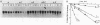Abstract
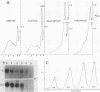
An increased level of unpolymerized tubulin caused by depolymerization of microtubules in sea urchin larvae resulted in a rapid loss of tubulin mRNA, which was prevented by nearly complete inhibition of protein synthesis. Results of an RNA run-on assay indicated that inhibition of protein synthesis does not alter tubulin gene transcription. Analysis of the decay of tubulin mRNA in embryos in which RNA synthesis was inhibited by actinomycin D indicated that inhibition of protein synthesis prevents the destabilization of tubulin mRNA. The effect was similar whether mRNA was maintained on polysomes in the presence of emetine or anisomycin or displaced from the polysomes in the presence of puromycin or pactamycin; thus, the stabilization of tubulin mRNA is not dependent on the state of the polysomes after inhibition of protein synthesis. Even after tubulin mRNA declined to a low level after depolymerization of microtubules, it could be rescued by treatment of embryos with inhibitors of protein synthesis. Tubulin mRNA could be induced to accumulate prematurely in gastrulae but not in plutei if protein synthesis was inhibited, an observation that is indicative of the importance of the autogenous regulation of tubulin mRNA stability during embryogenesis. Possible explanations for the role of protein synthesis in the control of mRNA stability are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandraki D., Ruderman J. V. Evolution of alpha q- and beta-tubulin genes as inferred by the nucleotide sequences of sea urchin cDNA clones. J Mol Evol. 1983;19(6):397–410. doi: 10.1007/BF02102315. [DOI] [PubMed] [Google Scholar]
- Alexandraki D., Ruderman J. V. Expression of alpha- and beta-tubulin genes during development of sea urchin embryos. Dev Biol. 1985 Jun;109(2):436–451. doi: 10.1016/0012-1606(85)90470-1. [DOI] [PubMed] [Google Scholar]
- Baumbach L. L., Marashi F., Plumb M., Stein G., Stein J. Inhibition of DNA replication coordinately reduces cellular levels of core and H1 histone mRNAs: requirement for protein synthesis. Biochemistry. 1984 Apr 10;23(8):1618–1625. doi: 10.1021/bi00303a006. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Bird R. C., Jacobs F. A., Sells B. H. Stability of histone mRNAs is related to their location in polysomes. Biochem Cell Biol. 1986 Feb;64(2):99–105. doi: 10.1139/o86-017. [DOI] [PubMed] [Google Scholar]
- Caron J. M., Jones A. L., Kirschner M. W. Autoregulation of tubulin synthesis in hepatocytes and fibroblasts. J Cell Biol. 1985 Nov;101(5 Pt 1):1763–1772. doi: 10.1083/jcb.101.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron J. M., Jones A. L., Rall L. B., Kirschner M. W. Autoregulation of tubulin synthesis in enucleated cells. Nature. 1985 Oct 17;317(6038):648–651. doi: 10.1038/317648a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Havercroft J. C. Is apparent autoregulatory control of tubulin synthesis nontranscriptionally regulated? J Cell Biol. 1983 Sep;97(3):919–924. doi: 10.1083/jcb.97.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981 Aug;25(2):537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- Conlon R. A., Tufaro F., Brandhorst B. P. Post-transcriptional restriction of gene expression in sea urchin interspecies hybrid embryos. Genes Dev. 1987 Jun;1(4):337–346. doi: 10.1101/gad.1.4.337. [DOI] [PubMed] [Google Scholar]
- Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle A. J., Graves R. A., Marzluff W. F., Johnson L. F. Regulation of histone mRNA production and stability in serum-stimulated mouse 3T6 fibroblasts. Mol Cell Biol. 1983 Nov;3(11):1920–1929. doi: 10.1128/mcb.3.11.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Dower W., Humphreys T. Normal synthesis, transport and decay of mRNA in the absence of its translation. Nature. 1975 Feb 27;253(5494):751–753. doi: 10.1038/253751a0. [DOI] [PubMed] [Google Scholar]
- Efrat S., Kaempfer R. Control of biologically active interleukin 2 messenger RNA formation in induced human lymphocytes. Proc Natl Acad Sci U S A. 1984 May;81(9):2601–2605. doi: 10.1073/pnas.81.9.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D. A., Yen T. J., Lau J. T., Cleveland D. W. Sequences that confer beta-tubulin autoregulation through modulated mRNA stability reside within exon 1 of a beta-tubulin mRNA. Cell. 1987 Aug 28;50(5):671–679. doi: 10.1016/0092-8674(87)90325-4. [DOI] [PubMed] [Google Scholar]
- Gong Z. Y., Brandhorst B. P. Stimulation of tubulin gene transcription by deciliation of sea urchin embryos. Mol Cell Biol. 1987 Dec;7(12):4238–4246. doi: 10.1128/mcb.7.12.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
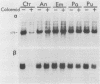
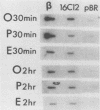
- Gong Z. Y., Brandhorst B. Autogenous regulation of tubulin synthesis via RNA stability during sea urchin embryogenesis. Development. 1988 Jan;102(1):31–43. doi: 10.1242/dev.102.1.31. [DOI] [PubMed] [Google Scholar]
- Graves R. A., Marzluff W. F. Rapid reversible changes in the rate of histone gene transcription and histone mRNA levels in mouse myeloma cells. Mol Cell Biol. 1984 Feb;4(2):351–357. doi: 10.1128/mcb.4.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves R. A., Pandey N. B., Chodchoy N., Marzluff W. F. Translation is required for regulation of histone mRNA degradation. Cell. 1987 Feb 27;48(4):615–626. doi: 10.1016/0092-8674(87)90240-6. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow P., Nemer M. Developmental and tissue-specific regulation of beta-tubulin gene expression in the embryo of the sea urchin Strongylocentrotus purpuratus. Genes Dev. 1987 Apr;1(2):147–160. doi: 10.1101/gad.1.2.147. [DOI] [PubMed] [Google Scholar]
- Hille M. B., Hall D. C., Yablonka-Reuveni Z., Danilchik M. V., Moon R. T. Translational control in sea urchin eggs and embryos: initiation is rate limiting in blastula stage embryos. Dev Biol. 1981 Aug;86(1):241–249. doi: 10.1016/0012-1606(81)90336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B., Gross P. R. The effect of protein synthesis inhibition on the entry of messenger RNA into the cytoplasm of sea urchin embryos. J Cell Biol. 1971 Jun;49(3):692–701. doi: 10.1083/jcb.49.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R., Shaw D. R., Ennis H. L. Role of protein synthesis in decay and accumulation of mRNA during spore germination in the cellular slime mold Dictyostelium discoideum. Mol Cell Biol. 1987 Feb;7(2):799–805. doi: 10.1128/mcb.7.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B. J., Chodchoy N., Marzluff W. F., Skoultchi A. I. Coupling of replication type histone mRNA levels to DNA synthesis requires the stem-loop sequence at the 3' end of the mRNA. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6189–6193. doi: 10.1073/pnas.84.17.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. F., Marzluff W. F. A factor in sea urchin eggs inhibits transcription in isolated nuclei by sea urchin RNA polymerase III. Biochemistry. 1983 Feb 1;22(3):645–653. doi: 10.1021/bi00272a019. [DOI] [PubMed] [Google Scholar]
- Pachter J. S., Yen T. J., Cleveland D. W. Autoregulation of tubulin expression is achieved through specific degradation of polysomal tubulin mRNAs. Cell. 1987 Oct 23;51(2):283–292. doi: 10.1016/0092-8674(87)90155-3. [DOI] [PubMed] [Google Scholar]
- Pittenger M. F., Cleveland D. W. Retention of autoregulatory control of tubulin synthesis in cytoplasts: demonstration of a cytoplasmic mechanism that regulates the level of tubulin expression. J Cell Biol. 1985 Nov;101(5 Pt 1):1941–1952. doi: 10.1083/jcb.101.5.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Analysis of interferon mRNA in human fibroblast cells induced to produce interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7426–7430. doi: 10.1073/pnas.78.12.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum I. Y., Mattson B. A., Heyner S. Stage-specific insulin binding in mouse preimplantation embryos. Dev Biol. 1986 Jul;116(1):261–263. doi: 10.1016/0012-1606(86)90063-1. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- Sariban E., Wu R. S., Erickson L. C., Bonner W. M. Interrelationships of protein and DNA syntheses during replication of mammalian cells. Mol Cell Biol. 1985 Jun;5(6):1279–1286. doi: 10.1128/mcb.5.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Heintz N., Roeder R. G. Regulation of human histone gene expression during the HeLa cell cycle requires protein synthesis. Mol Cell Biol. 1984 Dec;4(12):2723–2734. doi: 10.1128/mcb.4.12.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizz G., Roman D., Strauss A., Olson E. N. Serum and fibroblast growth factor inhibit myogenic differentiation through a mechanism dependent on protein synthesis and independent of cell proliferation. J Biol Chem. 1986 Jul 15;261(20):9483–9488. [PubMed] [Google Scholar]
- Stahl H., Gallwitz D. Fate of histone messenger RNA in synchronized HeLa cells in the absence of initiation of protein synthesis. Eur J Biochem. 1977 Jan;72(2):385–392. doi: 10.1111/j.1432-1033.1977.tb11263.x. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Lee K. L., Kenney F. T. Differential degradation of messenger RNAs in mammalian cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2634–2638. doi: 10.1073/pnas.73.8.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimac E., Groppi V. E., Jr, Coffino P. Inhibition of protein synthesis stabilizes histone mRNA. Mol Cell Biol. 1984 Oct;4(10):2082–2090. doi: 10.1128/mcb.4.10.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]