Abstract
The underwater adhesion of marine mussels relies on mussel foot proteins (mfps) rich in the catecholic amino acid 3, 4-dihydroxyphenylalanine (Dopa). As a side-chain, Dopa is capable of strong bidentate interactions with a variety of surfaces, including many minerals and metal oxides. Titanium is among the most widely used medical implant material and quickly forms a TiO2 passivation layer under physiological conditions. Understanding the binding mechanism of Dopa to TiO2 surfaces is therefore of considerable theoretical and practical interest. Using a surface forces apparatus, we explored the force-distance profiles and adhesion energies of mussel foot protein 3 (mfp-3) to TiO2 surfaces at three different pHs (pH3, 5.5 and 7.5). At pH3, mfp-3 showed the strongest adhesion force on TiO2, with an adhesion energy of ~ −7.0 mJ/m2. Increasing the pH gives rise to two opposing effects: (1) increased oxidation of Dopa, thus decreasing availability for the Dopa-mediated adhesion, and (2) increased bidentate Dopa-Ti coordination, leading to the further stabilization of the Dopa group and thus an increasing of adhesion force. Both effects were reflected in the resonance-enhanced Raman spectra obtained at the three deposition pHs. The two competing effects give rise to a higher adhesion force of mfp-3 on TiO2 surface at pH 7.5 than at pH 5.5. Our results suggest that Dopa-containing proteins and synthetic polymers have great potential as coating materials for medical implant materials, particularly if redox activity can be controlled.
Introduction
Mussels have mastered the art of wet adhesion, producing a bundle of threads tipped with adhesive pads, known collectively as the byssus, which serves as a robust holdfast in the often-treacherous environment of the intertidal zone. The byssus consists of a suite of proteins, having distinct localization and function, but united by the presence of the unusual modified amino acid 3,4-dihydroxyphenylalanine (Dopa). Mussel foot protein-3 fast (mfp-3f), a primary adhesive protein located at the plaque/substrate, has a Dopa content of 20 mol%, and has been shown to exhibit remarkable adhesive properties to mica surfaces. 1
The ability of Dopa to bind to surfaces with wide-ranging chemical and physical properties has inspired much research dedicated to understanding the mechanism of mussel adhesion1–2 as well as developing biomimetic adhesives for underwater and medical as well as dental applications.3 Titanium is widely used in medical implant devices. A 2 to 20nm thick TiO2 passivation layer is rapidly formed on titanium under physiological conditions, yielding a hydroxyl-terminated surface that is vital in promoting biocompatibility.4 Therefore, study of the interaction between Dopa-containing proteins/polymers and TiO2 substrates is of particular interest.
Dopa has a strong binding affinity to a variety of metal oxide surfaces due to the stable bidentate modes of H-bonding and metal coordination,5 therefore, Dopa containing proteins and polymers have great potential as molecular anchors of coatings on metal oxide surfaces. The coordination chemistry of Dopa/catecholic compounds has been studied extensively.6 AFM tests have shown that the pull-off of a single Dopa residue adsorbed to a wet titania surface requires a breaking force of nearly 1 nN and is completely reversible.2 Strong adhesion forces have also been reported by recent SFA tests of Dopa-grafted peptides and mfp-1 on TiO2 substrates.3a, 7
Density functional theory studies have shown that the binding of a Dopa group to a TiO2 surface involves at least three different forms: molecular adsorption (through H-bond), partially dissociated monodentate adsorption, and fully dissociated bidentate adsorption.8 In aqueous solutions, depending on the pH of the solution, either form of binding can be the dominating binding mechanism. At relatively low pH, pH < 5.5, the Dopa group is not ionized, with the two hydroxyl groups preferring to form two hydrogen bonds with the O atoms of the substrate. At higher pH (usually pH ≥8) and in the presence of appropriate metal ions, both hydroxyl groups undergo some degree of dissociation – the first, because pH is approaching the pKa (9.8 for Dopa),9 and, the second, because of the inductive effects of metal binding. The two phenolic O atoms form two coordination bonds (a charge-transfer complex with certain metals) with surface-bound, available Ti (Ti IV) sites. At an intermediate pH, a combination of one hydrogen bond and one coordination bond may be formed, resulting in a monodentate adsorption. The binding strength of a Dopa–TiO2 coordination bond (~44 kT) is significantly stronger than the Dopa-Ti hydrogen bond (~4–12 kT).10 Therefore the binding of a single Dopa to TiO2 substrate is much stronger at pH 7.5 than at pH 3.
Resonance Raman spectroscopy has been proven to be a powerful tool to observe and probe the mechanism and configuration of Dopa-metal coordination.3b, 6, 11 Mfp-1 and Dopa grafted synthetic polymers were shown to form stable bis- and tris- catecholate complexes with Fe(III) at pH 7.5 or higher in vitro and in situ (in the mussel plaque).3b, 11, 12 Resonance-enhanced signals assigned to mono- and bidentate coordination were seen for a Dopa-containing protein on TiO2.6 Despite having excellent binding ability to mica and TiO2 surfaces, Dopa has a confounding tendency that presents significant challenges to many potential applications: at alkaline pH and with trace oxidants such as O2, Dopa can readily undergo a two electron oxidation to Dopaquinone, which greatly diminishes the adhesion of mfp-3f to mica.1a Mussels have evolved a strategy to minimize Dopa oxidation by combining an acidic pH, imposed during the formation of the mussel plaque, with the co-secretion of an antioxidant protein, mfp-6. Mussels also use the Dopa-Fe3+ coordination complex to temporarily stabilize the Dopa in the plaques.11, 13 According to AFM single molecule studies, Dopa oxidation to Dopaquinone reduces binding to a TiO2 surface by 80%, which indicates that Dopa oxidation could also strongly perturb adhesion of mussel foot proteins to TiO2 or other metal oxide surfaces. Although much attention has been paid to engineering mussel mimetic functional coating materials, few if any studies have systematically examined the effect of Dopa oxidation on the performance of those coating materials on metal oxide surfaces.
To remedy this oversight, we investigated the adhesion of mfp-3f to TiO2 surfaces (RMS ~ 1nm, Fig. S1 and Fig. S2). SFA force measurements and resonance Raman spectroscopic investigations into the binding of mfp-3f on TiO2 surfaces indicate that although higher pH leads to increased Dopa oxidation, it also significantly increases the adhesion of surviving Dopa in mfp-3f on TiO2 surfaces.
Experimental Section
Protein purification
Purification of mfp-3f was achieved exactly as previously described.14 The purified proteins were suspended in buffer consisting of 0.1 M acetic acid (EMD Chemicals, Gibbstown, NJ), and 0.25 M potassium nitrate (Aldrich, St. Louis, MO) and with a pH of 3. The protein solutions were divided into small aliquots and stored at −50°C before experiments.
Surface forces apparatus
The adhesion of mfp-3f on TiO2 surfaces was studied using a surface forces apparatus 2000 (SFA 2000) with a reported geometry.15 A 10 nm thick TiO2 layer was deposited on the mica surfaces glued on the SFA disks using an E-Beam deposition method (Temescal system) at a constant rate of 0.1 nm/s and a pressure < 4×10−5 Torr, and was shown to be free of impurities by XPS (Kratos Ultra, Kratos Analytical Limited, S Figure 1). The surface roughness of the TiO2 substrate was 1 nm measured by AFM (Asylum MFP-3D-SA, S Figure 2). Prior to experiments, a droplet of mfp-3f solution with a mfp-3 concentration of 5μg/ml (pH = 3) was pipetted on top of one TiO2 surface, letting the protein adsorb for 20 min and ideally forming a monolayer of mfp-3 molecules on the surface. The TiO2 surfaces were then rinsed thoroughly with pH 3 buffer (0.1 M acetic acid, 0.25 M potassium nitrate, Sigma Aldrich) to remove the unabsorbed protein and then mounted into a SFA box. Buffer change was achieved by repeated injection and removal of buffer between the two surfaces. The distance D between two surfaces is measured with an optical interferometry technique (fringes of equal chromatic order, FECO). By applying the Derjaguin approximation, the normalized force F/R between the two cylindrical SFA surfaces is directly proportional to the energy between two flat surfaces with a simple relation of E(D)=F(D)/2πR. Buffer preparation: 0.1 M sodium acetate (EM Science, Gibbstown, NJ) and 0.25 M potassium nitrate titrated by acetic acid (PH 5.5); 0.016 M potassium phosphate monobasic (Mallinckrodt, Hazelwood, MO) and 0.084 M potassium phosphate dibasic (EMD Chemicals, Gibbstown, NJ) (PH 7.5). Milli-Q water (Millipore, Bedford, MA) was used for all the glassware cleaning and solution preparation.
Figure 1.
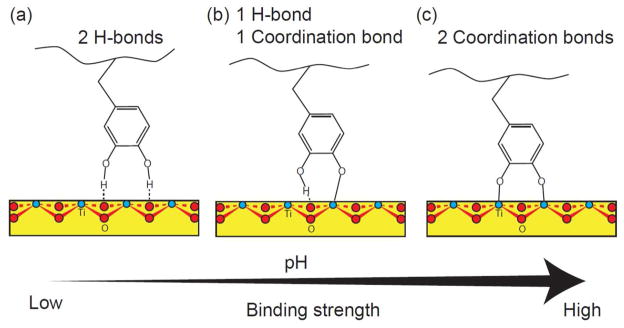
The possible modes of Dopa (catechol) binding to nonhydrated TiO2 surfaces. The catechol group can form molecular adsorption with (a) two hydrogen bonds, (b) monodentate adsorption with one hydrogen bond and one coordination bond, and (c) bidentate adsorption with two coordination bonds. Although which form the Dopa binds to a TiO2 surface depends is pH dependent: at lower pH (pH < 5.5), the molecular adsorption is preferred and at higher pH (pH > 7), the coordination charge transfer is more favorable. As marked, the red atoms are oxygens, the blue ones are titanium.
Figure 2.
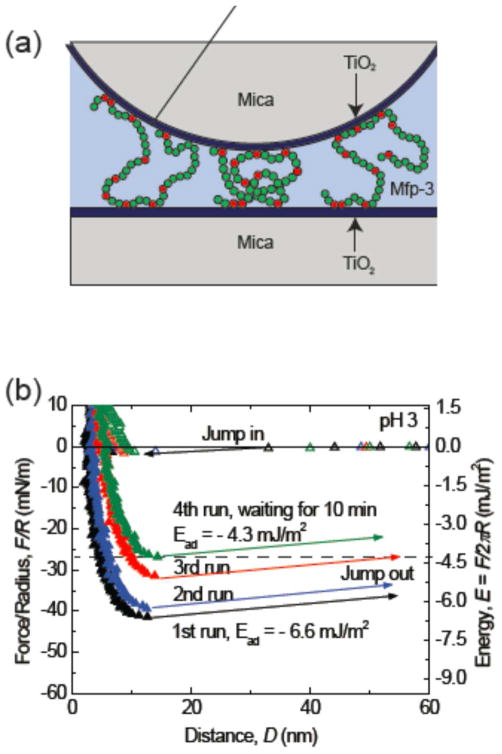
(a) The geometry of SFA experiments (Dopa residues in red). (b) The adhesion of mfp-3 on TiO2 surface in pH 3 buffer decreases with the force cycles/experiment time. The adhesion force dropped about 40% in about 1 hour. This is probably due to the light-induced oxidation of Dopa on TiO2.13
Raman spectroscopy
Raman spectra were collected with a confocal Raman microscope (alpha300; WITec) equipped with a Nikon objective (100X) and a laser excitation wavelength of 532 nm. Spectra were acquired with a CCD camera (DV401-BV; Andor) behind a spectrometer (UHTS 300; WITec) with a spectral resolution of 3 cm−1. Samples were prepared by drop-casting 20 μL of mfp-3f solution (10 μg/mL) in the appropriate buffer solution onto the TiO2-coated mica surfaces described for the SFA experiments, followed by incubation under ambient conditions for 20 minutes. After adsorption, surfaces were rinsed with the respective buffer solution and dried with a stream of N2. Samples were sensitive to burning by the laser beam; therefore, laser power was restricted to 10–20 mW. Each collected spectrum consisted of 120 accumulations; with an integration time of 1 s. ScanCtrlSpectroscopyPlus software (version 1.38, Witec) was used for measurement setup. The acquired spectra were analyzed and processed with Witec Project software (version 2.08). Spectra were background subtracted (average background subtract function) and smoothed with a 9-point Savitzky-Golay filter (4th order polynomial). Background spectra of TiO2-coated mica substrate with and without drop-cast buffer solution were also collected.
Results and discussion
Adhesion of mfp-3f on TiO2 surfaces at different pHs
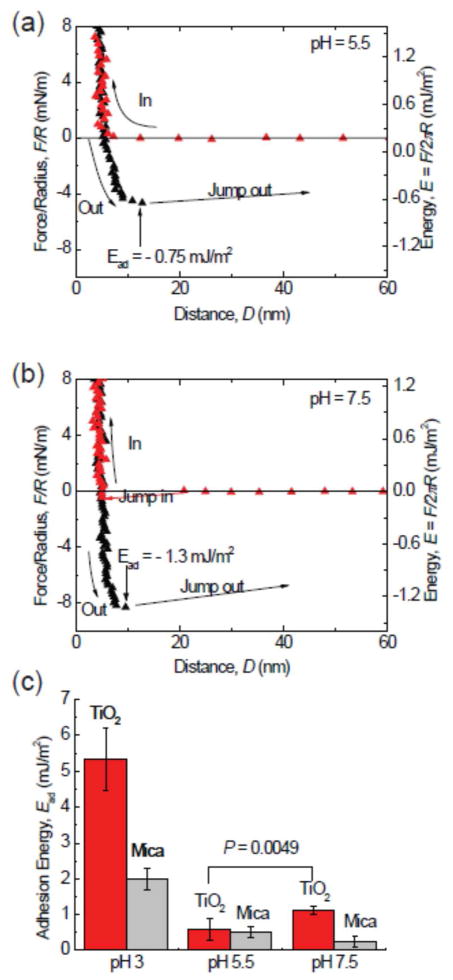
We have used the surface forces apparatus (SFA) to investigate adhesive interactions between mfp-3f and TiO2 at three pHs. As only monolayers of mfp-3f are deposited on TiO2, we can focus completely on their surface interactions. Cohesive changes resulting from protein cross-linking due to quinone formation arise primarily when multiple protein layers are present on the surfaces tested in the SFA. Strongest adhesion force with a corresponding adhesion energy of −6.6 mJ/m2 was measured at pH 3 after a brief compression of 1 min followed by separation of two surfaces. The thin hard wall (~ 5nm) of the force profile indicates that only a monomolecular layer of mfp-3f was deposited on the surface. Apparently, during contact, the mfp-3f molecules pre-absorbed onto one TiO2 surface can span the gap to bind to another surface, which gives rise to strong bridging adhesion. Interestingly, the adhesion force measured at pH 3 was not stable, showing strong time dependence. The adhesion force decreased with increasing the approach/separation force run cycles and/or the time of the experiment. In the third force run, the adhesion energy measured dropped to - 5 mJ/m2, about 25% decrease of the initial adhesion. Unlike on mica surfaces, a longer contact time did not enhance the adhesion force of mfp-3 on TiO2 surfaces: in the 4th force run, we kept the surfaces in contact for 10 min, and the adhesion force measured during separation decreased to - 4.5 mJ/m2.
The decrease in adhesion forces is probably due to the oxidation of Dopa on a TiO2 surface that is subjected to strong light intensity. Dopa does not undergo oxidation at pH3 in bulk solution; however, TiO2 is a good photo-oxidation catalyst.16 In the SFA, strong white light is used with the interferometric analyses of separation and force. Under such conditions, TiO2 can easily oxidize the Dopa group into Dopaquinone, which greatly diminishes the adhesion of Dopa to TiO2. Although the effect of photo-oxidation was very significant in our SFA tests, it is probably irrelevant to medical coatings applications since exposure to intense light is not typically involved in those processes.
The strong initial adhesion of mfp-3f on TiO2 surfaces at pH 3 dropped significantly to −0.7 mJ/m2 when the gap solution pH was increased to pH 5.5 (fig 3a). Previous SFA studies on mica surfaces have shown similar trends, due to the auto-oxidation of Dopa to Dopaquinone at high pH. Dopaquinone participates only weakly in all three types of adsorption, thereby auto-oxidation can greatly reduce the adhesion of mfp-3f to TiO2 surface.1 Surprisingly, increasing the pH to 7.5 did not further reduce adhesion, but instead increased it to −1.3 mJ/m2. Given that Dopa is more susceptible to auto-oxidation at pH 7.5, there should be fewer Dopa groups available for binding, and correspondingly lower adhesion; however, increasing the pH also increases the possibility of monodentate or bidentate binding of Dopa to the TiO2 surfaces,7–8, 17 thereby leading to much stronger binding than the molecular absorption through hydrogen bonds at the single molecular level. Tiiv-complexation by coordination may also significantly improve the stability of Dopa, as shown in mussel plaques. As a result of the two competing effects, the adhesion of mfp-3f on TiO2 surface is stronger at pH 7.5 than at pH 5.5.
Figure 3.
The pH dependence of adhesion of mfp-3F to TiO2. Reduced adhesion upon increasing the pH to 5.5 (a). A surprising increase in adhesion by almost 40% occurs upon bringing the pH up to 7.5 (b). Although Dopa loss to oxidation is more severe at high pH, the Dopa-TiO2 coordination bond gives higher adhesion forces. (c) A summary of the adhesion energies of mfp-3 on TiO2 in different pH buffers. The adsorption of Dopa on TiO2 surface is highly pH dependent. At low pH, the protonated Dopa predominates, whereas at pH 7.5, there exists a mixture of both half- and fully deprotonated catecholates.
To explore the correlation between the loss of adhesion and Dopa oxidation, we performed a control Dopa oxidation experiment using an oxidant, periodate, at pH 3 where the strongest adhesion was detected. After achieving a final stable adhesion energy of −3.6 mJ/m2, 20 μL periodate solution (100 μM) was injected into the gap between the surfaces (Figure 4). Only extremely weak adhesion force, corresponding to an energy of −0.15 mJ/m2, was detected in the separation after driving two surfaces into contact. The periodate oxidation experiment supports our proposition that oxidation of Dopa to Dopaquinone decreases mfp-3f adhesion to mica at pH 5.5 and 7.5, however, it does not preclude the possibility that electrostatic attraction also contributes to the increased adhesion at pH 7.5. The four-fold increase in the hardwall (from 10 to 40 nm) has been attributed the effect of dehydroDopa formation on backbone stiffening and is typically reversible with reduction.1
Figure 4.
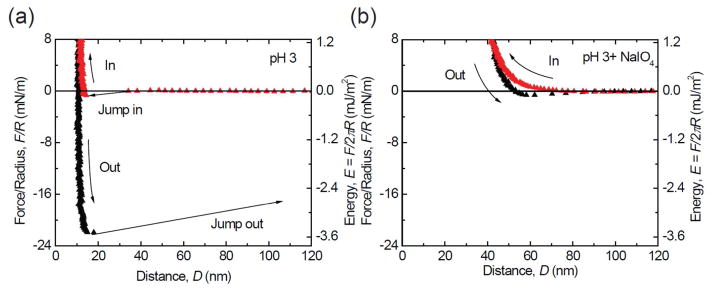
Periodate treatment as an independent measure of the effect of Dopa oxidation on adhesion. At pH 3, before adding periodate, strong adhesion was measured (a). The adhesion is almost completely abolished (96% decrease) by adding periodate at pH3 (b).
Raman spectroscopy study
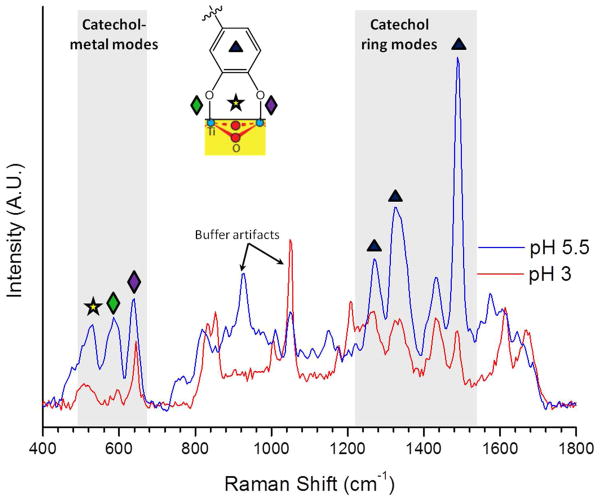
To further investigate the binding mechanism of mfp-3f to TiO2, we performed Raman spectroscopy measurements on mfp-3f adsorbed on TiO2 surfaces at three different pHs (pH 3, 5.5 and 7.5). Figure 5 shows background-subtracted Raman spectra for mfp-3f adsorbed at pH 3 and pH 5.5, normalized to the non-resonant CH vibrational peak (2800–3000 cm−1) (Fig S3). The spectrum for protein adsorption at pH 7.5 is shown in the Supporting Information (Fig. S4) Prominent peaks in the spectrum resulting from mfp-3f deposition at pH 5.5 are consistent with those seen for mfp-1-TiO2 adsorption, and share marked similarity to those originating from the complexation of mfp-1 and Fe3+.3b, 6, 11 Low energy resonance peaks (500–700 cm−1) have previously been assigned to vibrations of the oxygen–metal chelate bonds. Specifically, peaks at 585 cm−1 and 639 cm−1 indicate coordination by the oxygens on C3 and C4 of the catechol ring, respectively, whereas that of 530 cm−1 arises upon bidentate coordination and resulting charge transfer. Higher energy resonance peaks (1200–1500 cm−1) have been assigned to catechol ring vibrations.11 The pronounced spectral features in this region highlight the involvement of the catechol in the interfacial charge transfer upon coordination to TiO2. Namely, the strong ring stretch at 1488 cm−1, is indicative of the formation of a catechol-Ti charge transfer complex, and has previously been observed upon charge transfer following formation of tris-Dopa-Fe3+complexes.6, 11 Similarly, a strong feature at 1331 cm−1, attributed to a C-O stretching mode, indicates the vital role of the catecholate functionality in providing bridging adsorption.18
Figure 5.
Resonance Raman spectra of Dopa–TiO2 interactions in mfp-3f adsorbed at pH 3 and 5.5. Peaks at 1488 (▲; ν C-Carom + ν C-Harom), 1329 and 1261 cm−1 (▲; ν C-O + ν C-Carom) are characteristic catechol peaks which are enhanced upon coordination and charge transfer. Signals at 639 (◆; δ, ν C4-O), 585 (◆; δ, ν C3-O) and 530 cm−1 (✰; δ, ν C-O bidentate coordination) are characteristic catechol/metal chelate modes. Resonance enhancement of diagnostic modes for coordination is reduced at lower pH, suggesting predominant Ti-Dopa bridges at pH 5.5 and largely uncomplexed protein at pH 3. Peaks labeled represent artifacts from the buffer solutions (sodium acetate C-C at 930 cm−1 and nitrate N-O ν1 at 1050 cm−1).19
The spectra acquired at pH 3 also show characteristic enhanced signals in both the high and low energy regions shaded in Figure 3. The strength of these signals is notably diminished relative to that of mfp-3f adsorbed at pH 5.5. As anticipated, binding at pH 3 results in a sub-optimal coordination, as protons outcompete surface metal sites for the catecholate anions.
Mfp-3f deposited at pH 7.5 shows a strong fluorescent background (Fig. S4) that obscures all other signals; therefore, resonance-enhanced peaks signifying coordination could not be detected. However, it is anticipated that similar coordination peaks would indeed be seen at this pH, explaining the relatively robust adhesion measured in the SFA experiments.
Taken together, the Raman spectroscopy measurements support the SFA findings: increased pH favors the possibility of coordination binding of Dopa to the TiO2 surfaces while also increasing the rate of Dopa oxidation, which decreases the amount of Dopa residues available for binding. These opposing effects strongly alter the adhesive properties of mfp-3f and certainly have a similar effect on other Dopa-containing adhesives. This emphasizes the importance of well-controlled deposition conditions in forming robust interfacial binding. It is known that mussels regulate both the pH in the distal depression during protein secretion and the oxidation by co-secreting a protein, mfp-6, shown to have antioxidant properties at a wide range of pH. This study has confirmed the role of deposition pH on the adsorption and adhesive properties of mfp-3f to titania surfaces. Ongoing studies are geared towards the understanding of co-deposition strategies that retard the rate of oxidation at high pH, thereby allowing optimal surface complexation.
Finally, it should be noted that mussels use several different variants of mfp-3 as well as other mfps for adhesion to surfaces.14 The Dopa in some of these variants exhibits significantly shifted redox properties20 that could also show enhanced or altered binding to TiO2 surfaces and should be investigated in future studies.
Conclusion
Understanding the binding mechanism of Dopa to TiO2 surfaces is crucial for applying mussel inspired polymers as coating materials on TiO2. Our results show that mfp-3f binds strongly to TiO2 surfaces at low pH (pH 3). Raising the pH gives rise to two opposing effects: (1) Dopa oxidation, which decreases the adhesion of mfp-3f to TiO2 surfaces; (2) changing the binding nature of a single Dopa to the TiO2 from H-bonding to coordination bonding, and therefore increases the binding strength of a single Dopa group to the TiO2 surface. The two competing effects lead to a higher adhesion of mfp-3f on TiO2 surfaces at pH 7.5, whereas the lowest adhesion forces were measured at pH 5.5. Our results demonstrate that, by carefully controlling the Dopa redox activity, Dopa containing proteins and synthetic polymers can strongly bind to TiO2 surfaces, hence offer promise as coating materials for medical implants.
Supplementary Material
Acknowledgments
This work was supported by the Materials Research Science and Engineering Centers Program of the National Science Foundation under Award No. DMR 1121053, the IMI Program of the National Science Foundation under Award No. DMR 0843934, the National Institutes of Health under Grant R01-DE018468, and the UCSB-MPG Program for International Exchange in Materials Science. The authors thank Saurabh Das for the preparation of nanodeposited TiO2 layers onto the mica surfaces.
Footnotes
Supporting Information Available includes the following: S1. XPS analysis of the TiO2 layer; S2. A tapping mode image of the TiO2 surface; S3. Full window Raman spectra of mfp3f on TiO2 at pH 3 and 5.5; S4. The intrinsic fluorescence of mfp-3f following pH elevation to 7.5, and calculation of binding energy to TiO2. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.(a) Yu J, Wei W, Danner E, Ashley RK, Israelachvili JN, Waite JH. Mussel protein adhesion depends on interprotein thiol-mediated redox modulation. Nat Chem Biol. 2011;7(9):588–590. doi: 10.1038/nchembio.630. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yu J, Wei W, Danner E, Israelachvili JN, Waite JH. Effects of Interfacial Redox in Mussel Adhesive Protein Films on Mica. Adv Mater. 2011;23(20):2362. doi: 10.1002/adma.201003580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee H, Scherer NF, Messersmith PB. Single-molecule mechanics of mussel adhesion. P Natl Acad Sci USA. 2006;103(35):12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Anderson TH, Yu J, Estrada A, Hammer MU, Waite JH, Israelachvili JN. The Contribution of DOPA to Substrate-Peptide Adhesion and Internal Cohesion of Mussel-Inspired Synthetic Peptide Films. Adv Funct Mater. 2010;20(23):4196–4205. doi: 10.1002/adfm.201000932. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Holten-Andersen N, Harrington MJ, Birkedal H, Lee BP, Messersmith PB, Lee KYC, Waite JH. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. P Natl Acad Sci USA. 2011;108(7):2651–2655. doi: 10.1073/pnas.1015862108. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kim BH, Lee DH, Kim JY, Shin DO, Jeong HY, Hong S, Yun JM, Koo CM, Lee H, Kim SO. Mussel-Inspired Block Copolymer Lithography for Low Surface Energy Materials of Teflon, Graphene, and Gold. Adv Mater. 2011;23(47):5618. doi: 10.1002/adma.201103650. [DOI] [PubMed] [Google Scholar]; (d) Lee BP, Messersmith PB, Israelachvili JN, Waite JH. Mussel-Inspired Adhesives and Coatings. Annu Rev Mater Res. 2011;41:99–132. doi: 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Shao H, Stewart RJ. Biomimetic Underwater Adhesives with Environmentally Triggered Setting Mechanisms. Adv Mater. 2010;22(6):729. doi: 10.1002/adma.200902380. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Westwood G, Horton TN, Wilker JJ. Simplified polymer mimics of cross-linking adhesive proteins. Macromolecules. 2007;40(11):3960–3964. [Google Scholar]; (h) Stepuk A, Halter JG, Schaetz A, Grass RN, Stark WJ. Mussel-inspired load bearing metal-polymer glues. Chemical Communications. 2012;48(50):6238–6240. doi: 10.1039/c2cc31996a. [DOI] [PubMed] [Google Scholar]
- 4.(a) Larsson C, Thomson P, Lausmaa J, Rodahl M, Kasemo B, Ericson LE. Biomaterials. 1994;15:1062. doi: 10.1016/0142-9612(94)90092-2. [DOI] [PubMed] [Google Scholar]; (b) Pan J, Liao H, Leygraf C, Thierry D, Li J. J Biomed Mater Res. 1998;40:244. doi: 10.1002/(sici)1097-4636(199805)40:2<244::aid-jbm9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Waite JH. The Phylogeny and Chemical Diversity of Quinone-tanned Glues and Varnishes. Com Biochem Phys B. 1990;97(1):19–29. doi: 10.1016/0305-0491(90)90172-p. [DOI] [PubMed] [Google Scholar]
- 6.(a) Salameh S, Schneider J, Laube J, Alessandrini A, Facci P, Seo JW, Ciacchi LC, Mädler L. Adhesion Mechanisms of the Contact Interface of TiO2 Nanoparticles in Films and Aggregates. Langmuir. 2012;28(31):11457–11464. doi: 10.1021/la302242s. [DOI] [PubMed] [Google Scholar]; (b) Dalsin JL, Lin LJ, Tosatti S, Voros J, Textor M, Messersmith PB. Protein resistance of titanium oxide surfaces modified by biologically inspired mPEG-DOPA. Langmuir. 2005;21(2):640–646. doi: 10.1021/la048626g. [DOI] [PubMed] [Google Scholar]; (c) Gillich T, Benetti EM, Rakhmatullina E, Konradi R, Li W, Zhang A, Schluter AD, Textor M. Self-Assembly of Focal Point Oligo-catechol Ethylene Glycol Dendrons on Titanium Oxide Surfaces: Adsorption Kinetics, Surface Characterization, and Nonfouling Properties. J Am Chem Soc. 2011;133(28):10940–10950. doi: 10.1021/ja202760x. [DOI] [PubMed] [Google Scholar]; (d) Dalsin JL, Hu BH, Lee BP, Messersmith PB. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J Am Chem Soc. 2003;125(14):4253–4258. doi: 10.1021/ja0284963. [DOI] [PubMed] [Google Scholar]
- 7.Hwang DS, Harrington MJ, Lu QY, Masic A, Zeng HB, Waite JH. Mussel foot protein-1 (mcfp-1) interaction with titania surfaces. J Mater Chem. 2012;22(31):15530–15533. doi: 10.1039/C2JM32439C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Terranova U, Bowler DR. Adsorption of Catechol on TiO(2) Rutile (100): A Density Functional Theory Investigation. J Phys Chem C. 2010;114(14):6491–6495. [Google Scholar]; (b) Liu Y, Dadap JI, Zimdars D, Eisenthal KB. Study of interfacial charge-transfer complex on TiO2 particles in aqueous suspension by second-harmonic generation. J Phys Chem B. 1999;103(13):2480–2486. [Google Scholar]
- 9.(a) Martin RB. Zwitterion formation upon deprotonation in L-3,4-dihydroxyphenylalanine and other phenolic amines. J Phys Chem. 1971;75:2657–2661. doi: 10.1021/j100686a021. [DOI] [PubMed] [Google Scholar]; (b) Petit LD. Critical survey of formation constants of complexes of histidine, phenylalanine, tyrosine, L-DOPA and tryptophan. Pure & Appl Chem. 1984;56:247–292. [Google Scholar]
- 10.(a) Li SC, Chu LN, Gong XQ, Diebold U. Hydrogen Bonding Controls the Dynamics of Catechol Adsorbed on a TiO2(110) Surface. Science. 2010;328(5980):882–884. doi: 10.1126/science.1188328. [DOI] [PubMed] [Google Scholar]; (b) Valtiner M, Donaldson SH, Gebbie MA, Israelachvili JN. Hydrophobic Forces, Electrostatic Steering, and Acid-Base Bridging between Atomically Smooth Self-Assembled Monolayers and End-Functionalized PEGolated Lipid Bilayers. J Am Chem Soc. 2012;134(3):1746–1753. doi: 10.1021/ja209653n. [DOI] [PubMed] [Google Scholar]; (c) Israelachvili JN. Intermolecular and surface forces. 3. Academic Press; Burlington, MA: 2011. [Google Scholar]
- 11.Harrington MJ, Masic A, Holten-Andersen N, Waite JH, Fratzl P. Iron-Clad Fibers: A Metal-Based Biological Strategy for Hard Flexible Coatings. Science. 2010;328(5975):216–220. doi: 10.1126/science.1181044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng HB, Hwang DS, Israelachvili JN, Waite JH. Strong reversible Fe(3+)-mediated bridging between dopa-containing protein films in water. P Natl Acad Sci USA. 2010;107(29):12850–12853. doi: 10.1073/pnas.1007416107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang DS, Zeng HB, Masic A, Harrington MJ, Israelachvili JN, Waite JH. Protein- and Metal-dependent Interactions of a Prominent Protein in Mussel Adhesive Plaques. J Biol Chem. 2010;285(33):25850–25858. doi: 10.1074/jbc.M110.133157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao H, Robertson NB, Jewhurst SA, Waite JH. Probing the adhesive footprints of Mytilus californianus byssus. J Biol Chem. 2006;281(16):11090–11096. doi: 10.1074/jbc.M510792200. [DOI] [PubMed] [Google Scholar]
- 15.(a) Israelachvili J, Min Y, Akbulut M, Alig A, Carver G, Greene W, Kristiansen K, Meyer E, Pesika N, Rosenberg K, Zeng H. Recent advances in the surface forces apparatus (SFA) technique. Reports on Progress in Physics. 2010;73(3) [Google Scholar]; (b) Lin Q, Gourdon D, Sun CJ, Holten-Andersen N, Anderson TH, Waite JH, Israelachvili JN. Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3. P Natl Acad Sci USA. 2007;104(10):3782–3786. doi: 10.1073/pnas.0607852104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Nakata K, Fujishima A. TiO2 photocatalysis: Design and applications. Journal of Photochemistry and Photobiology C-Photochemistry Reviews. 2012;13(3):169–189. [Google Scholar]; (b) Jribi R, Barthel E, Bluhm H, Grunze M, Koelsch P, Verreault D, Sondergard E. Ultraviolet Irradiation Suppresses Adhesion on TiO2. J Phys Chem C. 2009;113(19):8273–8277. [Google Scholar]
- 17.Lana-Villarreal T, Rodes A, Perez JM, Gomez R. A spectroscopic and electrochemical approach to the study of the interactions and photoinduced electron transfer between catechol and anatase nanoparticles in aqueous solution. J Am Chem Soc. 2005;127(36):12601–12611. doi: 10.1021/ja052798y. [DOI] [PubMed] [Google Scholar]
- 18.Shoute LCT, Loppnow GR. Excited-state dynamics of alizarin-sensitized TiO2 nanoparticles from resonance Raman spectroscopy. J Chem Phys. 2002;117(2):842–850. [Google Scholar]
- 19.(a) Frost RL, Kloprogge JT. Raman spectroscopy of the acetates of sodium, potassium and magnesium at liquid nitrogen temperature. J Mol Struct. 2000;526:131–141. [Google Scholar]; (b) Liu D, Ullman FG, Hardy JR. Raman-Scattering and Lattice-Dynamic Calculations of Crystalline Kno3. Phys Rev B. 1992;45(5):2142–2147. doi: 10.1103/physrevb.45.2142. [DOI] [PubMed] [Google Scholar]
- 20.Wei W, Yu J, Broomell C, Israelachvili JN, Waite JH. Hydrophobic Enhancement of Dopa-Mediated Adhesion in a Mussel Foot Protein. J Am Chem Soc. 2013;135(1):377–383. doi: 10.1021/ja309590f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.