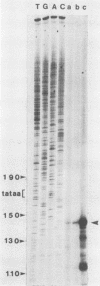
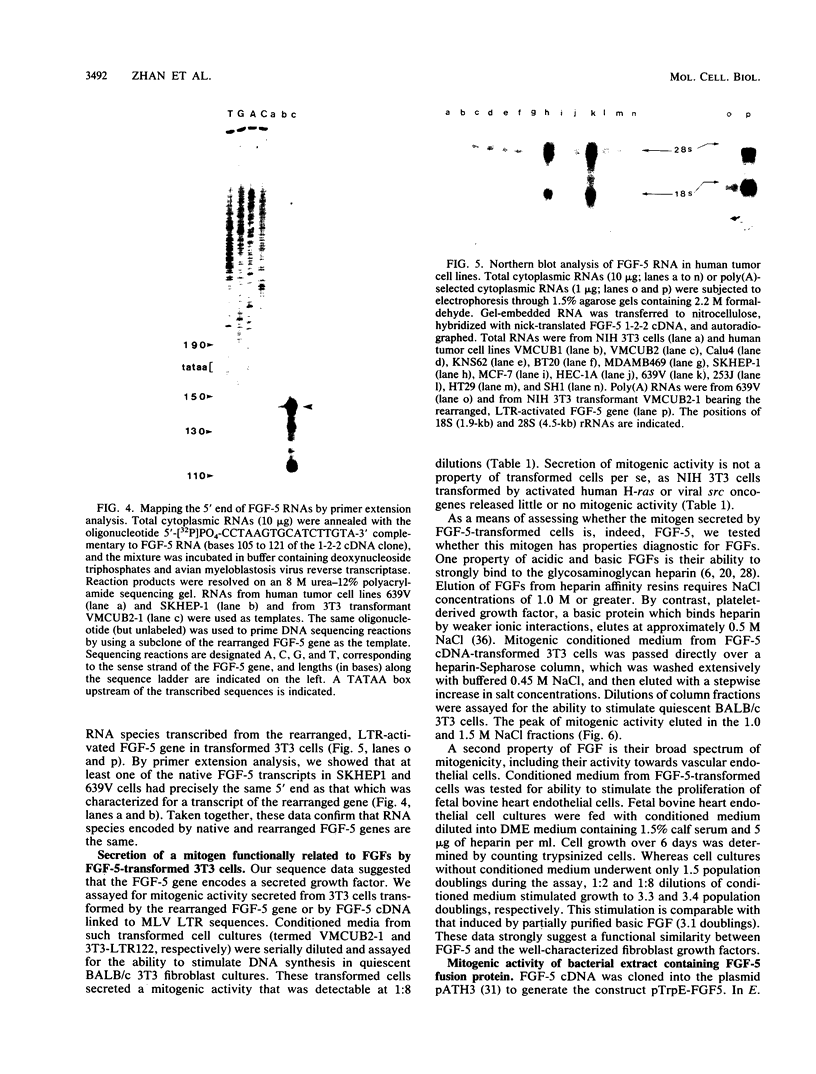
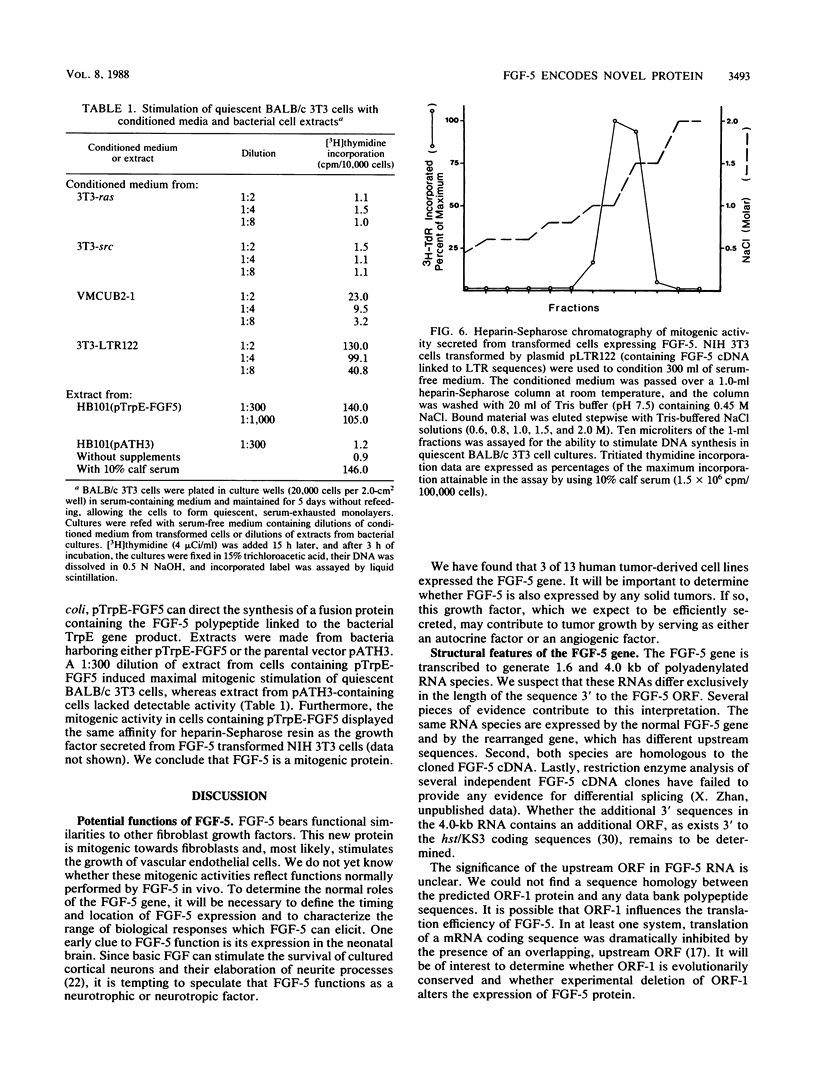
Abstract
We previously described the isolation of a human oncogene which had acquired transforming potential by a DNA rearrangement accompanying transfection of NIH 3T3 cells with human tumor DNA (X. Zhan, A. Culpepper, M. Reddy, J. Loveless, and M. Goldfarb, Oncogene 1:369-376, 1987). We now term this oncogene the FGF-5 gene, since it specifies the fifth documented protein related to fibroblast growth factors (FGFs. Two regions of the FGF-5 sequence, containing 122 of its 267 amino acid residues, were 40 to 50% homologous to the sequences of acidic and basic FGFs as well as to the sequences of the FGF-related oncoproteins int-2 and hst/KS3. The FGF-5 gene bears the three exon structures typical for members of this family. FGF-5 was found to be expressed in the neonatal brain and in 3 of the 13 human tumor cell lines examined. Several experiments strongly suggested that FGF-5 is a growth factor with properties common to those of acidic and basic FGFs. The rearrangement which activated the FGF-5 gene during DNA transfection had juxtaposed a retrovirus transcriptional enhancer just upstream from the native promoter of the gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Abraham J. A., Whang J. L., Tumolo A., Mergia A., Friedman J., Gospodarowicz D., Fiddes J. C. Human basic fibroblast growth factor: nucleotide sequence and genomic organization. EMBO J. 1986 Oct;5(10):2523–2528. doi: 10.1002/j.1460-2075.1986.tb04530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A., Ling N. Fibroblast growth factors are present in the extracellular matrix produced by endothelial cells in vitro: implications for a role of heparinase-like enzymes in the neovascular response. Biochem Biophys Res Commun. 1987 Jan 30;142(2):428–435. doi: 10.1016/0006-291x(87)90292-0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Walter P., Chang C. N., Goldman B. M., Erickson A. H., Lingappa V. R. Translocation of proteins across membranes: the signal hypothesis and beyond. Symp Soc Exp Biol. 1979;33:9–36. [PubMed] [Google Scholar]
- Conn G., Hatcher V. B. The isolation and purification of two anionic endothelial cell growth factors from human brain. Biochem Biophys Res Commun. 1984 Oct 15;124(1):262–268. doi: 10.1016/0006-291x(84)90946-x. [DOI] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Dickson C., Peters G. Potential oncogene product related to growth factors. 1987 Apr 30-May 6Nature. 326(6116):833–833. doi: 10.1038/326833a0. [DOI] [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh J. Cultivation, characterization, and identification of human tumor cells with emphasis on kidney, testis, and bladder tumors. Natl Cancer Inst Monogr. 1978 Dec;(49):5–9. [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Fogh J., Wright W. C., Loveless J. D. Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J Natl Cancer Inst. 1977 Feb;58(2):209–214. doi: 10.1093/jnci/58.2.209. [DOI] [PubMed] [Google Scholar]
- Gimenez-Gallego G., Rodkey J., Bennett C., Rios-Candelore M., DiSalvo J., Thomas K. Brain-derived acidic fibroblast growth factor: complete amino acid sequence and homologies. Science. 1985 Dec 20;230(4732):1385–1388. doi: 10.1126/science.4071057. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J., Braun D., Birdwell C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4120–4124. doi: 10.1073/pnas.73.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Sasse J., Sullivan R., Smith J. A. Human tumor cells synthesize an endothelial cell growth factor that is structurally related to basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2448–2452. doi: 10.1073/pnas.83.8.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol Cell Biol. 1987 Oct;7(10):3438–3445. doi: 10.1128/mcb.7.10.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R. R., Alderman E. M., Fett J. W. Induction of angiogenesis by bovine brain derived class 1 heparin-binding growth factor. Biochemistry. 1985 Sep 10;24(19):4969–4973. doi: 10.1021/bi00340a001. [DOI] [PubMed] [Google Scholar]
- Maciag T., Mehlman T., Friesel R., Schreiber A. B. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 1984 Aug 31;225(4665):932–935. doi: 10.1126/science.6382607. [DOI] [PubMed] [Google Scholar]
- Moore R., Casey G., Brookes S., Dixon M., Peters G., Dickson C. Sequence, topography and protein coding potential of mouse int-2: a putative oncogene activated by mouse mammary tumour virus. EMBO J. 1986 May;5(5):919–924. doi: 10.1002/j.1460-2075.1986.tb04304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. S., Sharma A., de Vellis J., Bradshaw R. A. Basic fibroblast growth factor supports the survival of cerebral cortical neurons in primary culture. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7537–7541. doi: 10.1073/pnas.83.19.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987 Jan 15;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Taira M., Yoshida T., Miyagawa K., Sakamoto H., Terada M., Sugimura T. cDNA sequence of human transforming gene hst and identification of the coding sequence required for transforming activity. Proc Natl Acad Sci U S A. 1987 May;84(9):2980–2984. doi: 10.1073/pnas.84.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanese N., Roth M. J., Goff S. P. Analysis of retroviral pol gene products with antisera raised against fusion proteins produced in Escherichia coli. J Virol. 1986 Aug;59(2):328–340. doi: 10.1128/jvi.59.2.328-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. A., Rios-Candelore M., Fitzpatrick S. Purification and characterization of acidic fibroblast growth factor from bovine brain. Proc Natl Acad Sci U S A. 1984 Jan;81(2):357–361. doi: 10.1073/pnas.81.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. A., Rios-Candelore M., Giménez-Gallego G., DiSalvo J., Bennett C., Rodkey J., Fitzpatrick S. Pure brain-derived acidic fibroblast growth factor is a potent angiogenic vascular endothelial cell mitogen with sequence homology to interleukin 1. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6409–6413. doi: 10.1073/pnas.82.19.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkles J. A., Friesel R., Burgess W. H., Howk R., Mehlman T., Weinstein R., Maciag T. Human vascular smooth muscle cells both express and respond to heparin-binding growth factor I (endothelial cell growth factor). Proc Natl Acad Sci U S A. 1987 Oct;84(20):7124–7128. doi: 10.1073/pnas.84.20.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Miyagawa K., Odagiri H., Sakamoto H., Little P. F., Terada M., Sugimura T. Genomic sequence of hst, a transforming gene encoding a protein homologous to fibroblast growth factors and the int-2-encoded protein. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7305–7309. doi: 10.1073/pnas.84.20.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Culpepper A., Reddy M., Loveless J., Goldfarb M. Human oncogenes detected by a defined medium culture assay. Oncogene. 1987;1(4):369–376. [PubMed] [Google Scholar]
- Zhan X., Goldfarb M. Growth factor requirements of oncogene-transformed NIH 3T3 and BALB/c 3T3 cells cultured in defined media. Mol Cell Biol. 1986 Oct;6(10):3541–3544. doi: 10.1128/mcb.6.10.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]