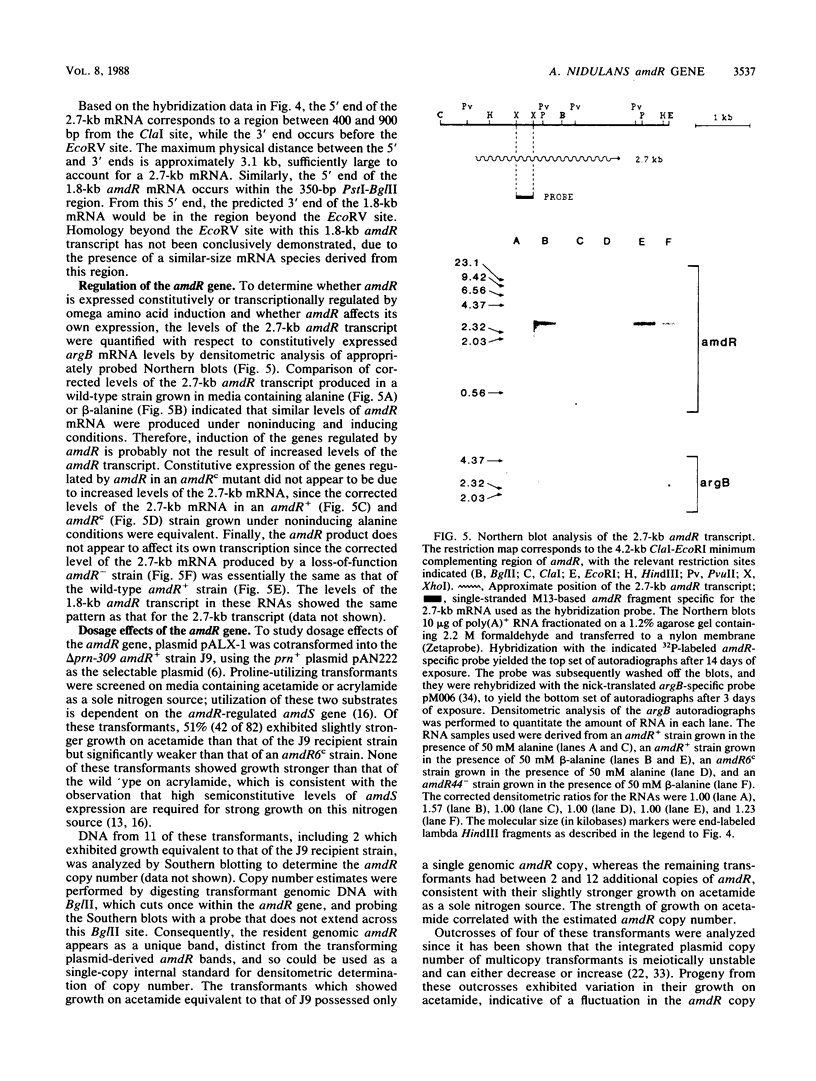
Abstract
The positively acting regulatory gene amdR of Aspergillus nidulans coordinately regulates the expression of four unlinked structural genes involved in acetamide (amdS), omega amino acid (gatA and gabA), and lactam (lamA) catabolism. By the use of DNA-mediated transformation of A. nidulans, the amdR regulatory gene was cloned from a genomic cosmid library. Southern blot analysis of DNA from various loss-of-function amdR mutants revealed the presence of four detectable DNA rearrangements, including a deletion, an insertion, and a translocation. No detectable DNA rearrangements were found in several constitutive amdRc mutants. Analysis of the fate of amdR-bearing plasmids in transformants showed that 10 to 20% of the transformation events were homologous integrations or gene conversions, and this phenomenon was exploited in developing a strategy by which amdRc and amdR- alleles can be readily cloned and analyzed. Examination of the transcription of amdR by Northern blot (RNA blot) analysis revealed the presence of two mRNAs (2.7 and 1.8 kilobases) which were constitutively synthesized at a very low level. In addition, amdR transcription did not appear to depend on the presence of a functional amdR product nor was it altered in amdRc mutants. The dosage effects of multiple copies of amdR in transformants were examined, and it was shown that such transformants exhibited stronger growth than did the wild type on acetamide and pyrrolidinone media, indicating increased expression of the amdS and lamA genes, respectively. These results were used to formulate a model for amdR-mediated regulation of gene expression in which the low constitutive level of amdR product sets the upper limits of basal and induced transcription of the structural genes. Multiple copies of 5' sequences from the amdS gene can result in reduced growth on substrates whose utilization is dependent on amdR-controlled genes. This has been attributed to titration of limiting amdR gene product. Strong support for this proposal was obtained by showing that multiple copies of the amdR gene can reverse this phenomenon (antititration).
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arst H. N., Jr Integrator gene in Aspergillus nidulans. Nature. 1976 Jul 15;262(5565):231–234. doi: 10.1038/262231a0. [DOI] [PubMed] [Google Scholar]
- Arst H. N., Jr, Penfold H. A., Bailey C. R. Lactam utilisation in Aspergillus nidulans: evidence for a fourth gene under the control of the integrator gene intA. Mol Gen Genet. 1978 Nov 9;166(3):321–327. doi: 10.1007/BF00267625. [DOI] [PubMed] [Google Scholar]
- Baum J. A., Geever R., Giles N. H. Expression of qa-1F activator protein: identification of upstream binding sites in the qa gene cluster and localization of the DNA-binding domain. Mol Cell Biol. 1987 Mar;7(3):1256–1266. doi: 10.1128/mcb.7.3.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddick M. X., Arst H. N., Jr, Taylor L. H., Johnson R. I., Brownlee A. G. Cloning of the regulatory gene areA mediating nitrogen metabolite repression in Aspergillus nidulans. EMBO J. 1986 May;5(5):1087–1090. doi: 10.1002/j.1460-2075.1986.tb04326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. A., Cobbett C. S., Hynes M. J. An amdS-lacZ fusion for studying gene regulation in Aspergillus. Gene. 1988 Mar 31;63(2):199–212. doi: 10.1016/0378-1119(88)90525-2. [DOI] [PubMed] [Google Scholar]
- Durrens P., Green P. M., Arst H. N., Jr, Scazzocchio C. Heterologous insertion of transforming DNA and generation of new deletions associated with transformation in Aspergillus nidulans. Mol Gen Genet. 1986 Jun;203(3):544–549. doi: 10.1007/BF00422084. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Marzluf G. A. Characterization of nit-2, the major nitrogen regulatory gene of Neurospora crassa. Mol Cell Biol. 1987 May;7(5):1691–1696. doi: 10.1128/mcb.7.5.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986 Sep 12;46(6):885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Huiet L., Giles N. H. The qa repressor gene of Neurospora crassa: wild-type and mutant nucleotide sequences. Proc Natl Acad Sci U S A. 1986 May;83(10):3381–3385. doi: 10.1073/pnas.83.10.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M. J. A cis-dominant regulatory mutation affecting enzyme induction in the eukaryote Aspergillus nidulans. Nature. 1975 Jan 17;253(5488):210–212. doi: 10.1038/253210a0. [DOI] [PubMed] [Google Scholar]
- Hynes M. J., Corrick C. M., King J. A. Isolation of genomic clones containing the amdS gene of Aspergillus nidulans and their use in the analysis of structural and regulatory mutations. Mol Cell Biol. 1983 Aug;3(8):1430–1439. doi: 10.1128/mcb.3.8.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M. J. Multiple independent control mechanisms affecting the acetamidase of Aspergillus nidulans. Mol Gen Genet. 1978 Apr 25;161(1):59–65. doi: 10.1007/BF00266615. [DOI] [PubMed] [Google Scholar]
- Hynes M. J. Mutants with altered glucose repression of amidase enzymes in Aspergillus nidulans. J Bacteriol. 1972 Sep;111(3):717–722. doi: 10.1128/jb.111.3.717-722.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M. J., Pateman J. A. The genetic analysis of regulation of amidase synthesis in Aspergillus nidulans. I. Mutants able to utilize acrylamide. Mol Gen Genet. 1970;108(2):97–106. doi: 10.1007/BF02430516. [DOI] [PubMed] [Google Scholar]
- Hynes M. J., Pateman J. A. The genetic analysis of regulation of amidase synthesis in Aspergillus nidulans. II. Mutants resistant to fluoroacetamide. Mol Gen Genet. 1970;108(2):107–116. doi: 10.1007/BF02430517. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S. A., Hopper J. E. Isolation of the yeast regulatory gene GAL4 and analysis of its dosage effects on the galactose/melibiose regulon. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6971–6975. doi: 10.1073/pnas.79.22.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S. A., Salmeron J. M., Jr, Dincher S. S. Interaction of positive and negative regulatory proteins in the galactose regulon of yeast. Cell. 1987 Jul 3;50(1):143–146. doi: 10.1016/0092-8674(87)90671-4. [DOI] [PubMed] [Google Scholar]
- Johnston S. A., Zavortink M. J., Debouck C., Hopper J. E. Functional domains of the yeast regulatory protein GAL4. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6553–6557. doi: 10.1073/pnas.83.17.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. M., Hynes M. J. Multiple copies of the amdS gene of Aspergillus nidulans cause titration of trans-acting regulatory proteins. Curr Genet. 1987;12(1):21–31. doi: 10.1007/BF00420723. [DOI] [PubMed] [Google Scholar]
- Laughon A., Gesteland R. F. Isolation and preliminary characterization of the GAL4 gene, a positive regulator of transcription in yeast. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6827–6831. doi: 10.1073/pnas.79.22.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockington R., Scazzocchio C., Sequeval D., Mathieu M., Felenbok B. Regulation of alcR, the positive regulatory gene of the ethanol utilization regulon of Aspergillus nidulans. Mol Microbiol. 1987 Nov;1(3):275–281. doi: 10.1111/j.1365-2958.1987.tb01933.x. [DOI] [PubMed] [Google Scholar]
- Ma J., Ptashne M. Deletion analysis of GAL4 defines two transcriptional activating segments. Cell. 1987 Mar 13;48(5):847–853. doi: 10.1016/0092-8674(87)90081-x. [DOI] [PubMed] [Google Scholar]
- Ma J., Ptashne M. The carboxy-terminal 30 amino acids of GAL4 are recognized by GAL80. Cell. 1987 Jul 3;50(1):137–142. doi: 10.1016/0092-8674(87)90670-2. [DOI] [PubMed] [Google Scholar]
- Oakley C. E., Weil C. F., Kretz P. L., Oakley B. R. Cloning of the riboB locus of Aspergillus nidulans. Gene. 1987;53(2-3):293–298. doi: 10.1016/0378-1119(87)90019-9. [DOI] [PubMed] [Google Scholar]
- Paietta J. V., Akins R. A., Lambowitz A. M., Marzluf G. A. Molecular cloning and characterization of the cys-3 regulatory gene of Neurospora crassa. Mol Cell Biol. 1987 Jul;7(7):2506–2511. doi: 10.1128/mcb.7.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. B., Giles N. H. Autogenous regulation of the positive regulatory qa-1F gene in Neurospora crassa. Mol Cell Biol. 1985 Dec;5(12):3593–3599. doi: 10.1128/mcb.5.12.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Case M. E., Dykstra C. C., Giles N. H., Kushner S. R. Identification and characterization of recombinant plasmids carrying the complete qa gene cluster from Neurospora crassa including the qa-1+ regulatory gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5086–5090. doi: 10.1073/pnas.78.8.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., Vollmer S. J. Molecular cloning of nit-2, a regulatory gene required for nitrogen metabolite repression in Neurospora crassa. Gene. 1986;46(2-3):291–295. doi: 10.1016/0378-1119(86)90414-2. [DOI] [PubMed] [Google Scholar]
- Tilburn J., Scazzocchio C., Taylor G. G., Zabicky-Zissman J. H., Lockington R. A., Davies R. W. Transformation by integration in Aspergillus nidulans. Gene. 1983 Dec;26(2-3):205–221. doi: 10.1016/0378-1119(83)90191-9. [DOI] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., Timberlake W. E. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1470–1474. doi: 10.1073/pnas.81.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton M. M., Timberlake W. E., Hondel C. A. A cosmid for selecting genes by complementation in Aspergillus nidulans: Selection of the developmentally regulated yA locus. Proc Natl Acad Sci U S A. 1985 Feb;82(3):834–838. doi: 10.1073/pnas.82.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]






