapping of mammalian genes by in situ hybridization to free chromatin. Proc Natl Acad Sci USA. 1992;89:9509–9513. doi: 10.1073/pnas.89.20.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen JO, Arumae U, Moshnyakov M, Saarma M. Expression of mRNAs for neurotrophins and their receptors in developing rat heart. Circ Res. 1996;79:930–939. doi: 10.1161/01.res.79.5.930. [DOI] [PubMed] [Google Scholar]
- Jongbloed MR, Schalij MJ, Poelmann RE, Blom NA, Fekkes ML, Wang Z, Fishman GI, Gittenberger-De Groot AC. Embryonic conduction tissue: A spatial correlation with adult arrhythmogenic areas. J Cardiovasc Electrophysiol. 2004;15:349–355. doi: 10.1046/j.1540-8167.2004.03487.x. [DOI] [PubMed] [Google Scholar]
- Jongbloed MR, Wijffels MC, Schalij MJ, Blom NA, Poelmann RE, van der Laarse A, Mentink MM, Wang Z, Fishman GI, Gittenberger-de Groot AC. Development of the right ventricular inflow tract and moderator band: A possible morphological and functional explanation for Mahaim tachycardia. Circ Res. 2005;96:776–783. doi: 10.1161/01.RES.0000162000.03997.65. [DOI] [PubMed] [Google Scholar]
- Kent WJ. BLAT—The BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima S, Miyagawa-Tomita S, Inoue T, Kanno J, Saga Y. Mesp1-nonexpressing cells contribute to the ventricular cardiac conduction system. Dev Dyn. 2006;235:395–402. doi: 10.1002/dvdy.20640. [DOI] [PubMed] [Google Scholar]
- Kupershmidt S, Yang T, Anderson ME, Wessels A, Niswender KD, Magnuson MA, Roden DM. Replacement by homologous recombination of the minK gene with lacZreveals restriction of minK expression to the mouse cardiac conduction system. Circ Res. 1999;84:146–152. doi: 10.1161/01.res.84.2.146. [DOI] [PubMed] [Google Scholar]
- Lee CT, Li L, Takamoto N, Martin JF, Demayo FJ, Tsai MJ, Tsai SY. The nuclear orphan receptor COUP-TFII is required for limb and skeletal muscle development. Mol Cell Biol. 2004;24:10835–10843. doi: 10.1128/MCB.24.24.10835-10843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C, Khoo WK, Cado D, Joyner AL. Two enhancer regions in the mouse En-2 locus direct expression to the mid/hindbrain region and mandibular myoblasts. Development. 1993;117:905–916. doi: 10.1242/dev.117.3.905. [DOI] [PubMed] [Google Scholar]
- Melia MJ, Bofill N, Hubank M, Meseguer A. Identification of androgen-regulated genes in mouse kidney by representational difference analysis and random arbitrarily primed polymerase chain reaction. Endocrinology. 1998;139:688–695. doi: 10.1210/endo.139.2.5763. [DOI] [PubMed] [Google Scholar]
- Morley GE, Danik SB, Bernstein S, Sun Y, Rosner G, Gutstein DE, Fishman GI. Reduced intercellular coupling leads to paradoxical propagation across the Purkinje-ventricular junction and aberrant myocardial activation. Proc Natl Acad Sci USA. 2005;102:4126–4129. doi: 10.1073/pnas.0500881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz IP, Pizard A, Patel VV, Bruneau BG, Kim JB, Kupershmidt S, Roden D, Berul CI, Seidman CE, Seidman JG. The T-Box transcription factor Tbx5 is required for the patterning and maturation of the murine cardiac conduction system. Development. 2004;131:4107–4116. doi: 10.1242/dev.01265. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Oohashi T, Sata M, Bekku Y, Hirohata S, Nakamura K, Yonezawa T, Kusachi S, Shiratori Y, Ninomiya Y. Lp3/Hapln3, a novel link protein that co-localizes with versican and is coordinately up-regulated by platelet-derived growth factor in arterial smooth muscle cells. Matrix Biol. 2004;23:287–298. doi: 10.1016/j.matbio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelmann RE, Jongbloed MR, Molin DG, Fekkes ML, Wang Z, Fishman GI, Doetschman T, Azhar M, Gittenberger-de Groot AC. The neural crest is contiguous with the cardiac conduction system in the mouse embryo: A role in induction? Anat Embryol (Berl) 2004;208:389–393. doi: 10.1007/s00429-004-0401-6. [DOI] [PubMed] [Google Scholar]
- Rentschler S, Vaidya DM, Tamaddon H, Degenhardt K, Sassoon D, Morley GE, Jalife J, Fishman GI. Visualization and functional characterization of the developing murine cardiac conduction system. Development. 2001;128:1785–1792. doi: 10.1242/dev.128.10.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentschler S, Zander J, Meyers K, France D, Levine R, Porter G, Rivkees SA, Morley GE, Fishman GI. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci USA. 2002;99:10464–10469. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler AP, Gilliard G, Xiong Y, Knudsen KA, Martin JL, De Suarez CB, Mota Gamboa JD, Mosca W, Zoppi LB. Overexpression of neural cell adhesion molecule in Chagas' myocarditis. Hum Pathol. 2001;32:149–155. doi: 10.1053/hupa.2001.21562. [DOI] [PubMed] [Google Scholar]
- St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Tamai I, Ohashi R, Nezu JI, Sai Y, Kobayashi D, Oku A, Shimane M, Tsuji A. Molecular and functional characterization of organic cation/carnitine transporter family in mice. J Biol Chem. 2000;275:40064–40072. doi: 10.1074/jbc.M005340200. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Timm M, Fallah-Najmabadi H. Cardiac expression of polysialylated NCAM in the chicken embryo: Correlation with the ventricular conduction system. Dev Dyn. 1992;194:128–141. doi: 10.1002/aja.1001940206. [DOI] [PubMed] [Google Scholar]
- Yang L, Cai CL, Lin L, Qyang Y, Chung C, Monteiro RM, Mummery CL, Fishman GI, Cogen A, Evans S. Isl1Cre reveals a common Bmp pathway in heart and limb development. Development. 2006;133:1575–1585. doi: 10.1242/dev.02322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.