Abstract
Biofabrication of living structures with desired topology and functionality requires the interdisciplinary effort of practitioners of the physical, life, medical and engineering sciences. Such efforts are being undertaken in many laboratories around the world. Numerous approaches are being pursued, such as those based on the use of natural or artificial scaffolds, decellularized cadaveric extracellular matrices and lately bioprinting. To be successful in this endeavor it is crucial to provide in vitro micro-environmental clues for the cells resembling those in the organism. Therefore scaffolds populated with differentiated cells or stem cells of increasing complexity and sophistication are being fabricated. However, scaffolds, no matter how sophisticated they are, can cause problems stemming from their degradation, eliciting immunogenic reactions and other a priori unforeseen complications. It is also being realized that ultimately the best approach is to rely on the self-assembly and self-organizing properties of cells and tissues and the innate regenerative capability of the organism itself, not just simply prepare tissue and organ structures in vitro followed by their implantation. Here we briefly review the different strategies for the fabrication of three-dimensional biological structures, in particular bioprinting. We detail a fully biological, scaffoldless, print-based engineering approach that uses self-assembling multicellular units as bioink particles and employs early developmental morphogenetic principles, such as cell sorting and tissue fusion.
Keywords: self-assembly, micro-environment, cell sorting, tissue fusion, bioprinting, bioink
1. Introduction
Self-assembly is the autonomous organization of components, from an initial state into final pattern or structure without external intervention [1-4]. Living organisms, in particular the developing embryo are quintessential self-organizing systems. Histogenesis and organogenesis are examples of self-assembly processes, in which, through cell-cell and cell-extracellular matrix (ECM) interactions, the developing organism and its parts gradually acquire their final shape. Ultimately the success of engineering and fabricating functional living structures will depend on understanding the principles of cellular self-assembly and our ability to employ them. This fact is being gradually recognized across the tissue engineering community, as except for a few spectacular successes [5-7] the field is yet to present viable solutions to the growing demand for novel regenerative technologies. Thus future biofabrication approaches (including but not restricted to the field of tissue engineering) aimed at re-establishing the functionality of damaged tissues and organs will need to focus on mobilizing developmental-morphogenetic processes coupled with requirements of adult biology, in short, the body's innate regenerative capability [8-10]. We will need to understand questions such as why the salamander can re-grow its limb but we cannot [11], or why the liver is the only internal human organ capable of regeneration [12]. This is the more important because though there is always a hope that in vitro tissue engineering efforts will eventually lead to the long awaited breakthrough, it is also possible that the multileveled and evolutionary established nature of cells and organisms will continue to defeat this hope.
In this article we overview recent progress in fabricating living structures of definite shape and functionality, in particular by implementing developmental principles and processes and describe a rapid prototyping technology, based on the bio-printing of self-assembling multicellular building blocks.
2. Fundamentals of tissue engineering
Tissue-engineering has emerged as an interdisciplinary field that applies the principles of engineering and life sciences toward the development of tissue substitutes [13, 14]. The fundamental goal of tissue engineering is to regenerate or replace defective, diseased or missing tissues and organs. Examples of grafts that are currently in pre-clinical studies include engineered skin, cartilage, bone, blood vessels, skeletal muscle, bladder, trachea, and myocardium [9, 15-19]. Three “classical” tissue-engineering approaches include [20] (i) use of an instructive environment (e.g., bioactive material) to recruit and guide host cells to regenerate a tissue; (ii) delivery of repair cells and/or bioactive factors into the damaged area; (iii) cultivation of cells on a biomaterial scaffold in a culture system (bioreactor), under conditions designed to engineer a functional tissue for implantation. In all cases ((i) and (ii) in vivo, (iii) in vitro) the engineered environments are designed to direct the cells – added from exogenous sources or mobilized from the host – to regenerate a specific tissue structure and function [9, 21-23]. When a tissue is engineered in vitro, cells (the actual “tissue engineers”) are placed into a biomaterial scaffold (providing the structural and logistic template for tissue formation) and cultured in a bioreactor (providing microenvironmental control and the necessary molecular and physical regulatory signals). Once a desired developmental stage is achieved (in most cases measured by critical functional properties), the tissue construct is implanted into the host, where further maturation and integration are anticipated.
At this time, tissue engineering opens several exciting possibilities: (i) to create functional grafts suitable for implantation and repair of failing tissues, (ii) to study stem cell behavior and developmental processes in the context of controllable three dimensional (3D) models of engineered tissues, and (iii) to utilize engineered tissues as models for studies of physiology and disease [22-27].
In what follows we briefly review some fundamentals of classical tissue engineering and present examples of self-assembly-based approaches.
2.1. Biomimetic approach to tissue engineering
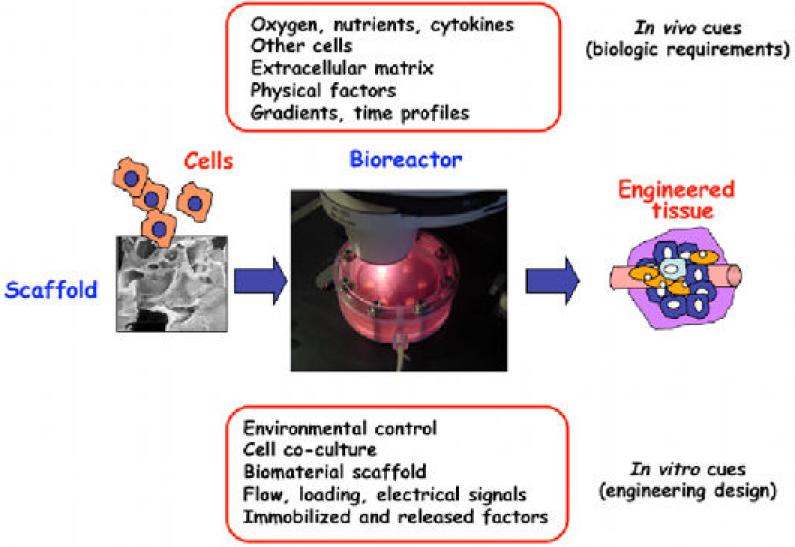
In living organisms, tissue development is orchestrated by numerous regulatory factors, dynamically interacting at multiple levels, in space and time. Recent developments in the field of tissue engineering are aimed at designing a new generation of tissue engineering systems with in vivo like, but controllable cell environment. Such “biomimetic” environment, as a result of biology and engineering interacting at multiple levels [23, 26], should be suitable to direct the cells to differentiate at the right time, in the right place, and into the right phenotype and eventually to assemble functional tissues by using biologically derived design requirements (figure 1). Thus these environments must be able to provide the cells with the same regulatory factors - molecular, structural, and physical – that govern the in vivo cellular processes [21, 24, 27], and thereby unlock their full potential for tissue development and regeneration. Progress in the environmental control of tissue growth and maturation in bioreactors, directing cell fate using soluble clues (e.g. morphogens), mechanical or electrical stimulation, are increasingly orienting the field towards biologically inspired designs with a real-time insight [9, 27].
Figure 1.
Biomimetic paradigm. In vivo, the progression of tissue development and remodeling depends on the interaction of time and space gradients of multiple factors that are not entirely known. The “biomimetic” approach to tissue engineering aims to utilize these same factors through the design of biomaterial scaffolds (providing structural, mechanical and logistic templates for cell attachment and tissue formation) and bioreactors (providing environmental control, exchange of nutrients and metabolites, and the molecular and physical regulatory signals). This way, the biological requirements inspire the design of tissue engineering systems, whereas tissue engineering provides controllable models of high fidelity for regenerative biology studies (reproduced from [2] with permission of Elsevier).
2.2 Cell sources
The choice of repair cells is central to any of the many different modalities of tissue engineering - injection of repair cells (with or without biomaterial), implantation of a fully formed cell-based graft, or mobilization of the host cells into the site of injury [20, 23, 26, 28]. One obvious requirement is that of immune tolerance of the repair cells. Utilization of autologous adult stem cells (i.e., cells obtained from the patient) has the advantage of being patient-specific and alleviating immune rejection or transmission of disease. While adult stem cells were first isolated from the bone marrow, recent literature supports their presence in a variety of extramedullary organs including adipose tissue, dental pulp, circulating blood, amniotic fluid, and joint synovium [29-33].
In general, adult human mesenchymal stem cells have documented capacity to form a number of tissues (including cartilage, bone, fat, and blood vessels), but not necessarily cardiac muscle, nerves, hepatocytes or Langerhans islets. Embryonic-like stem cells, including the newly derived induced pluripotent cells (iPS) [34, 35] have essentially unlimited potential for expansion in vitro. Recent exciting developments in iPS cells from various human tissues opens the possibility of creating autologous embryonic-like cells for cardiac regeneration, with the caveat that the derivation of iPS cells involves, at least at this time, genetic transformation. It is expected however, that the use of small molecules will gradually and completely replace gene transfer in the derivation protocols of patient-specific adult human cells.
We propose that the “conditioning” of stem cells – both adult and embryonic like - by biophysical stimulation in 3D culture settings may result in the stabilization and maturation of differentiated cell phenotypes. Several promising paths towards deriving the “right” cells for the repair of a range of human tissues and the right technology to utilize their potential need to be pursued until one or more of these options is translated into clinical practice.
2.3. Tissue engineering scaffolds
Tissue replacements are created by culturing cells on three-dimensional scaffolds to develop functionality, and then implanted in vivo. Depending on what tissue is to be replaced, the properties of scaffolds will vary along several parameters, including biological substances used, porosity, elasticity, stiffness, and specific anatomical shapes. To make a scaffold, biomaterials are processed into 3D architectures suitable for cell seeding and cultivation.
The choice of biomaterial is generally guided by the need to restore tissue-specific structure and cell signaling, and to match the necessary physical behavior (such as load-bearing, or signal propagation). Scaffolds also need to serve as “logistic templates”, by providing the cells with specific topological features (from nano to micro and macro scale), mechanical environment (which cells can sense at multiple levels), surface ligands, and the ability to release cytokines. Some of the novel scaffold designs enable an active and dynamic interplay with the cells [21]. As the cells deposit their own extracellular matrix, the biomaterial is expected to fully degrade, and a tissue-like structure to form and progressively integrate within the surrounding host tissue upon implantation. This approach has been implemented in the reconstruction of various tissues including skin, bone, cartilage, meniscus, bladder, and has, in some cases, been further translated to clinical practice[5, 36-39].
Scaffold-based tissue-engineering faces some challenges [40, 41], including: (i) immunogenicity, (ii) acute and long-term inflammatory response resulting from the host response to the scaffold and its biodegradation products, (iii) mechanical mismatch with the surrounding tissue, (iv) difficulties in incorporating high numbers of cells uniformly distributed within the scaffold, and (v) limitations in introducing multiple cell types with positional specificity. These difficulties, along with the increased understanding of developmental and morphogenetic processes, have led many groups towards the development of “self-assembly” approaches in which individual cells organize into multicellular subunits (e.g. spheroids, sheets or cylinders) [42-45]. The individual subunits further arrange themselves into larger tissue structures with little intervention, and in most cases, without the use of exogenous scaffolds [46, 47].
2.4. Scaffold-free tissue engineering: fabricating cardiovascular grafts
Functional small-caliber arteries were engineered by cell cultivation on biodegradable fibrous polyglycolic acid (PGA) scaffolds in a bioreactor with dynamic pulsatile flow [48]. Collagen deposition and alignment during construct maturation is crucial for achieving adequate mechanical strength (e.g. burst pressure) needed for implantation. Maturation of vascular constructs under cyclic radial strain allowed for circumferential assembly and alignment of collagen in a helicoidal pattern similar to native blood vessels [49]. However the use of biodegradable scaffolds can lead to the residual presence of polymer fragments disrupting the normal organization of the vascular wall [50]. For such a mechanically demanding application, balancing the degradation rate of the scaffold material with tissue remodeling in the patient remains a challenge.
In parallel, self-assembly (in particular, scaffold-free) approaches demonstrate that fully biological tissues can be engineered with specific compositions and shapes, by exploiting cell-cell adhesion and the ability of cultured cells to grow their own ECM, and thereby help reduce and mediate inflammatory responses. One of the impressive examples of self-assembly methods is the sheet-based tissue-engineering technology developed by L'Heureux and colleagues [43]. Using this approach, sheets of human smooth muscle cells (SMC) and fibroblasts were grown on culture plates in the presence of ascorbic acid to enhance collagen production, detached and wrapped around a porous, tubular mandrel to form the equivalents of media and adventitia of blood vessels. After several weeks of maturation in a bioreactor, lumens of the constructs were seeded with endothelial cells (EC). The resulting structures displayed strong mechanical properties, well-defined multilayer organization and an abundant, organized ECM. Autologous small-diameter vascular grafts engineered using this method are currently in clinical trials for hemodialysis access [44].
Okano and colleagues have developed a similar “self-assembled” sheet based approach for cardiac tissue-engineering. In this approach, neonatal rat cardiomyocytes were cultured on temperature-responsive culture dishes [51-53]. After harvest, electrical coupling of layered cardiomyocyte sheets occurred quickly through functional gap junction formation [54] and subsequent implantation in subcutaneous position demonstrated that pulsatile, layered cardiomyocyte sheets survived and grew for a prolonged period of time [55]. The versatility of this method has made it a good candidate to create functional and transplantable tissue sheets obtained from various cell types including epidermal keratinocytes [56], kidney epithelial cells [57], and periodontal ligaments [58, 59]. It has already been used in a clinical trial involving corneal transplantation, which promoted the recovery of weakened vision [60].
2.5 Need for functional vascularization
One of the most critical present problems in the field of tissue engineering is to provide vascular supply to thick constructs [61, 62], as molecular diffusion can assure the exchange of nutrients and oxygen only within approximately ~100 μm thick layer of viable tissue [63, 64]. The provision of vascular conduits that have been engineered in vitro to pre-vascularize tissues [65-68] is a remarkable but not sufficient advancement, as the immediate connection to blood flow upon implantation still remains a problem.
For one application, self-assembly of cells into sheets provided an indirect solution to this problem [69] by sequential implantation of multiple cardiac sheets. Because the thickness of a single cardiac sheet (<80 μm) was thinner than the diffusional penetration depth of oxygen, the host vasculature was given time (1-3 days) to vascularize each individual transplanted sheet before the next one was added during the following surgery. This way, 1mm-thick vascularized myocardium was obtained. While achieving the goal of functional vascularization of thick tissue-engineered constructs in vivo, this method is clearly not applicable to patients due to the medical risk of multiple surgical procedures.
Another approach enabled the generation of fully viable, thick and functional cardiac tissue constructs in vitro using microchanneled scaffolds and bioreactor perfusion systems [70, 71]. The functional assembly of engineered cardiac muscle in vitro was enhanced by oxygen supply provided by mechanisms resembling those in normal vascularized tissues. To mimic the capillary network, cardiomyocytes and fibroblasts isolated from neonatal rat hearts were cultured on a highly porous elastomer with a parallel array of channels that were perfused with culture medium. To mimic oxygen supply by hemoglobin, culture medium was supplemented with a perfluorocarbon (PFC) emulsion. The structural and functional properties of the constructs were markedly improved, in a manner correlated to the improved supply of oxygen to the cells. It was postulated that the channel arrays can also serve as precursors for the formation of the vascular network.
However the transition and integration of a tissue from an in vitro to an in vivo setting still needs to be addressed. From a surgical point of view, a tissue-engineered graft would contain a hierarchical macro- to micro-vascular tree ending by a connectable artery and vein so that perfusion of the whole graft could be immediately restored upon implantation. In order to form such a construct, three gradual components of the vascular tree have to be considered: the capillary network (~10-20 μm, by induction of sprouting either in co-culture or by cytokines), intermediate microvessels (50-500 μm, by microfabrication), and the macrovasculature (up to 2 mm, by tissue engineering). Capillary networks can form in co-cultures of osteoblasts or cardiomyocytes with endothelial cells, by natural cell assembly in either sheet [65, 72] or spheroid [73, 74] cell culture systems. Despite major improvements allowing design of microvascular networks in vitro using microfluidic technologies [75], pre-vascularized tissues still face the challenge of functional anastomosis to the host vasculature. Recent clinical trials with engineered blood vessels using scaffold-based [7] and sheet-based methods [76] illustrate that engineering of the macro-vasculature including the connectable artery and vein for surgical anastomosis seems to be within reach. It is hoped that these vessels will exhibit mechanical competence (e.g. burst pressure and compliance, suturability) and antithrombogenic properties [77, 78]. However, it remains unclear how can the macrovasculature be connected to the capillary tree and perfused with blood.
2.6. Self-assembly of vascular networks
Self-assembly approaches may have major impact on the development of the intermediate vasculature, which is the “missing link” for establishing a perfused vascular tree. Sefton and colleagues have reported a method based on the modular assembly of endothelialized microtissues to form macrotissues [79]. In this approach, modular tissue-engineered constructs were assembled from sub-millimeter-sized cylindrical modules of collagen or gelatin seeded with cells, and endothelialized at the surfaces [79, 80]. EC-covered modules then randomly self-assembled into a modular construct with interstitial spaces that enabled perfusion with medium or whole blood. It remains to be seen how stable is the self-assembled microvasculature on the long-term and how it can integrate components enabling direct anastomosis to the host vasculature in vivo. In a similar fashion, several groups, including ours, are currently working on the fusion of endothelialized spheroids as means for creating microvessels in vitro [81-83].
To develop tissue-engineered constructs of clinically relevant size with a fully functional vasculature that can eventually be anastomosed to the host vasculature in vivo, we suggest a self-assembly approach shown in figure 2.
Figure 2.
Model for thick construct tissue-engineering using a self-assembly approach. (a) A macrovascular network (from a few mm to less than 1 mm in diameter) is built using bioprinting, then matured for several weeks in a perfusion bioreactor (b), in order to achieve adequate mechanical properties needed for future implantation in the host. (c) Micro-vascularized multicellular cylindrical modules (200 μm) are obtained from coaggregation of endothelial cells and the cell type of interest (ex: hepatocytes), and their surface is endothelialized. (d) EC-covered modules are then randomly assembled into a modular construct within each lumen of the macrovascular network (only one shown explicitly), and form interconnected channels (intermediate microvasculature: 50- 500 μm) under medium perfusion. (e) The entire construct is then surgically connected to the host vasculature in an arterio-venous position.
A network of macrovessels including a connectable artery and vein would be build (by bioprinting, see below), and then matured using a perfusion bioreactor to achieve mechanical properties necessary for implantation. Parenchymal and endothelial cells would be co-cultured to produce micro-vascularized units (in the form of cylindrical or spherical multicellular aggregates), sized to be below the diffusion limitation. After the surfaces are endothelialized, pre-vascularized units would be packed into the lumen of the matured macro-vessels and perfused to promote self-assembly of intermediate microvessels and their connection to capillary network, to enable implantation by direct anastomosis to the host vasculature.
Cellular self-assembly approaches represent an alternative and offer a complement to scaffold-based tissue engineering. They allow establishing high cell-density, controlled deposition of extracellular matrix, and positional specificity of cell patterning. Translation of cell and tissue self-assembly approaches into the clinical field will necessarily depend on our ability to understand the principles underlying such approaches.
In what follows we present examples of early developmental self-assembly processes that will be utilized for the ‘biofabrication by bioprinting technology’.
3. Developmental mechanisms of cellular self-assembly
It is through cellular self-assembly that the morphologically featureless zygote evolves into the fully developed organism with its numerous structures of widely varied shapes and forms. Although the sequence of morphogenetic processes that underlies early development is under strict genetic control, additional physical mechanisms are mobilized to move mass and make shapes. In turn, the changes brought about by physical processes (e.g. diffusion, changes in shape, molecular and ion concentration) provide feedback to gene expression. The interplay of genetic and physical processes is the hallmark of embryonic development. A better understanding of this interplay would enhance our ability to employ the principles of cellular self-assembly in the biofabrication of living constructs. In this section we review characteristic morphogenetic mechanisms that are utilized in the biofabrication technology to be discussed in the next section.
3.1. Cell sorting
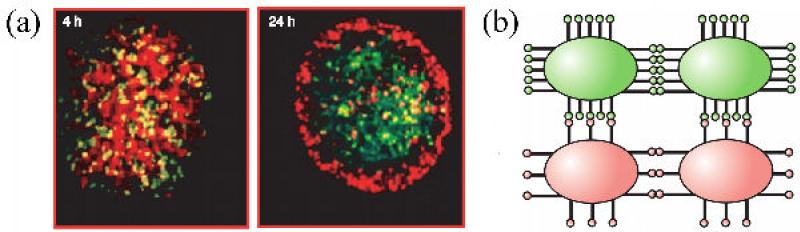
Cell sorting is a self-assembly process providing a common mechanism to establish cellular compartments and boundaries between distinct tissues [84-87]. To understand the molecular basis of cell sorting, let us consider lumen or cavity formation. As cells in an initially homogeneous cell population differentiate they may become polarized and express cell adhesion molecules only on restricted parts of their surface. As a consequence, if minimization of configurational energy is the driving force in cell rearrangement, a lumen is bound to appear (figure 3).
Figure 3.
Schematics of lumen formation as a consequence of differential adhesion. The shading in some cells in the middle panel represents the lack of adhesion molecules on corresponding regions of the cells. These cells sort through the mixture and orient themselves to form a lumen or cavity. [88]. [Figure reproduced with permission of Cambridge University Press.]
Lumen formation is a simple manifestation of the differential adhesion hypothesis (DAH; [89, 90]), which postulates that cells of different type adhere to each other with different strength (such as the polarized and non-polarized cells in figure 3). The DAH may also provide a simple molecular explanation for cell sorting: an initially mixed population of differentially adhesive cells, either due to quantitative ([90]; figure 4) or qualitative ([91, 92]; figure 5; see also figure 8) differences in cell surface adhesion molecules, evolves to a compartmentalized state in which the more adhesive cells aggregate and become surrounded by the less adhesive ones. As sorting requires the cells to be motile, this morphogenetic mechanism is most active during embryonic development, where adhesive contacts (through cell adhesion molecules such as cadherins or selectins) are not yet at the mature stage as in an adult organism (e.g. tight junctions, gap junction) [88].
Figure 4.
Sorting in a mixture of cells based on differential adhesiveness. (a) Sorting of two genetically transformed Chinese Hamster Ovary (CHO) cell populations with ~30% difference in N-cadherin expression (green cells contain more N-cadherins). Aggregates (200 μm in diameter) contain equal number (~6,000) of the two cell types (i.e. green and red), and sort within ~24 hrs. (b) Schematic illustration of adhesion molecules (bars ending in circles) on the surfaces of the two CHO cell populations. To minimize the configurational energy, highly adhesive cells tend to group together. [Figure reproduced with permission of Cambridge University Press.]
Figure 5.
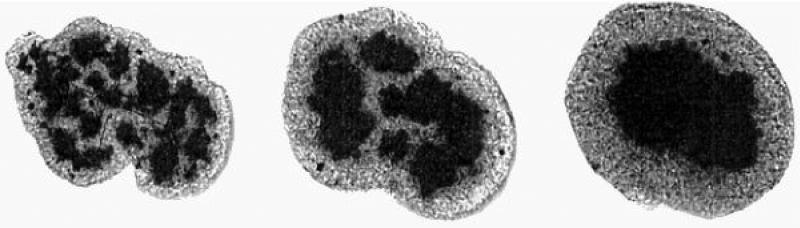
The images show the full cross-section of a 200 μm diameter spheroidal aggregate consisting of a mixture of chicken embryonic pigmented epithelial (dark) and neural retinal (light) cells. The three panels correspond to the times of 17, 42 and 73 hrs after the initiation of sorting [96]. [Figure reproduced with permission of PNAS].
Figure 8.
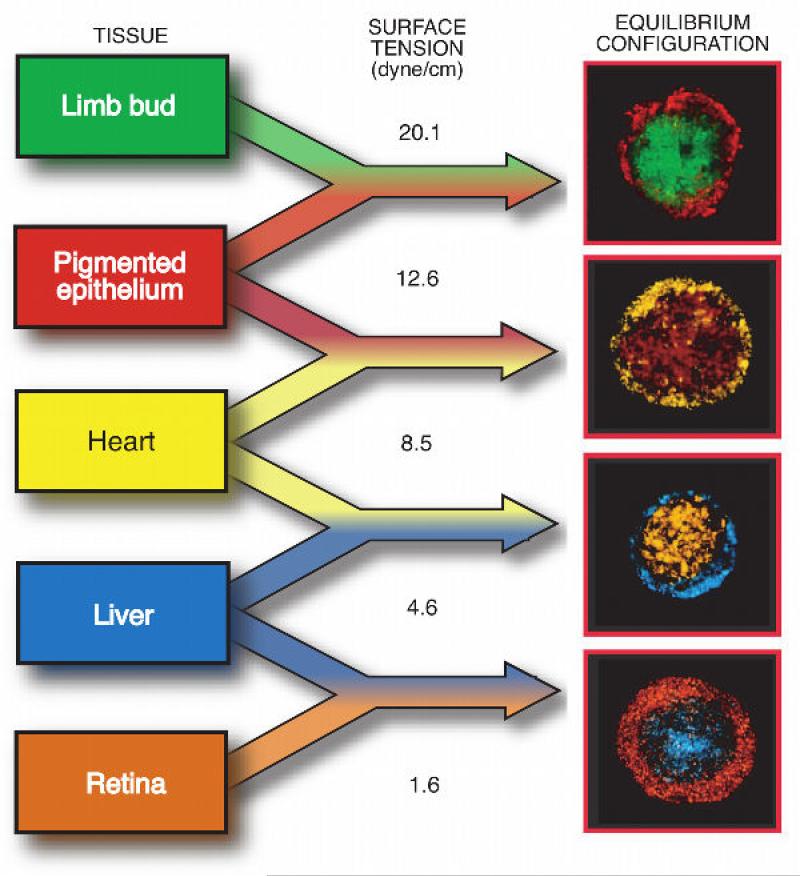
Correspondence between the sorted patterns and apparent tissue surface tension. Data are shown for five chicken embryonic tissues [88, 101]. [Figure reproduced with permission of Cambridge University Press].
Though interpretation of sorting by DAH is appealing and elegant, recent developments suggest the situation may be more complicated; compartment boundaries between tissues may result from the synergistic effect of differential cell adhesion and cellular tensile forces generated by acto-myosin-dependent cell cortex tensions [91-95].
3.2. Tissue fusion
Tissue fusion is a self-assembly process in which two or more distinct cell populations make contact and coalesce. The fusion process underlies the formation of numerous structures in the embryo (for specific examples see [86]). Figure 6 illustrates the in vitro fusion of two multicellular aggregates [97]. A striking in vivo example is shown in figure 7 [81].
Figure 6.
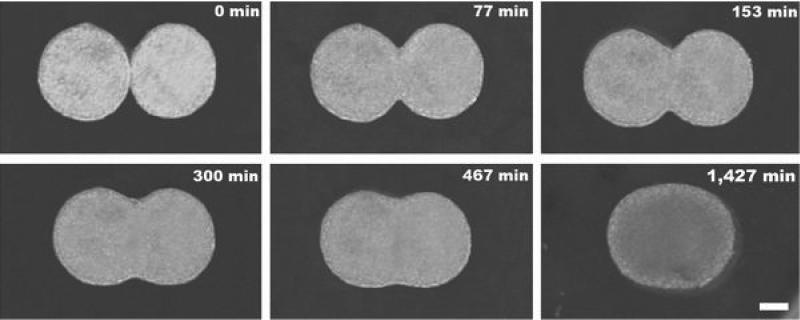
In vitro fusion of two embryonic cell aggregates. Snapshots of two apposed embryonic cushion tissue spheroids cultured in a hanging drop configuration fuse into a single spheroid over a period of ~1 day [42]. Scale bar: 100 μm.
Figure 7.
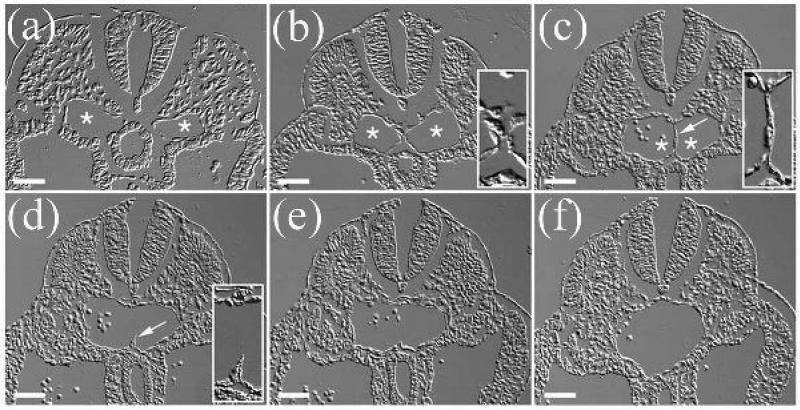
Formation of the descending aorta by fusion of the pair of dorsal aortae (29). Panels represent a series of cross-sections of the posterior-anterior axis in an E9.5 mouse embryo, arranged in a posterior to anterior direction. (a-c) Asterisks indicate the lumens of the paired dorsal aortae; arrow in (c) shows the cellular septum separating the two aortae. Insets in (b) and (c) show high-magnification views of the interface region between fusing dorsal aortae. (d) Arrow shows a remnant of the cellular septum. Inset in (d) shows a high-magnification view of the remnant of the cellular septum in the dorsal aorta. Scale bars: 50 μm. [Reproduced with permission of Wiley Interscience Publishing Co.].
3.3. Apparent tissue liquidity
During sorting and fusion, the cellular system evolves in time from an initial state to a structurally and energetically more stable final state. It is an intriguing question what drives the underlying equilibration processes. As sorting and fusion strikingly resemble, respectively, the phase separation and coalescence in liquids, it was proposed that adhesive and motile cell populations have apparent liquid-like properties [98-100]. Based on this analogy, cell sorting and tissue fusion should be driven by apparent surface and interfacial tension. An apparent tissue surface tension, has been measured using methods applicable to liquids [101-103] for a large variety of naturally occurring tissues [102, 104-106] and tissue cell aggregates [101, 103, 107-110].
It was suggested that the notion of apparent tissue surface tension is intimately related to DAH [108]: differences in apparent tissue surface tension are quantitative measures of differential cell adhesion. In particular, the measured values of these tensions are consistent with the observed sorting behavior: the tissue of higher surface tension is surrounded by the one of lower surface tension as shown in figure 8, similarly to immiscible liquids, such as oil and water. Furthermore, using engineered cell lines it was demonstrated that is proportional to the number of cell surface adhesion molecules [108], implying that the more cohesive the tissue the higher its surface tension. The final sorted and fused tissue configurations, respectively shown in figure 8 and figure 6 can then be understood as equilibrium states with the lowest interfacial energy. As gravitational forces are negligible and other external forces do not act, in these states the cellular assemblies assume spherical shape.
The spontaneous rounding up of embryonic tissue fragments (figure 9) and multicellular aggregates was one of the first indications of the liquid-like nature of such cellular assemblies. Cellular spheroids are extensively used as close-to-physiological three-dimensional cell culture models that can be easily and reproducibly fabricated using a variety of rapid prototyping methods [45, 46, 111] (see Section 4).
Figure 9.
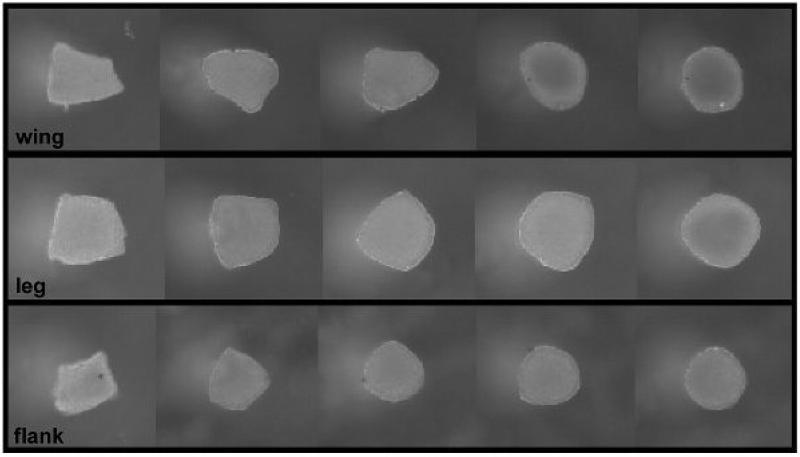
The spontaneous rounding of tissue fragments. The rounding of tissue fragments (~300 Sm in size) of 4-day embryonic chick leg, wing and flank tissue that occurs within 24 h in vitro, is viewed as an indication of liquid-like behavior. The fragments were photographed successively after explant, and at 5 h, 11.5 h, 17.5 h and 24 h thereafter [104].
It is important to note that the concept of tissue liquidity establishes analogy as opposed to identity between true liquids and cellular assemblies. The motion of liquid molecules is driven by thermal fluctuations with characteristic energy kBT (kB - Boltzmann constant, T - absolute temperature), whereas the motion of cells is driven by metabolic energy resulting from ATP hydrolysis. Therefore it is even more striking that not only the final sorted and fused configurations are liquid-like but so is the approach to these final states. Indeed, the time sequence shown in figure 6 can be described by the theory of highly viscous liquids [112-115]. Tissue liquidity, albeit a useful and convenient concept, is still not a universal morphogenetic principle. Processes that are consistent with tissue liquidity may act at one stage of early development but not at another. Indeed, apparent surface tension is typically a meaningful quantity only for a period of time that depends on tissue type [101]. Nevertheless it is exciting option that cellular properties can be summarized using just a few parameters (e.g. surface tension, viscosity, elastic constants), to describe large-scale tissue behavior with relevance to the fabrication of multicellular living constructs.
4. Engineering and fabricating tissues by bioprinting
Recently, several research groups have embarked on engineering three-dimensional living structures using bioprinting. Two main distinct technologies have emerged. One relies on the use of inkjet printing [116-124]. In this technology either individual cells or small clusters are printed. The method is rapid, versatile and cheap. Its disadvantage is that it is difficult to assure high cell density needed for the fabrication of solid organ structures. Furthermore, due to the high speed of cell deposition considerable damage is caused to cells, although the latest developments in the field have led to considerable improvement in cell survival. Finally, to achieve appropriate structural organization and functionality remain a challenge.
In the other approach mechanical extruders [125, 126] are used to place “bioink” particles, multicellular aggregates of definite composition into a supporting environment, the “biopaper”, according to computer-generated templates consistent with the topology of the desired biological structure [42, 45, 97]. Organoids form by the postprinting fusion of the bioink particles and the sorting of cells within the bioink particles. The advantage of this technology is that as bioink particles represent small three-dimensional tissue fragments. Thus cells in them are in a more physiologically relevant arrangement, with adhesive contacts with their neighbors, which may assure the transmission of vital molecular signals. The method employs early developmental mechanisms, such as tissue fusion and cell sorting. The disadvantage of the method is associated with the relatively high cost of the printers. Both inkjet and extruder bioprinting are compatible with rapid prototyping.
In this section we review the latest developments in extruder-based bioprinting technology developed in our laboratory (figure 10). In particular, we first describe how the multicellular bioink particles are prepared and subsequently discuss how the special-purpose extruder printers deliver them according to computer-generated templates. For specificity we will use the process of engineering vascular grafts.
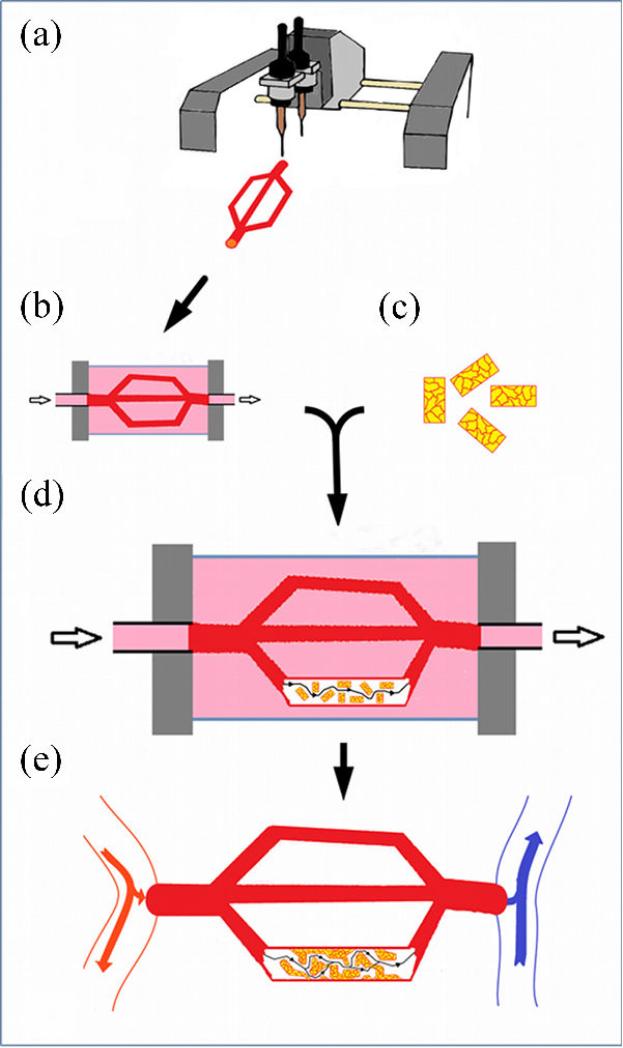
Figure 10.
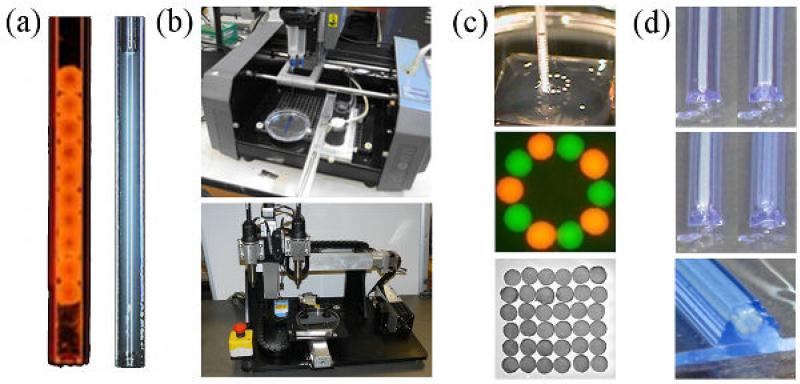
Components of the print-based tissue engineering technology. (a) The bio-ink-filled micropipette printer cartridge filled with multicellular building blocks that can be spheroidal (left) or cylindrical (right) depending on the method of preparation (b) The bio-printer. Three-dimensional printing is achieved by displacementof the 3-axis positioning system (stage in y and printing heads along x and z (top: Neatco, Carlisle, Canada; bottom: Organovo-Invetech, San Diego)). (c) Spheroids are delivered one by one into the hydrogel bio-paper (itself printed) according to a computer script. (d) Layer-by-layer deposition of cylindrical units of biopaper (shown in blue) and multicellular cylindrical building blocks. The outcome of printing (spheroids in panel (c), multicellular cylinders in panel (d)) is a set of discrete units, which postprinting fuse to form a continuous structure.
4.1. Fabrication of self-assembling, multicellular building blocks
Multicellular bioink building blocks are prepared from cell suspensions. They can be homogeneous, containing a single cell type or heterogeneous, made from a mixture of several cell types. Bioink particles typically used in our laboratory are either spherical or cylindrical in shape. Multiple methods have been described to prepare spherical aggregates [45, 46, 111, 127].
Here we briefly describe one method for the preparation of the spherical or cylindrical units (figure 11). The cell suspension is centrifuged and the resulting pellet is transferred into a capillary micropipette. After a short incubation in medium at 37°C, cell-cell interactions are restored and the cylindrical slurry becomes sturdy enough to be extruded into liquid. The spherical building blocks are obtained by mechanically cutting uniform fragments that spontaneously round-up as a manifestation of tissue liquidity. If the slurry is composed of multiple cell types, sorting and rounding will occur in parallel. As for embryonic tissues, the sorting behavior is driven by differences in tissue surface tension of the cell aggregates (figure 12). The fabrication of cylindrical building blocks requires maturation of the slurries in a non-adhesive mold overnight to improve their cohesivity. Automation of the deposition step into the mold has been achieved and was important for the high quality of cellular cylinders and the rate of their production.
Figure 11.
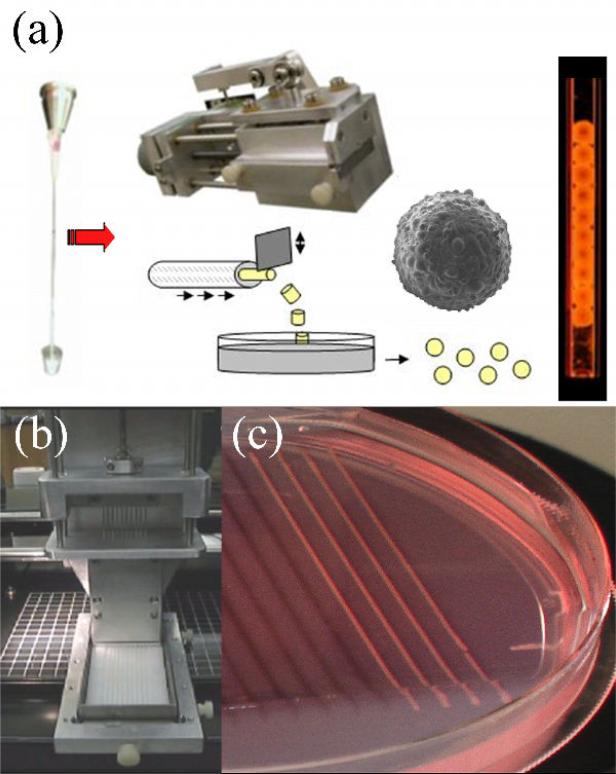
Preparation of the multicellular building blocks. (a) Preparation of spherical building blocks. The cell slurry extruded from a micropipette is cut into cylindrical fragments (with identical diameter and height) of equal size using a custom device. Spherical bio-ink particles are formed by rounding of the cylinders upon overnight incubation on a gyratory shaker. A scanning electron microscopy picture of a 500 μm spheroid composed of endothelial cells is shown. The spheroids are packaged into the bio-ink-filled micropipette printer cartridge just before printing. (b) Preparation of cylindrical building blocks. Up to ten multicellular slurries can be simultaneously extruded into a non-adhesive agarose mold using a customized attachment to the bioprinter. After overnight maturation, the multicellular cylinders are strong enough to be printed.
Figure 12.
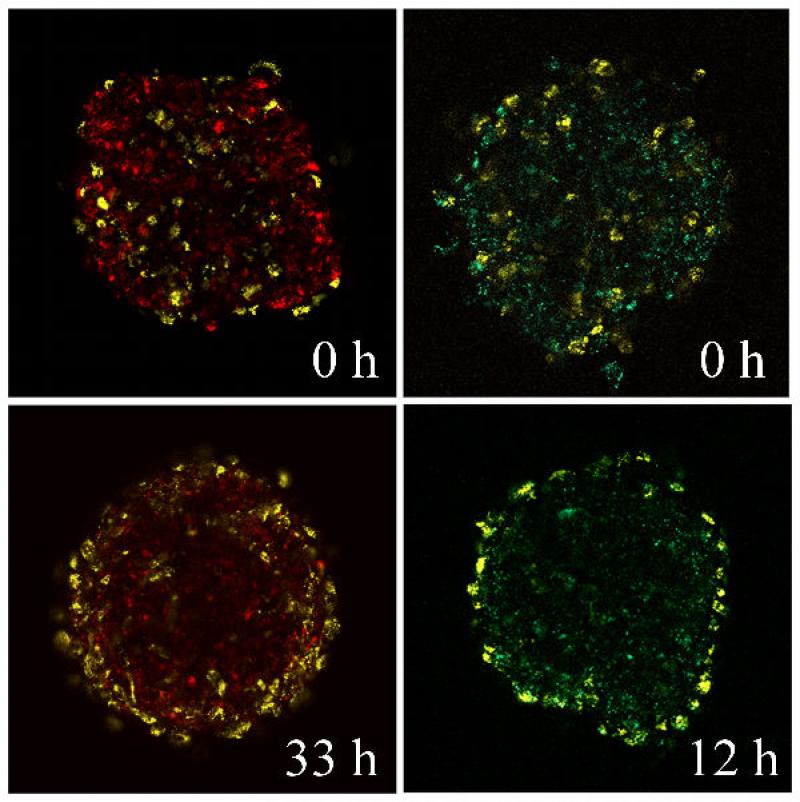
Time evolution of cell sorting in multicellular aggregates. Aggregates composed of endothelial cells (yellow) and fibroblasts (red) (left column) and endothelial cells (EC) (yellow) and smooth muscle cells (SMC) (green) (right column) are shown. The sorting pattern can be attributed to the differences in the apparent surface tension of multicellular assemblies prepared from the two cell types, similarly to the situation shown in figure 8 (endothelial cell aggregates: 12 dyne/cm; fibroblast aggregates: 72.7 dyne/cm, smooth muscle cell aggregates: 279 dyne/cm).
4.2.Bioink deposition
The extrusion-based bioprinting described here represents an automated deposition method that enables the building of 3D custom-shaped tissue and organ modules without the use of any scaffold. In this way, a fully biological construct is generated that can be structurally and functionally close to a native tissue. Spherical or cylindrical multicellular units – the bioink – are delivered according to a computer-generated template together with hydrogel – the biopaper – serving as support material.
Different deposition schemes have been employed for 3D tissue bioprinting as the technology evolved. The scheme shown in figure 13 was used initially to establish proof of concept. Despite some successful outcomes [42], the rapidity, reproducibility and scalability of the technique were challenged by a few shortcomings.
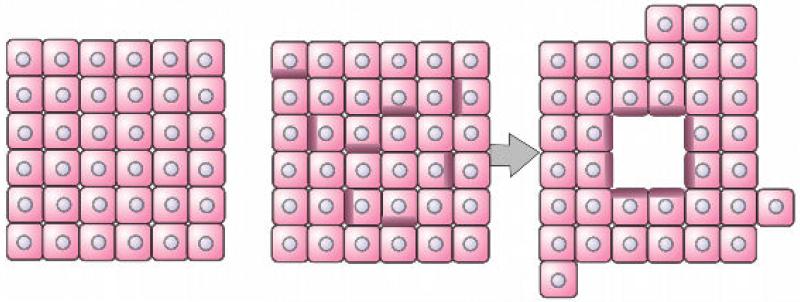
Figure 13.
Layer by layer deposition. (a): A sheet of biocompatible hydrogel is printed, and the building blocks (a) and (b) are embedded into the collagen sheet. (b)(c). The alternate deposition of layers of hydrogel and building blocks is pursued according to the predefined blueprint of the desired 3D structure (here, a tubular construct). (d). Fusion of the building blocks and removal of the hydrogel result in a hollow tube after a few days.
One limitation of this early system was that the success of printing depended strongly on the control of the gelation state of the collagen layers. Uneven gelation compromised the spatial accuracy of the construct in particular for constructs thicker than a few layers [42]. In addition, collagen was remodeled by the cells and integrated into the final structure, such that its removal was challenging. Another limitation arose during printing constructs of larger size and more complex patterns (i.e. branching tubes). The preparation of spheroids in large quantities (>1,000 for branched tubular structures) became excessively time-consuming. In addition, the manual filling of the micropipette printer cartridge with the cellular spheroids (one by one) represented a serious challenge (e.g. it had to be assured that no gaps between the spheroids appeared upon their aspiration into the micropipette).
To overcome the above limitations, we replaced the collagen sheets with agarose rods (figure 14) and we used cylindrical multicellular building blocks instead of spheroids (figure 10 and 14). The agarose rods are formed in situ and deposited by the bioprinter automatically, rapidly and accurately. Agarose is an inert and biocompatible hydrogel that cells neither invade nor rearrange. The agarose rods kept their integrity during post-printing fusion, and were easily removed to free the fused multicellular construct.
Figure 14.
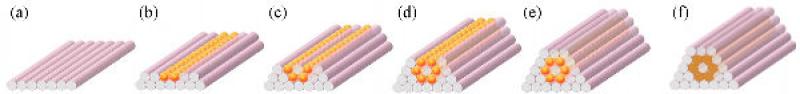
Horizontal layer-by-layer deposition of the building blocks. (a-e) A possible deposition scheme for a tubular structure built of agarose rods and spherical multicellular building blocks. (f) The same tubular configuration printed with cylindrical multicellular building blocks.
4.3 Self-assembly of the building blocks
A living cell based construct that results from the described printing process and the postprinting fusion of the bioink particles is subsequently placed in an incubator where it achieves its final 3D structure and through maturation develops appropriate biomechanical properties. Proof of concept studies have been carried out to build tissue toroids, thick sheets and straight and branched tubes ([42, 45]; figure 15).
Figure 15.
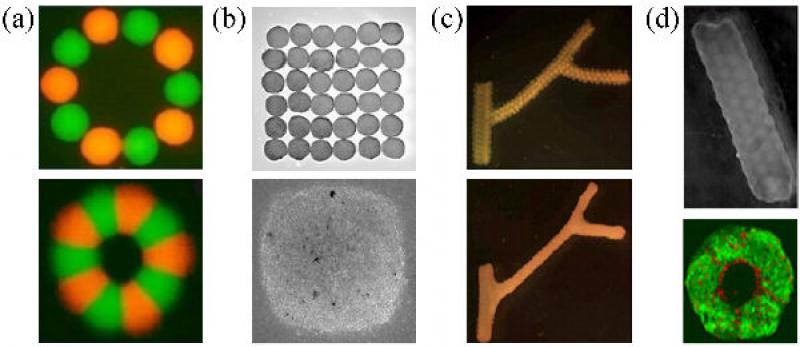
Printing of geometrically complex multicellular constructs. (a) 500 μm bio-ink particles (top), composed of Chinese Hamster Ovary (CHO) cells were printed on 1.3 mg/ml collagen bio-paper, in circle (aggregates are labeled alternatively with red and green dyes). A fused toroid, after ~60h (bottom) [42]. (b) Chick cardiac cell aggregates printed into sheet (top). A fused sheet, after ~70h (bottom) [42].(c) A branching conduit printed using spherical units; bottom panel shows the fused structure. (d) A 12-layered printed CHO tube using spherical units (top). Cross section of the fused tube made with units composed of SMCs (green) and ECs (red) (bottom).
It was demonstrated that no biological functionality was lost in the bioprinting process when the sheet obtained by the fusion of chick cardiac cell spheroids exhibited synchronous macroscopic beating throughout the construct. When endothelial cells were mixed with the cardiac cells, self-assembly has led to the formation of vessel-like conduits [42].
Our current efforts are focused on the bioprinting of small and intermediate diameter blood vessel substitutes ([45], figure 16a). As the print-based technology exploits intrinsic self-organizing properties of cells and tissues, it incorporates some of the natural vessel forming processes. During embryonic vasculogenesis and angiogenesis, the initial steps are performed by the ECs as they form the elementary tubular conduits. Under shear stress, the ECs secrete the growth factors PDGF and TGFβ and the associated molecular signals are responsible for the recruitment of SMCs and the induction of ECM deposition (i.e. collagen and elastin). In particular TGFβ plays a key role in the production of elastin, an ECM component that not only is responsible for the elastic properties of the vessels but also regulates the sequestration and activation of the growth factors [128].
Figure 16.
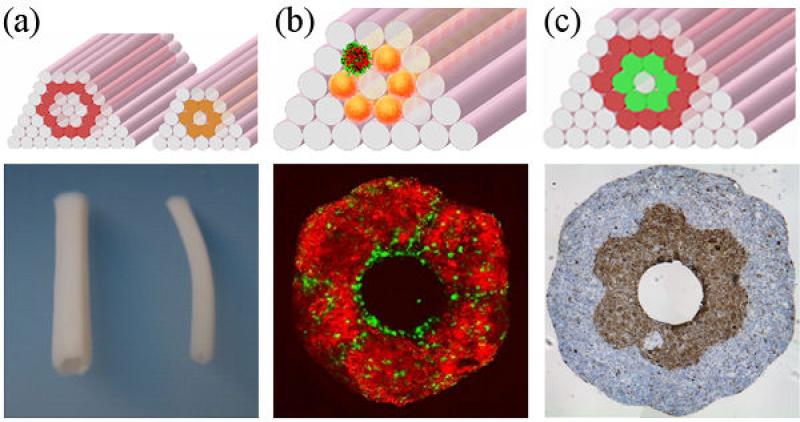
(a) Design templates (top) and fused constructs (bottom) of different vessel diameters built with cylindrical bioink. (b) The top image shows a template to build a construct with spheroids composed of SMC (red) and ECs (green). A transversal section after fusion (bottom) shows that the lumen is composed predominantly of endothelial cells. (c) Template to construct a double-layered vascular tube (top). The inner layer is constructed of SMC building blocks (green), the second of fibroblast building blocks (red). The transversal section (bottom) shows fusion and the segregation of the two cell types mimicking the media and adventitia of blood vessels.
With the described print-based technology, tubular structures can be built from mixtures of randomly distributed ECs and SMCs, which offers a unique opportunity to exploit the EC/SMC interaction from the initial step of vessel formation. Endothelium is expected to form during the postprinting fusion and sorting (figure 16b). Applying flow to the forming endothelium early on will stimulate ECs to behave as they do in vivo. The anticipated benefits will be the production of a more physiological ECM (both in composition and organization), better attachment of the endothelium and faster maturation. To mimic the blood vessel structure, a double-layered vascular tube has been constructed (figure 16c).
In conclusion, the novel print-based tissue engineering technology has several distinct features with great potential for the generation of tissue and organ structures: (i) it represents an approach for producing fully biological (scaffold-free) small diameter vessels; (ii) it utilizes natural shape-forming (i.e. morphogenetic) processes, that are present during normal development; (iii) it can provide organoids of complex topology (i.e, branching tubes); (iv) it is scalable and compatible with methods of rapid prototyping.
5. Future perspectives
While regenerative medicine is far from providing “replacement parts” and tissue regeneration therapies ready to be used in patients, we seem to be getting increasingly close to the development of treatment modalities for re-establishing tissue structure and function. The rapid development of the field is largely driven by the medical needs of our aging population, and by recent developments in stem cell biology and technologies of cell and tissue engineering. In the last years, tissue engineering went through some major changes that might be indicative of the future trends in this field.
Overall, a largely empirical approach to tissue engineering (“tissue try this”, as cleverly described by Shannon Dahl) is being replaced by the development of developmentally inspired technologies (approaches based on bioprinting and self-assembly being remarkable examples). Consequently, one major focus is on the cells. With the living cell being the ultimate “tissue engineer”, our effort is being directed to providing the conditions, so that the cells do the engineering. Another focus is on technology. After major breakthroughs in stem cell biology, we are now developing technologies for “instructing” the cells to regenerate defective tissues by providing a highly engineered environmental milieu.
These focus points are illustrations of the paradigm shift in the field towards the establishment of a “biomimetic “ approach, that is based on the premise that unlocking the full biological potential of stem cells (in vitro or in vivo), necessitates an environment mimicking that present during native development. This paradigm is guiding many current tissue engineering approaches, including those based on cell self-assembly that holds promise of building fully biological tissues with structural and functional specification. We described the potential of the self-assembly-based technology for the formation of a hierarchically defined vascular tree perfused with blood, one of the main unsolved problems in tissue engineering. It is highly possible that in the future this technology can also help build other complex tissues – such as stratified and anisotropic structures consisting of multiple cell types and tissue subunits. For this to happen, we will need to continue to cross the boundaries between the disciplines, to take advantage of the synergism of developmental and adult biology, biomaterials science, biomedical engineering and medicine.
Acknowledgments
The authors particularly wish to acknowledge the contribution of enthusiastic undergraduate students Troy Young, Lydia Beck and Timea Kosztin. This research was supported by NSF under grant EF0256854 and NIH under grants HL076485, EB002520, HL089913 and HL 095191.
References
- 1.Boncheva M, Andreev SA, Mahadevan L, Winkleman A, Reichman DR, Prentiss MG, Whitesides S, Whitesides GM. Magnetic self-assembly of three-dimensional surfaces from planar sheets. 2005;102:3924–9. doi: 10.1073/pnas.0500807102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boncheva M, Gracias DH, Jacobs HO, Whitesides GM. Biomimetic self-assembly of a functional asymmetrical electronic device. PNAS. 2002;99:4937–40. doi: 10.1073/pnas.032667599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitesides GM, Boncheva M. Beyond molecules: Self-assembly of mesoscopic and macroscopic components. 2002;99:4769–74. doi: 10.1073/pnas.082065899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitesides GM, Grzybowski B. Self-assembly at all scales. 2002;295:2418–21. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- 5.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. 2006;367:1241–6. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 6.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, Hollander AP, Mantero S, Conconi MT, Birchall MA. Clinical transplantation of a tissue-engineered airway. 2008;372:2023–30. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 7.Shin'Oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery(5) 2001;344:532–3. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 8.Hellman KB, Nerem RM. Advancing tissue engineering and regenerative medicine. 2007;13:2823–4. doi: 10.1089/ten.2007.1504. [DOI] [PubMed] [Google Scholar]
- 9.Ingber DE, Mow VC, Butler D, Niklason L, Huard J, Mao J, Yannas I, Kaplan D, Vunjak-Novakovic G. Tissue engineering and developmental biology: going biomimetic. 2006;12:3265–83. doi: 10.1089/ten.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- 10.Vunjak-Novakovic G, Kaplan DL. Tissue engineering: The next generation. 2006;12:3261–3. doi: 10.1089/ten.2006.12.3261. [DOI] [PubMed] [Google Scholar]
- 11.Morrison JI, Loof S, He P, Simon A. Salamander limb regeneration involves the activation of a multipotent skeletal muscle satellite cell population. 2006;172:433–40. doi: 10.1083/jcb.200509011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otu HH, Naxerova K, Ho K, Can H, Nesbitt N, Libermann TA, Karp SJ. Restoration of liver mass after injury requires proliferative and not embryonic transcriptional patterns. 2007;282:11197–204. doi: 10.1074/jbc.M608441200. [DOI] [PubMed] [Google Scholar]
- 13.Griffith L, Naughton G. Tissue engineering - current challenges and expanding opportunities. 2002;295:1009–14. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 14.Langer R, Vacanti JP. Tissue engineering. 1993 May 14;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 15.Grayson WL, Chao PH, Marolt D, Kaplan DL, Vunjak-Novakovic G. Engineering custom-designed osteochondral tissue grafts. 2008;26:181–9. doi: 10.1016/j.tibtech.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, Schoen FJ, Toner M, Mooney D, Atala A, Van Dyke ME, Kaplan D, Vunjak-Novakovic G. Engineering complex tissues. 2006;12:3307–39. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. 2004;101:18129–34. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tandon N, Cannizzaro C, Chao PH, Maidhof R, Marsano A, Au HT, Radisic M, Vunjak-Novakovic G. Electrical stimulation systems for cardiac tissue engineering. 2009;4:155–73. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vunjak-Novakovic G, Altman G, Horan R, Kaplan DL. Tissue engineering of ligaments. 2004;6:131–56. doi: 10.1146/annurev.bioeng.6.040803.140037. [DOI] [PubMed] [Google Scholar]
- 20.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freytes DO, Wan LQ, Vunjak-Novakovic G. Geometry and force control of cell function. 2009;108:1047–58. doi: 10.1002/jcb.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grayson WL, Frohlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, Wan LQ, Liu XS, Guo XE, Vunjak-Novakovic G. Regenerative Medicine Special Feature: Engineering anatomically shaped human bone grafts. 2010;107:3299–304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens T, Radisic M. Challenges in Cardiac Tissue Engineering. 2009 doi: 10.1089/ten.teb.2009.0352. Epub ahead of print 2009 Aug 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cimetta E, Figallo E, Cannizzaro C, Elvassore N, Vunjak-Novakovic G. Micro-bioreactor arrays for controlling cellular environments: design principles for human embryonic stem cell applications. 2009;47:81–9. doi: 10.1016/j.ymeth.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Figallo E, Cannizzaro C, Gerecht S, Burdick JA, Langer R, Elvassore N, Vunjak-Novakovic G. Micro-bioreactor array for controlling cellular microenvironments. 2007;7:710–9. doi: 10.1039/b700063d. [DOI] [PubMed] [Google Scholar]
- 26.Godier AF, Marolt D, Gerecht S, Tajnsek U, Martens TP, Vunjak-Novakovic G. Engineered microenvironments for human stem cells. 2008;84:335–47. doi: 10.1002/bdrc.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grayson WL, Martens TP, Eng GM, Radisic M, Vunjak-Novakovic G. Biomimetic approach to tissue engineering. 2009;20:665–73. doi: 10.1016/j.semcdb.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. 2007;104:11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. 2007;100:1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai HH. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. 2003;102:1548–9. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 31.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pei M, He F, Vunjak-Novakovic G. Synovium-derived stem cell-based chondrogenesis. 2008;76:1044–56. doi: 10.1111/j.1432-0436.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang S, Wang D, Estrov Z, Raj S, Willerson JT, Yeh ET. Both cell fusion and transdifferentiation account for the transformation of human peripheral blood CD34-positive cells into cardiomyocytes in vivo. 2004;110:3803–7. doi: 10.1161/01.CIR.0000150796.18473.8E. [DOI] [PubMed] [Google Scholar]
- 34.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. 2008;322:949–53. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Kerker JT, Leo AJ, Sgaglione NA. Cartilage repair: Synthetics and scaffolds basic science, surgical techniques, and clinical outcomes. 2008;16:208–16. doi: 10.1097/JSA.0b013e31818cdbaa. [DOI] [PubMed] [Google Scholar]
- 37.Lee K, Chan C,K, Patil N, Goodman S,B. Cell therapy for bone regeneration - Bench to bedside. 2009;89B:252–63. doi: 10.1002/jbm.b.31199. [DOI] [PubMed] [Google Scholar]
- 38.Priya SG, Jungvid H, Kumar A. Skin tissue engineering for tissue repair and regeneration. 2008;14:105–18. doi: 10.1089/teb.2007.0318. [DOI] [PubMed] [Google Scholar]
- 39.van Tienen TG, Hannink G, Buma P. Meniscus Replacement Using Synthetic Materials. 2009;28:143–56. doi: 10.1016/j.csm.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. 2006;103:2480–7. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langer R. Editorial: Tissue Engineering: Perspectives, challenges, and future directions. 2007;13:1–2. doi: 10.1089/ten.2006.0219. [DOI] [PubMed] [Google Scholar]
- 42.Jakab K, Norotte C, Damon B, Marga F, Neagu A, Besch-Williford CL, Kachurin A, Church KH, Park H, Mironov V, Markwald R, Vunjak-Novakovic G, Forgacs G. Tissue engineering by self-assembly of cells printed into topologically defined structures. 2008;14:413–21. doi: 10.1089/tea.2007.0173. [DOI] [PubMed] [Google Scholar]
- 43.L'Heureux N, Pâquet S, Labbé R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 44.McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, Antonelli J, Kocher A, Zembala M, Cierpka L, de la Fuente LM, L'Heureux N. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. 2009;373:1440–6. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- 45.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. 2009;30:5910–7. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: Tissue spheroids as building blocks. 2009;30:2164–74. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Yamato M, Shimizu T, Sekine H, Ohashi K, Kanzaki M, Ohki T, Nishida K, Okano T. Reconstruction of functional tissues with cell sheet engineering. 2007;28:5033–43. doi: 10.1016/j.biomaterials.2007.07.052. [DOI] [PubMed] [Google Scholar]
- 48.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. 1999;284:489–93. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 49.Dahl SLM, Vaughn ME, Niklason LE. An ultrastructural analysis of collagen in tissue engineered arteries. 2007;35:1749–55. doi: 10.1007/s10439-007-9340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dahl SLM, Rhim C, Song YC, Niklason LE. Mechanical properties and compositions of tissue engineered and native arteries. 2007;35:348–55. doi: 10.1007/s10439-006-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hannachi I, Yamato M, Okano T. Cell sheet technology and cell patterning for biofabrication. 2009;1:022002. doi: 10.1088/1758-5082/1/2/022002. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu T, Yamato M, Akutsu T, Shibata T, Isoi Y, Kikuchi A, Umezu M, Okano T. Electrically communicating three-dimensional cardiac tissue mimic fabricated by layered cultured cardiomyocyte sheets. 2002;60:110–7. doi: 10.1002/jbm.1284. [DOI] [PubMed] [Google Scholar]
- 53.Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, Kikuchi A, Umezu M, Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. 2002;90 doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 54.Haraguchi Y, Shimizu T, Yamato M, Kikuchi A, Okano T. Electrical coupling of cardiomyocyte sheets occurs rapidly via functional gap junction formation. 2006;27:4765–74. doi: 10.1016/j.biomaterials.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu T, Sekine H, Isoi Y, Yamato M, Kikuchi A, Okano T. Long-term survival and growth of pulsatile myocardial tissue grafts engineered by the layering of cardiomyocyte sheets. 2006;12:499–507. doi: 10.1089/ten.2006.12.499. [DOI] [PubMed] [Google Scholar]
- 56.Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A, Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. 2001;7:473–80. doi: 10.1089/10763270152436517. [DOI] [PubMed] [Google Scholar]
- 57.Kushida A, Yamato M, Isoi Y, Kikuchi A, Okano T. A noninvasive transfer system for polarized renal tubule epithelial cell sheets using temperature-responsive culture dishes. 2005;10:23–30. doi: 10.22203/ecm.v010a03. [DOI] [PubMed] [Google Scholar]
- 58.Akizuki T, Oda S, Komaki M, Tsuchioka H, Kawakatsu N, Kikuchi A, Yamato M, Okano T, Ishikawa I. Application of periodontal ligament cell sheet for periodontal regeneration: A pilot study in beagle dogs. 2005;40:245–51. doi: 10.1111/j.1600-0765.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- 59.Hasegawa M, Yamato M, Kikuchi A, Okano T, Ishikawa I. Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. 2005;11:469–78. doi: 10.1089/ten.2005.11.469. [DOI] [PubMed] [Google Scholar]
- 60.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H, Okano T, Tano Y. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. 2004;351:1187–96. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 61.Ko HC, Milthorpe BK, McFarland CD. Engineering thick tissues - the vascularisation problem. 2007;14:1–18. doi: 10.22203/ecm.v014a01. discussion-9. [DOI] [PubMed] [Google Scholar]
- 62.Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. 2008;26:434–41. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 63.Haraguchi Y, Sekine W, Shimizu T, Yamato M, Miyoshi S, Umezawa A, Okano T. Development of a new assay system for evaluating the permeability of various substances through 3-dimensional tissue. 2009. [DOI] [PubMed]
- 64.Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. 2004;86-A:1541–58. doi: 10.2106/00004623-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 65.Black AF, Berthod F, L'Heureux N, Germain L, Auger FA. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. 1998;12:1331–40. doi: 10.1096/fasebj.12.13.1331. [DOI] [PubMed] [Google Scholar]
- 66.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. 2007;100:263–72. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 67.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D'Amore PA, Langer R. Engineering vascularized skeletal muscle tissue. 2005;23:879–84. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 68.Unger RE, Sartoris A, Peters K, Motta A, Migliaresi C, Kunkel M, Bulnheim U, Rychly J, Kirkpatrick CJ. Tissue-like self-assembly in cocultures of endothelial cells and osteoblasts and the formation of microcapillary-like structures on three-dimensional porous biomaterials. 2007;28:3965–76. doi: 10.1016/j.biomaterials.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E, Okano T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. 2006;20:708–10. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 70.Radisic M, Marsano A, Maidhof R, Wang Y, Vunjak-Novakovic G. Cardiac tissue engineering using perfusion bioreactor systems. 2008;3:719–38. doi: 10.1038/nprot.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. 2006;12:2077–91. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 72.Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, Yamato M, Kurosawa H, Kobayashi E, Okano T. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. 2008;118:S145–52. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 73.Wenger A, Kowalewski N, Stahl A, Mehlhorn AT, Schmal H, Stark GB, Finkenzeller G. Development and characterization of a spheroidal coculture model of endothelial cells and fibroblasts for improving angiogenesis in tissue engineering. 2005;181:80–8. doi: 10.1159/000091097. [DOI] [PubMed] [Google Scholar]
- 74.Wenger A, Stahl A, Weber H, Finkenzeller G, Augustin HG, Stark GB, Kneser U. Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. 2004;10:1536–47. doi: 10.1089/ten.2004.10.1536. [DOI] [PubMed] [Google Scholar]
- 75.Fidkowski C, Kaazempur-Mofrad MR, Borenstein J, Vacanti JP, Langer R, Wang Y. Endothelialized microvasculature based on a biodegradable elastomer. 2005;11:302–9. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 76.L'Heureux N, McAllister TN, de la Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. 2007;357:1451–3. doi: 10.1056/NEJMc071536. [DOI] [PubMed] [Google Scholar]
- 77.Haruguchi H, Teraoka S. Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: a review. 2003;6:227–35. doi: 10.1007/s10047-003-0232-x. [DOI] [PubMed] [Google Scholar]
- 78.Sarkar S, Salacinski HJ, Hamilton G, Seifalian AM. The mechanical properties of infrainguinal vascular bypass grafts: their role in influencing patency. 2006;31:627–36. doi: 10.1016/j.ejvs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 79.McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. 2006;103:11461–6. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McGuigan AP, Sefton MV. Modular tissue engineering: fabrication of a gelatin-based construct. 2007;1:136–45. doi: 10.1002/term.14. [DOI] [PubMed] [Google Scholar]
- 81.Fleming P,A, Argraves WS, Gentile C, Neagu A, Forgacs G, Christopher JD. Fusion of uniluminal vascular spheroids: A model for assembly of blood vessels. 2010;239:398–406. doi: 10.1002/dvdy.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gentile C, Fleming PA, Mironov V, Argraves KM, Argraves WS, Drake CJ. VEGF-mediated fusion in the generation of uniluminal vascular spheroids. 2008;237:2918–25. doi: 10.1002/dvdy.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Inamori M, Mizumoto H, Kajiwara T. An approach for formation of vascularized liver tissue by endothelial cell-covered hepatocyte spheroid integration. 2009;15:2029–37. doi: 10.1089/ten.tea.2008.0403. [DOI] [PubMed] [Google Scholar]
- 84.Godt D, Tepass U. Drosophila oocyte localization is mediated by differential cadherin-based adhesion. 1998;395:387–91. doi: 10.1038/26493. [DOI] [PubMed] [Google Scholar]
- 85.Gonzalez-Reyes A, St Johnston D. Patterning of the follicle cell epithelium along the anterior-posterior axis during Drosophila oogenesis. 1998;125:2837–46. doi: 10.1242/dev.125.15.2837. [DOI] [PubMed] [Google Scholar]
- 86.Perez-Pomares JM, Foty RA. Tissue fusion and cell sorting in embryonic development and disease: Biomedical implications. 2006;28:809–21. doi: 10.1002/bies.20442. [DOI] [PubMed] [Google Scholar]
- 87.Suwinska A, Czolowska R, Ozdzeński W, Tarkowski AK. Blastomeres of the mouse embryo lose totipotency after the fifth cleavage division: Expression of Cdx2 and Oct4 and developmental potential of inner and outer blastomeres of 16- and 32-cell embryos. 2008;322:133–44. doi: 10.1016/j.ydbio.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 88.Forgacs G, Newman S. Biological physics of the developing embryo. Cambridge University Press; Cambridge: 2005. [Google Scholar]
- 89.Steinberg MS. Differential adhesion in morphogenesis: a modern view. 2007;17:281–6. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 90.Steinberg MS. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movementsand the sorting out of embryonic cells may have a common explanation. 1963;141:401–8. doi: 10.1126/science.141.3579.401. [DOI] [PubMed] [Google Scholar]
- 91.Brodland GW. The Differential Interfacial Tension Hypothesis (DITH): A comprehensive theory for the self-rearrangement of embryonic cells and tissues. 2002;124:188–97. doi: 10.1115/1.1449491. [DOI] [PubMed] [Google Scholar]
- 92.Krieg M, Arboleda-Estudillo Y, Puech PH, Käfer J, Graner F, Müller DJ, Heisenberg CP. Tensile forces govern germ-layer organization in zebrafish. 2008;10:429–36. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 93.Lecuit T. “Developmental mechanics”: cellular patterns controlled by adhesion, cortical tension and cell division. 2008;2:72–8. doi: 10.2976/1.2896332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. 2007;8:633–44. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 95.Martin A, Wieschaus E. Tensions divide. 2010;12:5–7. doi: 10.1038/ncb0110-5. [DOI] [PubMed] [Google Scholar]
- 96.Beysens DA, Forgacs G, Glazier JA. Cell sorting is analogous to phase ordering in fluids. 2000;97:9467–71. doi: 10.1073/pnas.97.17.9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jakab K, Neagu A, Mironov V, Markwald RR, Forgacs G. Engineering biological structures of prescribed shaped using self-assembling multicellular systems. 2004;101:2864–9. doi: 10.1073/pnas.0400164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Phillips HM, Steinberg MS. Embryonic tissues as elasticoviscous liquids. I. Rapid and slow shape changes in centrifuged cell aggregates. 1978;30:1–20. doi: 10.1242/jcs.30.1.1. [DOI] [PubMed] [Google Scholar]
- 99.Phillips HM, Steinberg MS, Lipton BH. Embryonic tissues as elasticoviscous liquids. II. Direct evidence for cell slippage in centrifuged aggregates. 1977;59:124–34. doi: 10.1016/0012-1606(77)90247-0. [DOI] [PubMed] [Google Scholar]
- 100.Steinberg MS, Poole T. Liquid behavior of embryonic tissues. Cambridge University Press; Cambridge: 1982. [Google Scholar]
- 101.Foty RA, Pfleger CM, Forgacs G, Steinberg MS. Surface tensions of embryonic tissues predict their mutual envelopment behavior. 1996;122:1611–20. doi: 10.1242/dev.122.5.1611. [DOI] [PubMed] [Google Scholar]
- 102.Kalantarian A, Ninomiya H, Saad SMI, David R, Winklbauer R, Neumann AW. Axisymmetric drop shape analysis for estimating the surface tension of cell aggregates by centrifugation. 2009;96:1606–16. doi: 10.1016/j.bpj.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mgharbel A, Delanoë-Ayari H, Rieu J. Measuring accurately liquid and tissue surface tension with a compression plate tensiometer. 2009;3:213–21. doi: 10.2976/1.3116822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Damon BJ, Mezentseva NV, Kumaratilake JS, Forgacs G, Newman SA. Limb bud and flank mesoderm have distinct “physical phenotypes” that may contribute to limb budding. 2008;321:319–30. doi: 10.1016/j.ydbio.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 105.Jakab K, Damon B, Marga F, Doaga O, Mironov V, Kosztin I, Markwald R, Forgacs G. Relating cell and tissue mechanics: Implications and applications. 2008;237:2438–49. doi: 10.1002/dvdy.21684. [DOI] [PubMed] [Google Scholar]
- 106.Schötz E, Burdine R, Jülicher F, Steinberg M, Heisenberg C, Foty R. Quantitative differences in tissue surface tension influence zebrafish germ layer positioning. 2008;2:42–56. doi: 10.2976/1.2834817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Duguay D, Foty RA, Steinberg MS. Cadherin-mediated cell adhesion and tissue segregation: Qualitative and quantitative determinants. 2003;253:309–23. doi: 10.1016/s0012-1606(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 108.Foty RA, Steinberg MS. The differential adhesion hypothesis: A direct evaluation. 2005;278:255–63. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 109.Foty RA, Steinberg MS. Measurement of tumor cell cohesion and suppression of invasion by E- or P-cadherin. 1997;57:5033–6. [PubMed] [Google Scholar]
- 110.Hegedus B, Marga F, Jakab K, Sharpe-Timms KL, Forgacs G. The interplay of cell-cell and cell-matrix interactions in the invasive properties of brain tumors. 2006;91:2708–16. doi: 10.1529/biophysj.105.077834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. 2008;3:1172–84. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 112.Flenner E, Marga F, Neagu A, Kosztin I, Forgacs G. Relating Biophysical Properties Across Scales. Current Topics in Developmental Biology. 2008:461–83. doi: 10.1016/S0070-2153(07)81016-7. [DOI] [PubMed] [Google Scholar]
- 113.Forgacs G, Foty RA, Shafrir Y, Steinberg MS. Viscoelastic properties of living embryonic tissues: A quantitative study. 1998;74:2227–34. doi: 10.1016/S0006-3495(98)77932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Frenkel J. Viscous flow of crystalline bodies under the action of surface tension. 1945;9:385–91. [Google Scholar]
- 115.Marmottant P, Mgharbel A, Kafer J, Audren B, Rieu JP, Vial JC, van der Sanden B, Maree AF, Graner F, Delanoe-Ayari H. The role of fluctuations and stress on the effective viscosity of cell aggregates. 2009;106:17271–5. doi: 10.1073/pnas.0902085106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boland T, Xu T, Damon B, Cui X. Application of inkjet printing to tissue engineering. 2006;1:910–7. doi: 10.1002/biot.200600081. [DOI] [PubMed] [Google Scholar]
- 117.Campbell PG, Weiss LE. Tissue engineering with the aid of inkjet printers. 2007;7:1123–7. doi: 10.1517/14712598.7.8.1123. [DOI] [PubMed] [Google Scholar]
- 118.Nakamura M, Kobayashi A, Takagi F, Watanabe A, Hiruma Y, Ohuchi K, Iwasaki Y, Horie M, Morita I, Takatani S. Biocompatible inkjet printing technique for designed seeding of individual living cells. 2005;11:1658–66. doi: 10.1089/ten.2005.11.1658. [DOI] [PubMed] [Google Scholar]
- 119.Nishiyama Y, Nakamura M, Henmi C, Yamaguchi K, Mochizuki S, Nakagawa H, Takiura K. Development of a three-dimensional bioprinter: construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. 2009;131:035001. doi: 10.1115/1.3002759. [DOI] [PubMed] [Google Scholar]
- 120.Phillippi JA, Miller E, Weiss L, Huard J, Waggoner A, Campbell P. Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. 2008;26:127–34. doi: 10.1634/stemcells.2007-0520. [DOI] [PubMed] [Google Scholar]
- 121.Saunders RE, Gough JE, Derby B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. 2008;29:193–203. doi: 10.1016/j.biomaterials.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 122.Xu T, Gregory CA, Molnar P, Cui X, Jalota S, Bhaduri SB, Boland T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. 2006;27:3580–8. doi: 10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 123.Xu T, Jin J, Gregory C, Hickman JJ, Boland T. Inkjet printing of viable mammalian cells. 2005;26:93–9. doi: 10.1016/j.biomaterials.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 124.Yamazoe H, Tanabe T. Cell micropatterning on an albumin-based substrate using an inkjet printing technique. 2009;91:1202–9. doi: 10.1002/jbm.a.32312. [DOI] [PubMed] [Google Scholar]
- 125.Smith CM, Christian JJ, Warren WL, Williams SK. Characterizing environmental factors that impact the viability of tissue-engineered constructs fabricated by a direct-write bioassembly tool. 2007;13:373–83. doi: 10.1089/ten.2006.0101. [DOI] [PubMed] [Google Scholar]
- 126.Smith CM, Stone AL, Parkhill RL, Stewart RL, Simpkins MW, Kachurin AM, Warren WL, Williams SK. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. 2004;10:1566–76. doi: 10.1089/ten.2004.10.1566. [DOI] [PubMed] [Google Scholar]
- 127.Marga F, Neagu A, Kosztin I, Forgacs G. Developmental biology and tissue engineering. 2007;81:320–8. doi: 10.1002/bdrc.20109. [DOI] [PubMed] [Google Scholar]
- 128.Kielty CM, Stephan S, Sherratt MJ, Williamson M, Shuttleworth CA. Applying elastic fibre biology in vascular tissue engineering. 2007;362:1293–312. doi: 10.1098/rstb.2007.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]