Abstract
Immunodominance is a fundamental property of CD8+ T cell responses to viruses and vaccines. It had been observed that route of administration alters immunodominance after vaccinia virus (VACV) infection, but only a few epitopes were examined and no mechanism was provided. We re-visited this issue, examining a panel of 15 VACV epitopes and four routes, namely intradermal (i.d.), subcutaneous (s.c.), intraperitoneal (i.p.) and intravenous (i.v.) injection. We found that immunodominance is sharpened following peripheral routes of infection (i.d. and s.c.) compared with those that allow systemic virus dissemination (i.p. and i.v.). This increased immunodominance was demonstrated with native epitopes of VACV and with herpes simplex virus glycoprotein B when expressed from VACV. Responses to some subdominant epitopes were altered by as much as fourfold. Tracking of virus, examination of priming sites, and experiments restricting virus spread showed that priming of CD8+ T cells in the spleen was necessary, but not sufficient to broaden responses. Further, we directly demonstrated that immunodomination occurs more readily when priming is mainly in lymph nodes. Finally, we were able to reduce immunodominance after i.d., but not i.p. infection, using a VACV expressing the costimulators CD80 (B7-1) and CD86 (B7-2), which is notable because VACV-based vaccines incorporating these molecules are in clinical trials. Taken together, our data indicate that resources for CD8+ T cell priming are limiting in local draining lymph nodes, leading to greater immunodomination. Further, we provide evidence that costimulation can be a limiting factor that contributes to immunodomination. These results shed light on a possible mechanism of immunodomination and highlight the need to consider multiple epitopes across the spectrum of immunogenicities in studies aimed at understanding CD8+ T cell immunity to viruses.
Author Summary
During an infection, the adaptive immune system responds to many epitopes of the pathogen but the strength of these responses varies widely. This unequal distribution of responses across a range of epitopes is known as immunodominance and understanding why it occurs is a fundamental problem in immunology. It is also relevant to vectored vaccines where the intention is to raise immunity against an antigen of choice, but responses to vector epitopes may dominate. We show that the route of infection changes the extent to which the strongest epitope can dominate CD8+ T cell responses to vaccinia virus (VACV). The cause of this phenomenon is linked to virus spread and therefore the different lymphoid organs that prime T cell responses for each route. We also show that local draining lymph nodes are sites of more robust competition between T cells compared with the spleen, explaining why immunodominance differs according to route. Finally the normally heightened immunodominance after peripheral VACV infection can be reduced by expression the costimulators CD80 (B7-1) and CD86 (B7-2) from the virus. In summary, we have carefully dissected immunodominance using VACV as a model and in doing so exposed general features of CD8+ T cell immunity to pathogens.
Introduction
Immunodominance is a term used to describe the preferential recognition of some epitopes over others in a complex antigen and is a fundamental property of all immune responses. CD8+ T cell responses to viruses are no exception and immunodominance has been noted for many viruses in mice and humans [1], [2]. Immunodominance arises due to factors that affect either 1) the amount of peptide-MHC (pMHC) complexes, including abundance of parent antigen, ease of processing and affinity of peptides for MHC [3]–[17] or 2) the quantity or quality of T cells in the naive repertoire that recognize these pMHC complexes [5], [8], [10], [11], [18]–[29]. An additional determinant that emerges from the intersection of the factors above is immunodomination, which is the ability of T cells with dominant specificities to inhibit responses to less-dominant epitopes. This is observed most clearly in secondary infections, where some memory T cells are clearly less able to compete [30]–[34]. However, it must also operate in primary infection, because deletion of immunodominant epitopes allows responses to subdominant epitopes to increase [10], [30], [35]. Further in some, but not all cases pre-priming of individual epitopes can lead to radically altered dominance hierarchies, presumably because the already primed T cells have an advantage over other specificities [5], [36], [37]. Finally, competition amongst the various clones recognising the same specificity can be directly observed during infection by monitoring the expansion adoptively transferred TCR transgenic T cells compared with the endogenous polyclonal response [38]. While the mechanism of immunodomination remains obscure, it can be relieved if the epitopes are presented on separate antigen presenting cells (APCs). Therefore it is most likely due to competition for resources either on APCs or released by APCs in the immediate environment, but these remain undefined [36], [38]–[40].
Vaccinia virus (VACV) was used as the live vaccine to eradicate smallpox and attenuated strains are now being used as vectors for recombinant vaccines. In understanding both the historical and contemporary usage of VACV, CD8+ T cell responses are of interest. Further, VACV provides an excellent model for studies of immunodominance because it has a large genome with many mapped epitopes and infections are entirely acute. These attributes set it apart from the well-studied RNA viruses such as influenza or lymphocytic choriomeningitis viruses, with genomes less than a tenth the size and the herpesviruses, all of which cause latent/persistent infections. Of the roughly 50 CD8+ T cell epitopes for VACV identified in the C57BL/6 mouse, B8R20 (TSYKFESV) is by far the most dominant [41]–[45]. Depending on the estimate of the total anti-VACV CD8+ T cell population, 10–25% of all anti-viral CD8+ T cells are specific for this epitope during acute infection and we refer to this as the immunodominant epitope (IDE) [42]. The rest of the mapped epitopes can be considered to be sub-dominant epitopes (SDE) and more than 20 of these induce easily measurable responses that range from 2–0.2% of CD8+ T cell at acute times [41]. In the work characterizing the first five CD8+ T cell epitopes for VACV in C57BL/6, it was noted that different routes of infection appeared to alter immunodominance with intradermal (i.d.) infection favoring B820, the IDE, over the SDE compared to intraperitoneal (i.p.) infection [42]. Responses to an IDE were also favored by the i.d. route in VACV infections of BALB/c mice [46]. That this phenomenon was observed in two strains of mice (with different sets of epitopes) suggests that it was genuinely linked to immunodominance.
Here we confirm, explore and explain the route-related effects on CD8+ T cell immunodominance during VACV infection. We find that immunodominance is linked to the sites of antigen presentation and that individual draining lymph nodes (LN) are environments where there is more competition between T cells of differing specificities than the spleen. Finally we show that the effects of this competition that occurs after i.d. (but not i.p.) infection are reduced by a VACV that expresses the costimulators CD80 and CD86 (B7-1 and 2).
Results
Peripheral routes of infection sharpen immunodominance
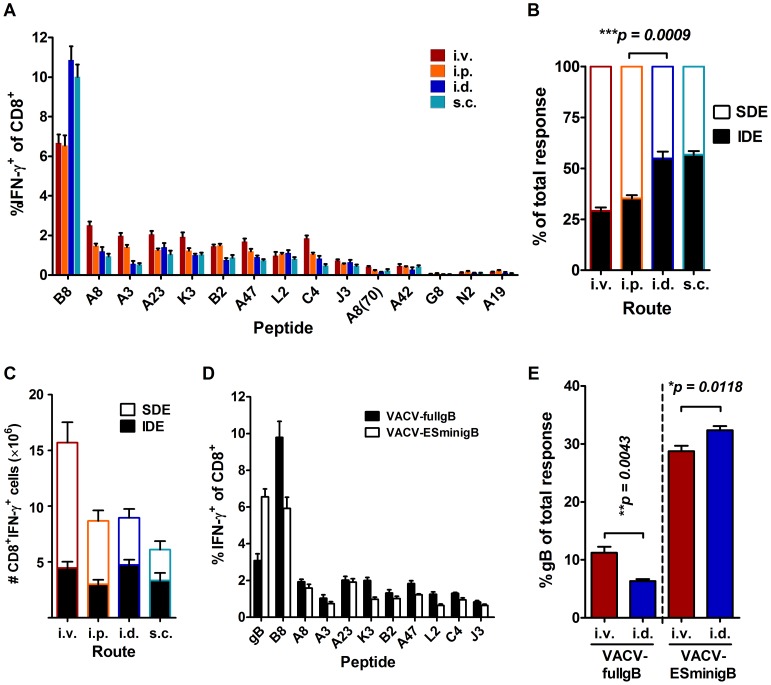
To extend published results suggesting an effect of vaccination route on CD8+ T cell immunodominance, groups of C57BL/6 mice were infected with 1×106 plaque forming units (pfu) vaccinia virus Western Reserve (WR) strain by i.p., i.d., intravenous (i.v.) and subcutaneous (s.c.) injection. After 7 days, CD8+ T cell responses to 15 VACV epitopes (Table 1) were measured using brief ex vivo stimulation of splenocytes followed by intracellular staining for IFN-γ (ICS) (Figure 1A). This method allows very accurate enumeration of epitope-specific CD8+ T cells at acute times after infection [47]. At a broad scale, the overall hierarchy of the 15 epitopes was similar for the four routes, with B820 being the IDE and the other 14 epitopes being SDEs. However, on closer inspection the previously noted increased dominance of the IDE, B820 over the SDE in i.d. compared with i.p. infected mice was repeated here. There were statistically significant differences for several epitopes (including B8) between i.v., i.p. and i.d. routes, but none differed between i.d. and s.c. infections (statistics not shown). To see the ratio of IDE to SDE responses more clearly, the data were plotted to show B820–specific responses as a fraction of the sum of responses to all epitopes (Figure 1B). When viewed this way, responses in mice infected by the two peripheral routes (i.d. and s.c.) had identical IDE∶SDE ratios of around 50%, but use of the systemic i.p. and to a slightly greater extent i.v. route reduced the dominance of B820 to around 30%. Differences between the peripheral and systemic routes were statistically significant (p<0.01). When the data were analyzed to show the total number of CD8+ T cells responding to each epitope, it could be seen that summed responses to the subdominant epitopes are reduced when peripheral routes were used (Figure 1C). The basic phenomenon of skewing towards B820 after i.d. compared with i.p. infection has also been observed in memory CD8+ T cell responses, as measured at 28 days after infection and so is not an artifact of the acute infection (not shown).
Table 1. Synthetic peptides used in this study.
| Name | Sequence | H-2 | Gene name |
| B820 | TSYKFESV | Kb | VACWR190 |
| A8189 | ITYRFYLI | Kb | VACWR127 |
| A3270 | KSYNYMLL | Kb | VACWR122 |
| A23297 | IGMFNLTFI | Db | VACWR148 |
| K36 | YSLPNAGDVI | Db | VACWR034 |
| B254 | YSQVNKRYI | Db | VACWR184 |
| A47138 | AAFEFINSL | Kb | VACWR173 |
| L253 | VIYIFTVRL | Kb | VACWR089 |
| C4125 | LNFRFENV | Kb | VACWR024 |
| J3289 | SIFRFLNI | Kb | VACWR095 |
| A870 | IHYLFRCV | Kb | VACWR127 |
| A4288 | YAPVSPIVI | Db | VACWR167 |
| G834 | LMYIFAAL | Kb | VACWR086 |
| N260 | FLMMNKDEL | Db | VACWR029 |
| A1947 | VSLDYINTM | Kb | VACWR139 |
| gB498 | SSIEFARL | Kb | HSV-1 gB |
| OVA257 | SIINFEKL | Kb | ovalbumin |
Figure 1. CD8+ T cell Immunodominance is altered by the route of infection for VACV.
(A–C) CD8+ T cell responses to VACV epitopes were determined in C57BL/6 mice infected with 1×106 PFU of VACV WR by i.v., i.p., i.d., or s.c. injection seven days after infection. Data are from two or more experiments and total mice for each route were: i.v. 8, i.p. 16, i.d. 6 and s.c. 10. A) Means and SEM of percent of CD8+ T cells responding to each peptide. B) The fraction of all measured responses (sum of responses to all 15 VACV peptides) accounted for by B820-specific (IDE) and the sum of SDE-specific CD8+ T cells. C) Responses to VACV epitopes broken down into IDE and SDE as in panel B, but shown as total numbers of CD8+IFN-γ+ cells per spleen. (D–E) Responses to VACV and HSV gB498 epitopes seven days after infection of mice with 1×106 PFU of recombinant VACV-fullgB or VACV-ESminigB. Data are from two experiments with a total of 6 mice per virus. D) Means and SEM of percent of CD8+ T cells responding to each peptide after i.v. infection (10 of 14 VACV epitopes shown). E) Response to gB498 shown as a fraction of the total measured epitope-specific responses (VACV+gB498). Selected statistical significance denoted with p value over appropriate bars.
Next we wanted to explore whether this phenomenon was related to the particular selection of epitopes used or would apply to other IDE and SDE when expressed as foreign antigens from VACV. One of the most immunodominant CD8+ T cell epitopes mapped to date is the gB498–505 epitope (SSIEFARL) of herpes simplex virus glycoprotein B (HSVgB) and we had two recombinant VACVs expressing this epitope. These express full length HSVgB (VACVfullgB) or the gB498 epitope as an endoplasmic reticulum-targeted ‘minigene’ (VACV-ESminigB) [48]. Infection of mice with these viruses showed that gB498 was a SDE when it required processing from full HSVgB, but became co-dominant with B820 when expressed as an ER-targeted minigene (Figure 1D). These viruses then provided the opportunity to test the effect of route of infection on immunodominance using a single epitope that was either an IDE or a SDE depending on the context. CD8+ T cell responses to the gB498 epitope were then compared to the sum of responses to all 15 VACV epitopes for the two viruses after i.d. and i.v. infection. In the context of full HSVgB, where gB498 ranks as an SDE, responses to this epitope were enhanced roughly two-fold by i.v. compared with i.d. infection, but the opposite result (albeit with a narrower difference) was obtained for the IDE minigene version (Figure 1E). Taken together, the conclusion from these experiments is that VACV infection by peripheral, compared with systemic routes sharpens immunodominance and this can predict which route will maximise responses to foreign antigens expressed from VACV vectors.
Virus dissemination after infection by different routes
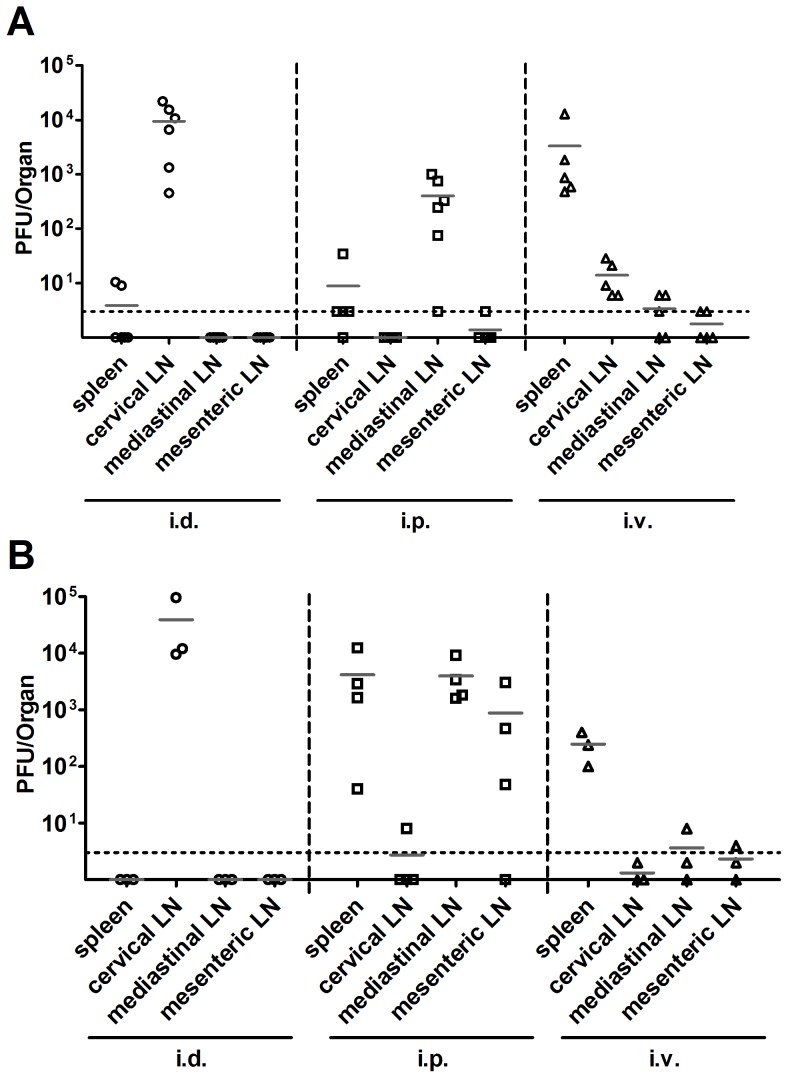
VACV has more opportunity to spread after i.p. and i.v. injection, compared with infection by a peripheral route like i.d. [49]–[51]. We reasoned that virus dissemination might be linked to differences in immunodominance if it affected the range of secondary lymph organs that have access to virus antigen. To test the extent to which the inoculated virus spread to lymph organs, mice were infected by i.d., i.p. and i.v. routes and six hours later, virus titers were measured in cervical, mediastinal and mesenteric LNs and spleen (Figure 2A). As expected, after i.v. injection virus titers were highest in the spleen but virus was also found in each LN. For i.d. and i.p. routes, the highest titers of virus were in the cervical and mediastinal LN respectively, which is consistent with the lymphatic drainage of these injection sites. For i.d. infection, spread of inoculum beyond the cervical LN was seen for two of five mice with low titers found in the spleen. After i.p. infection virus was found in the spleen in four of five and mesenteric LN in one of five mice. Next, spread of virus during the first day of infection was explored by quantifying virus 24 hours after infection by the same three routes (Figure 2B). At this later time point virus spread remained wide after i.v. injection (though titers dropped substantially) and this broad distribution was also seen for mice infected by the i.p. route. In fact the titers were generally higher after i.p. compared with i.v. infection. In contrast, virus was entirely restricted to the cervical lymph node after i.d. infection. These results demonstrate that there was wider spread of virus and potential for antigen presentation after i.v. and i.p. than i.d. infection.
Figure 2. VACV spreads more widely after i.v. or i.p. infection.
C57BL/6 mice were infected with 1×106 PFU of VACV WR by i.v., i.p. or i.d. injection. At 6 (A) or 24 (B) hours after infection, spleen and lymph nodes were collected and infectious virus measured by plaque assay. Titers from each organ are plotted with the average for each group shown by a grey bar. The dotted line represents the limit of detection (3 PFU/Organ).
Systemic routes prime CD8+ T cells in more lymphoid organs early after infection
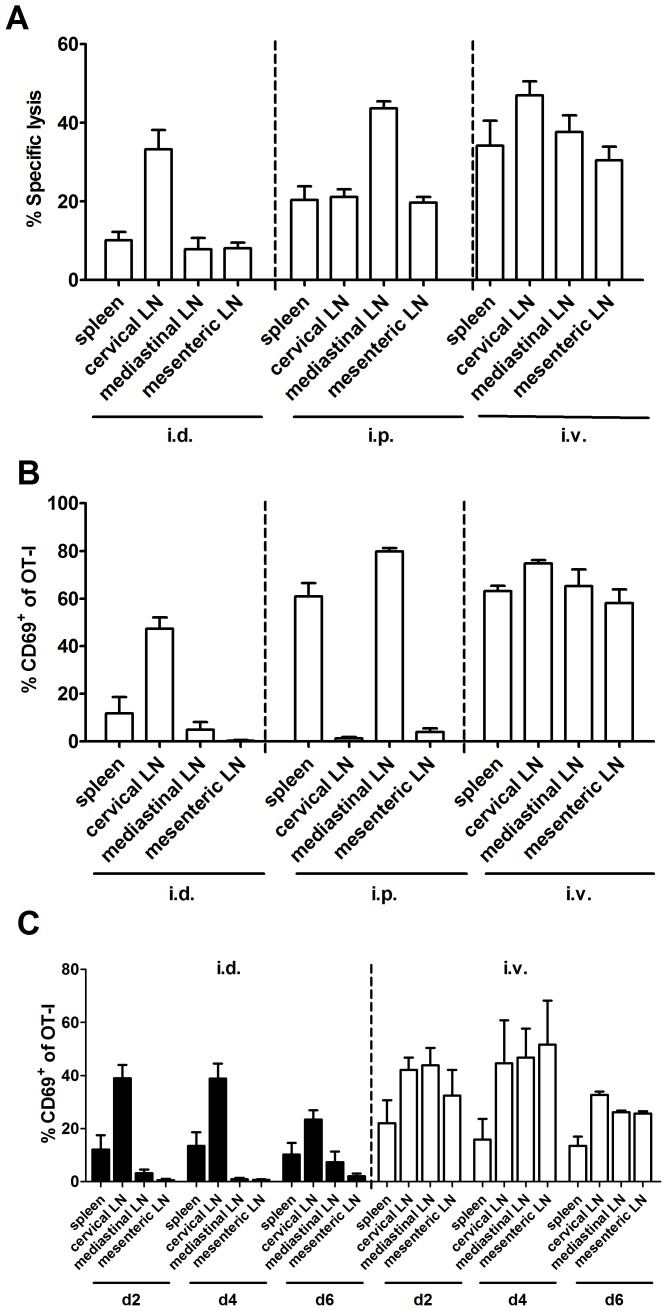
Having shown wider spread of virus after i.v. and i.p. routes we wanted to know if this was reflected in the sites where CD8+ T cells were primed. The first method used was the in vivo cytotoxicity (CTL) assay, which has been used previously to track sites of T cell priming [52]. In the first set of experiments, specific killing of B820-pulsed targets in various lymphoid organs was determined at different times after i.d. infection (Figure S1 in Supporting Information). This showed that at two days after i.d. infection, B820-specific killing activity was already found in the cervical LN, but it took several more days until it was detected at similar levels in other LN and spleen, similar to a report with dermal HSV infection [52]. The killing seen at the other LN and spleen on later days presumably reflects the recirculation of primed CD8+ T cells once they leave the site of initial priming. We then examined killing two days after infection by the i.v. and i.p. routes (Figure 3A). Two days after infection by the i.v. route, B820-specific killing was high in all LN and spleen, suggesting priming of CD8+ T cells in all these sites. Infection by the i.p. route produced the highest B820-specific killing in the mediastinal LN, but killing was also strong (around 20%) in spleen and other LN. These data suggested a difference in the amount of priming at sites beyond the local draining LN after i.p., compared with i.d. infection and very widespread priming after i.v. injection.
Figure 3. Wider spread but not longevity of antigen presentation is associated with systemic routes.
A) Cytotoxicity in lymphoid organs of mice infected with 1×106 PFU of VACV WR by the routes shown was determined by measuring the specific lysis of B820 epitope–pulsed splenocytes two days after infection. Data are from two or more experiments and total mice for each route were: i.d. 7, i.p. 9 and i.v. 7. Killing in the spleen was significantly lower for i.d. compared with i.p. (p<0.05) or i.v. (p<0.01) infected mice. B) B6.SJL mice were given 5×106 OT-I CD8+ T cells (CD45.2+), then the following day infected with 1×106 PFU of VACV NP-S-GFP by the routes shown. At 24 hours post-infection, expression of CD8, CD45.2 and CD69 were measured by flow cytometry on cells from spleens and LNs. Mean and SEM of percent of OT-I CD8+ cells expressing CD69 is shown. Data are from two or more experiments and total mice for each route were: i.d. 5, i.p. 5 and i.v. 3. Priming of OT-I in the spleen was significantly (p<0.05) lower for i.d. compared with i.p. (p<0.01) or i.v. (p<0.001) infected mice. C) B6.SJL mice were infected with 1×106 PFU of VACV NP-S-GFP by the routes shown then given 5 × 106 OT-I CD8+ T cells (CD45.2+) 1, 3 and 5 days later. Expression of CD8, CD45.2 and CD69 were measured by flow cytometry on cells from spleens and LNs 24 hours after transfer to give times 2, 4 and 6 as shown on the graph. Means and SEM of percent of OT-I CD8+ cells expressing CD69 are shown.
It was unclear in these experiments if the roughly 10% killing in the spleen and non-draining LN after i.d. infection was due to the early migration of some effectors or was background as an artifact of the assay. To look at the sites of priming even earlier, a recombinant VACV (NP-S-GFP) expressing the ovalbumin257 (SIINFEKL; OVA257) epitope [53], [54] was used in combination with transferred naive OT-I T cells. The dominance of B820 after infection of mice with VACV NP-S-GFP by i.v., i.p. and i.d. routes was similar to that seen for non-recombinant WR (Figure S2 in Supporting Information). To detect the earliest events of priming we looked for CD69 up-regulation on the OT-I cells 24 hours after infection of mice by the three routes (Figure 3B). After i.d. infection, priming of OT-I T cells, as indicated by CD69 up-regulation, was very low in all sites other than the cervical LN. In contrast, i.p. infection primed over 60% of OT-I cells both in the mediastinal LN and the spleen. As expected, i.v. infection was able to prime OT-I in all sites. So by this method, priming of CD8+ T cells at sites beyond the local draining LN is efficient after the systemic i.v. and i.p. routes, but not the peripheral i.d. route of infection. While we saw differences in the spread of priming sites, it remained possible that the length of antigen presentation also differed and that this might drive changes in immunodominance. To test this we used the OT-I model again, but this time did transfers at various times after infection (day 1, 3 and 5) by i.d. or i.v. route and looked for CD69 up regulation 24 hours later (Figure 3C). Overall, there was no difference in kinetics of presentation between the routes and priming of OT-I was observed on all days, though it began to wane at the latest time (day 5 to 6). However, we were surprised to see that priming in the spleen was relatively poor at all times after i.v. infection in this experiment, which was in contrast to findings earlier after infection (Figure 3B). To ensure that this was sound, an experiment was done to include a day 1 readout of OT-I activation side-by-side with later times after i.v. infection and this confirmed that priming in the spleen declines more rapidly than in LN (not shown). Interestingly, by day 3–4, there is little difference in priming in the spleen between the i.v. and i.d. infected mice. All together these experiments show that a difference between the systemic and peripheral routes is the extent to which antigen spread allows CD8+ T cell priming to take place at early times in lymphoid organs beyond the LN and perhaps especially in the spleen.
Reduced immunodominance is associated with robust priming in the spleen
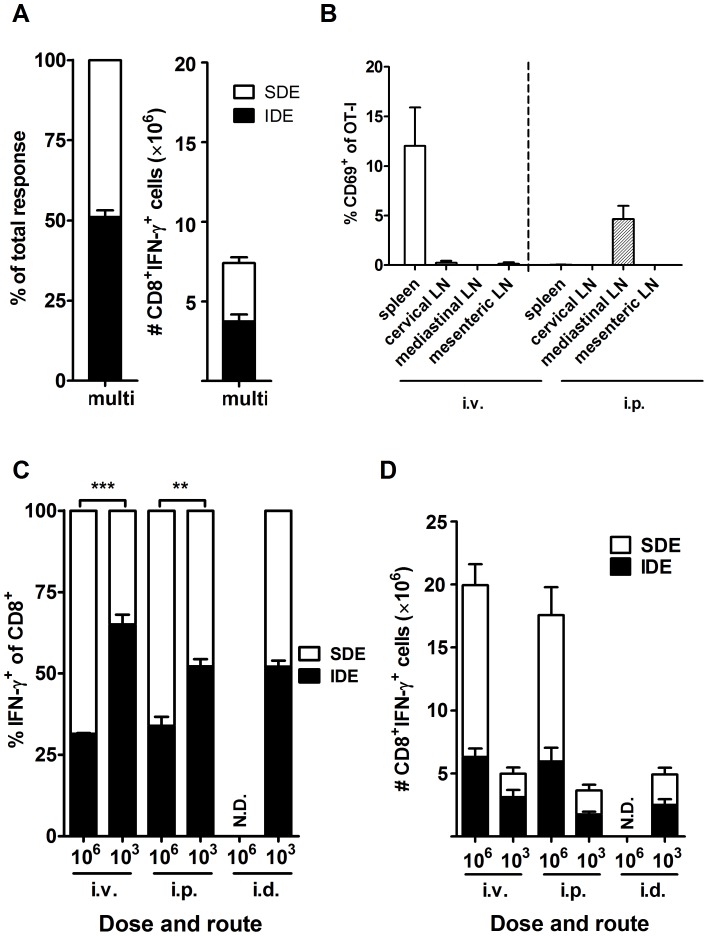
Next we wanted to explore whether the number of priming sites and/or levels of presentation lead to reduced immunodominance. First, peripheral routes were explored and priming in more LN was achieved by simultaneous injections (two i.d. and two s.c.) at four different sites. Seven days later, responses to the panel of 15 VACV peptides were determined. In mice that received these multiple concurrent injections, responses to the VACV epitopes were similar to those in mice infected at a single site, with B820–specific CD8+ T cells accounting for 50% of the total epitope-specific response (Figure 4A and compare with Figure 1B and C). This suggested that if priming was restricted to LN, increasing the number of lymphoid organs where priming occurs does not reduce immunodominance. In the second experiment, the dose of virus given was reduced to 1×103 PFU, which we reasoned would stop the spread of virus to the spleen after i.p. injection and also greatly limit the number of APCs irrespective of route. First, VACV NP-S-GFP and OT-I transfer was used to directly examine sites of priming after this low-dose infection (Figure 4B). At 24 hours after i.p. infection, only modest priming (5% of OT-I activated) was observed with this dose and it was exclusively in the mediastinal LN. When a similar low dose was used by the i.v. route, again priming was poor (10% of OT-I), but in this case it was found only in the spleen. Therefore the reduced dose clearly limited the spread of antigen and the levels of presentation. Next we examined the effect of this reduced dose on immunodominance and found that mice infected with a low-dose by the i.p. or i.v. routes had CD8+ T cell responses more heavily dominated by the IDE, which now accounted for 50% and 60% of the epitope-specific response respectively (Figure 4C). This is similar, if not more extreme domination by B820 as seen for i.d. infection at the standard high dose. By contrast, reducing the dose to 1×103 PFU in i.d. infection did not further sharpen immunodominance for this route. The lower dose given by all routes resulted in much lower total numbers of epitope-specific CD8+ T cells and again in the case of i.p. and i.v. routes it was SDE-specific responses that were most reduced (Figure 4D). For example reducing dose in the i.v. route roughly halved the number of B820-specific CD8+ T cells, but reduced the number of SDE-specific T cells by around three quarters. In case the dose changed the kinetics of response for the various epitopes, the immunodominance hierarchy was examined at days 6, 7, 8, 9 and 11 after i.p. infection and the dominance of B820 was remarkably stable across these five times (Figure S3 in Supporting Information). From these data it was concluded that simply increasing the number of lymph organs where priming occurs does not reduce immunodominance, neither does priming in the spleen guarantee this outcome. Rather, only where priming in the spleen is substantial can immunodominance be reduced. This suggests a requirement for a higher number of APCs being involved in priming in one organ (as offered by the spleen), rather than there being a special property of splenic versus LN APCs.
Figure 4. Immunodominance is reduced by robust priming in the spleen.
A) CD8+ T cell responses to 15 VACV epitopes were measured by intracellular staining of IFN-γ seven days after infection with 1×106 PFU of VACV WR in total spread equally across four peripheral sites (left and right ear pinnae, i.d. and shanks, s.c.). The fraction of the total measured response accounted for by the IDE-specific and the sum of SDE-specific CD8+ T cells is shown on the left. On the right, responses are again broken down into IDE and SDE, but shown as total numbers of CD8+IFN-γ+ cells per spleen. Mean and SEMs of 5 mice from two experiments are shown. B) Priming of OT-I CD8+ T cells as demonstrated by upregulation of CD69 expression in the lymphoid organs shown 24 hours after infection with 1×103 PFU (low dose) of VACV NP-S-GFP by i.v. or i.p. injection (as for figure 3B). Data are from more than two experiments; 4 mice for i.p. and 3 for i.v. route. C-D) CD8+ T cell responses to 15 VACV epitopes in mice infected with 1×103 or 1×106 PFU of VACV WR by the routes shown, as described previously. Data are from more than one experiment and numbers of mice were: i.v. 106 = 3, 103 = 7; i.p. 106 = 5, 103 = 18; i.d. 106 = not done (N.D.), 103 = 11. C) Data shown are the fraction of the total measured response accounted for by the IDE-specific and the sum of SDE-specific CD8+ T cells. D) Responses are again broken down into IDE and SDE, but shown as total numbers of CD8+IFN-γ+ cells per spleen. Statistical significance denoted with p value over appropriate bars.
Immunodomination is greater after peripheral infection
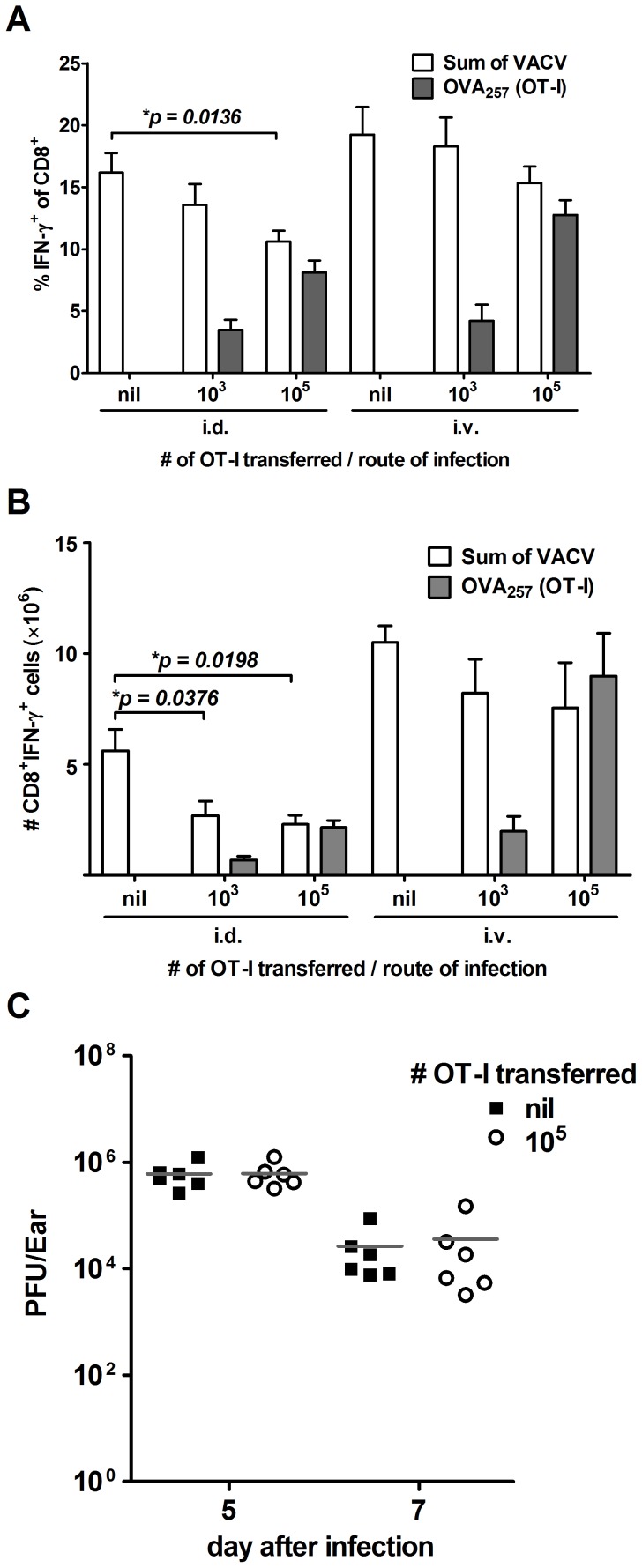
The results thus far suggested that the sharpened immunodominance after i.d. infection could be due to a limiting resource needed for priming (e.g. number of APCs) in LN and therefore greater potential for immunodomination. To examine immunodomination directly, cross competition between transferred OT-I cells responding to OVA257 expressed from VACV NP-S-GFP and endogenous CD8+ T cells responding to the VACV epitopes was examined. This approach was originally used to examine competition between T cell clones for OVA257, but OT-Is can also cross-compete with native virus epitopes co-expressed with OVA257 [38], [55]. Two doses (1×103 and 1×105) of congenically marked OT-I T cells (CD45.2+) were transferred into B6.SJL mice (CD45.1+), which were infected with VACV NP-S-GFP a day later. After seven days, splenic responses to the set of 15 VACV peptides (CD45.1+) and the number of responding OT-I T cells (CD45.2+) were measured by ICS. This experiment was done with mice infected by the i.d. route and the i.v. route to represent the greatest and least domination by B820 in the experiments shown thus far. When data were analyzed either as a percent of CD8+ T cells (Figure 5A) or as total number of CD8+ T cells (Figure 5B), responses to VACV epitopes were significantly reduced by the presence of competing OT-I T cells after i.d., but not i.v. infection. This is despite the relatively larger expansion of the transferred OT-I cells in i.v. infected mice. No significant suppression of VACV-specific responses by OT-I transfer was seen when mice were infected with a control virus that did not express the OVA257 peptide (Figure S4 in Supporting Information). It was possible that the transfer of so many OT-I cells might influence the amount of virus growth and therefore antigen presentation during i.d. infection. This was tested by removing the ears from i.d. infected mice that had received OT-I or no transferred cells and determining virus titers by plaque assay. There was no difference in the amount of infectious virus found in ears of mice that received OT-I cells, compared with controls (Figure 5C). Together these data suggest that CD8+ T cell responses are more prone to immunodomination after i.d., compared with i.v. infection.
Figure 5. Greater cross-competition from transferred OT-I cells after peripheral infection.
A–B) B6.SJL mice were infected with 1×106 PFU of VACV NP-S-GFP by i.d. or i.v. injection a day after receiving the numbers of OT-I CD8+ T cells as shown. Seven days later, CD8+ T cell responses to 15 VACV epitopes plus OVA257 were measured by intracellular staining of IFN-γ. OT-I T cells were identified by expression of CD45.2. CD8+ T cells responding to VACV (as a sum of all 15 peptides) and OT-I CD8+ T cells responding to OVA257 are show as a percent of all CD8+ T cells (A) and as a total number of CD8+ IFN-γ+ cells per spleen (B). Graph shows the average ± SEM and data come from two independent experiments and a total of at least 4 mice for each route and dose of OT-I cells. Statistical significance denoted with p value over appropriate bars. C) B6.SJL mice that received the number of OT-I CD8+ cells as shown were infected with 1×106 PFU of VACV NP-S-GFP i.d.. On day 5 and 7 post-infection, virus titers in the ear pinnae were determined by plaque assay. Data are titers from individual ears and the grey bar represents the average.
Costimulation reduces immunodominance after i.d. but not i.p. infection
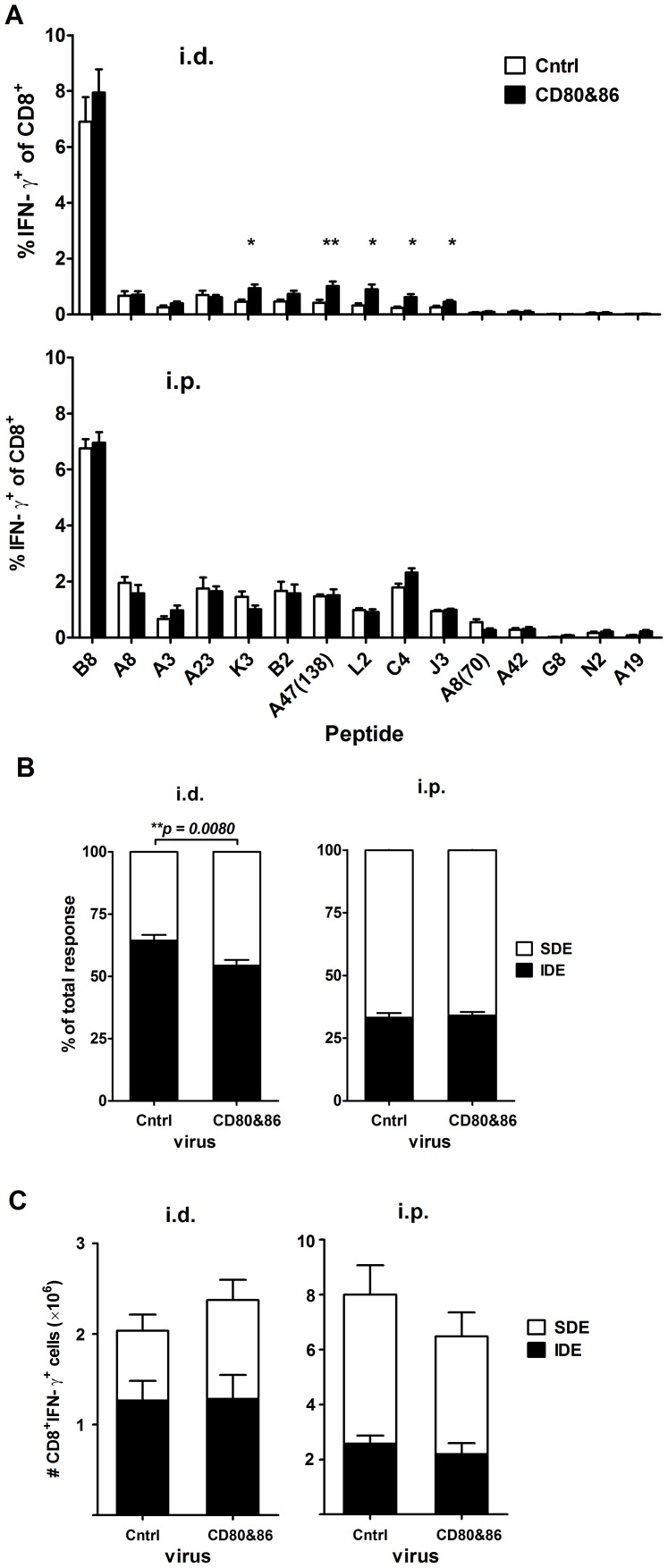
Immunodomination is most likely the result of T cells competing for limited resources on or very close to APCs, but none have been identified. One essential resource required for CD8+ T cell priming that has been proposed, but not shown to be involved in immunodominance is costimulation [56]–[58]. These studies used mice deficient in CD28 or treated mice with soluble reagents that block the interaction between CD28 and the costimulators CD80 (B7-1) and CD86 (B7-2). However, this approach only tests the effect of eliminating costimulation and so cannot reveal whether costimulation under normal conditions can be limiting and might be a resource for which CD8+ T cells compete. To test this possibility, we used a recombinant VACV expressing CD80 and CD86 (VACV-CD80&86) and infected mice by the i.d. and i.p. routes. Priming of CD8+ T cells by VACV is thought to be largely via direct presentation, so this virus should increase the amount of costimulation available on each APC [53], [59], [60]. Consistent with a role for limiting CD80 and CD86 playing a role in immunodomination, mice infected i.d. with VACV-CD80&86 had higher responses to SDEs and the ratio of IDE to SDE was significantly lower than in mice infected with the control VACV (Figure 6). By contrast, in the i.p. infected mice there was no enhanced response to any epitope and immunodominance was unaltered. Further when analyzed by total number of VACV-specific CD8+ T cells, in mice infected by the id. but not i.p. route expression of CD80 and CD86 improved the sum of responses to SDEs, but not to B820. From these results we conclude that expression of costimulators by a recombinant VACV reduces immunodomination.
Figure 6. Immunodominance in i.d. but not i.p. infected mice was reduced by over-expression of CD80 and CD86.
CD8+ T cell responses to VACV epitopes were determined in C57BL/6 mice infected i.d. with 1×104 PFU of VACV-CD80&86 or control virus (VSC-8) or i.p. with 1×106 PFU of the same viruses. Seven days later, CD8+ T cell responses to 15 VACV epitopes were measured by intracellular staining of IFN-γ. Data are from two experiments and a total of 8 and 6 mice per virus for i.d. and i.p. respectively; route is shown above each graph. A) Means and SEM of percent of CD8+ T cells responding to each peptide. * p<0.05, ** p<0.01. B) The fraction of all measured responses accounted for by B820-specific (IDE) and the sum of SDE-specific CD8+ T cells. Statistical significance denoted with p value over appropriate bars. C) Responses to VACV epitopes broken down into IDE and SDE as in panel B, but shown as total numbers of CD8+IFN-γ+ cells per spleen.
Discussion
Infection route has been suggested to alter several aspects of CD8+ T cell responses to a variety of viruses including priming mechanism, magnitude and quality [61]–[65]. Here we demonstrate clearly that for VACV, immunodominance also needs to be considered. This leads to the first important conclusion of this work, which is that where magnitude of response is a primary read-out, examining responses to a single epitope can be misleading. For example, if B820 was used as a sole epitope in the experiment shown in Figure 1A, one would conclude that i.d. or s.c. injections of VACV were most immunogenic. In contrast if the majority of the SDE were chosen, the opposite conclusion would be drawn. The size of differences in response for individual SDE varied, but were up to four-fold for C4125 across the routes. Analyzing the total number of epitope-specific CD8+ T cells removes the apparent improvement of B820-specific response seen by the peripheral routes, but makes the suppressive effect of these routes on the SDE more pronounced, with the difference for C4125 being more than seven-fold. So while as an overall picture, the change in dominance profile across the doses seems quite modest compared with the very big differences in virus spread and number of priming sites, effects were substantial for some individual epitopes. Much VACV immunology in the past has used recombinant viruses and responses to a single foreign epitope were monitored. In the light of our results some conclusions from these earlier experiments may need to be reconsidered. Indeed the experiments shown here with HSVgB498 demonstrate that different forms of antigen can change the dominance ranking of an epitope dramatically in the context of a recombinant VACV. This in turn alters its competitiveness as an immunogen differentially according to route. There are also lessons here for studies of immunodominance using other viruses where few epitopes are known or used.
The original experiments that indicated a role for route in VACV immunodominance used only dermal scarification and i.p. routes [42]. By including i.v. and s.c. routes here, the association between virus spread and immunodominance was noted and then confirmed virologically. The lack of spread after i.d. infection was not surprising [49]. However, the much larger amount of virus found in all organs 24 hours after i.p., compared with i.v. injection was less expected. The reduction in virus in all lymph organs from 6 hrs to 24 hrs after i.v. injection suggests that these sites do not sustain VACV replication. Therefore the higher amounts of virus found a day after i.p. injection are most likely the result of continued draining of virus generated at other sites, rather than infection of the lymph organs. This is an advantageous feature of the VACV model in that findings made in lymph organs are not complicated by these also being major sites of virus infection. In terms of CD8+ T cell immunity, presence of any virus in lymphoid organs was a useful a guide for defining priming sites, even if the amounts measured were poorly predictive of antigen presentation levels. For example, despite very low levels of infectious virus being found in the various LN after i.v. infection, priming was robust at all these sites. Perhaps in this situation many APCs are reached by the ample (1×106 PFU) inoculum and despite undergoing abortive infections, these cells persist long enough to prime effectively. Alternatively there may be a reservoir of antigen that is cross presented, but the evidence thus far suggests that direct priming is more important for VACV-specific CD8+ T cells [60]. The kinetics of antigen presentation as determined for the i.d. and i.v. routes were remarkably similar with sustained activation of OT-I cells seen until day 5–6, though it was decreasing by this time. This result rules out the premature loss of antigen presentation in LN, perhaps as a result of killing of APCs by IDE-specific T cells, as a mechanism for increased immunodominance associated with the i.d. route. Further the one organ where presentation seemed to decay the fastest was the spleen after i.v. infection and antigen presentation levels there became very similar for i.d. and i.v. routes by day 3–4. This points to early antigen presentation events being more important in setting the immunodominance hierarchy, possibly reflecting the requirement for only a brief encounter with antigen to drive CD8+ T cell responses [66], [67].
We considered the possibility that there was something qualitatively different between the APCs in the spleen and LN that leads to reduced immunodominance. If this were correct, reduced immunodominance should be the hallmark of any responses primed in the spleen. However, reducing virus dose by i.v. and i.p. routes and thereby restricted priming to the spleen and a LN respectively, sharpened immunodominance in both cases. On the other hand, the lower doses also greatly limited the number of APCs available at any priming site, as demonstrated by poor priming of OT-I cells. Strikingly, restricting priming to the mediastinal LN with low dose i.p. infection lead to exactly the same ratio of IDE∶SDE as found for any dose of virus injected i.d. where priming also occurs in a single LN. Further, increasing the number of LNs involved in priming and thereby total APC numbers by infecting multiple peripheral sites did not reduce immunodominance. To reduce immunodominance, a large number of APCs in a single lymph organ were required and this was only provided by the spleen when there was an abundance of antigen. Finally, reducing the virus dose given i.d. did not further sharpen immunodominance, which together with other results here suggests there is a limit to immunodomination. We speculate that the architecture and size of LNs limit APCs and/or some associated essential resources required for priming over a wide range of antigen doses and increase competition between T cells. The possibility of a more competitive environment in LN was confirmed by subjecting VACV-specific CD8+ T cells to rivalry from transferred OT-I T cells. The finding that VACV responses were more easily suppressed by OT-I after i.d. but not i.v. infection is consistent with greater competition across specificities when priming is restricted to LN. This leads to the conclusion that immunodomination in LNs, which are the main priming sites after i.d. infection, suppresses responses to SDE.
Having established i.d. infection with VACV as a setting where immunodomination can occur in primary responses, we decided to take advantage of this model to examine the role of costimulation in immunodominance. Our data suggest that increasing levels of costimulators to APCs that directly prime CD8+ T cells in a LN reduces the level of immunodomination by the IDE. Conversely, there was no advantage for SDE (or the IDE) when these constimulators were expressed by a virus given by the i.p. route. This effect for the i.d. route might be achieved either by reducing competition for costimulation on individual APCs or possibly extending the number of APCs that have adequate levels of costimulators to prime CD8+ T cells. Several groups have demonstrated that CD8+ T cell responses can occur in the absence of costimulation via CD80/CD86 and CD28, but that these responses are substantially compromised [58], [68]–[71]. The only report to examine multiple epitopes came to the conclusion that costimulation affected IDE and SDE equally [58]. This is not necessarily inconsistent with our findings or conclusions. We are not suggesting that IDE and SDE have a differential requirement for costimulation, but rather that in the context of priming in LNs, costimulation is limiting and IDE have an advantage in competing for this resource. The VACV strains that express CD80 and CD86 used here were originally developed to be improved vaccine vectors [72]. Other reports with similar viruses suggest that they enhance magnitude and avidity of CD8+ T cell responses to model antigens that are co-expressed from the same virus [73], [74]. While we have not tested avidity, our data on magnitude are consistent with these published reports: the epitopes examined previously would rank as SDE and the immunizations published were done using a peripheral route (s.c.). However, the assumption made until now was that all CD8+ T cell responses could be boosted by expression of the costimulators. Here we show that the benefit of expressing costimulators, at least in terms of magnitude of response may be for SDE only. This reinforces again the importance of examining responses to multiple epitopes before drawing conclusions about the benefit of different immunization strategies based on pre-clinical models in mice.
In conclusion, we show here that route-related changes in immunodominance after primary infection with VACV are the result of differential spread of virus antigen, which determines the sites of CD8+ T cell priming. CD8+ T cell priming is more competitive when it is mainly limited to LNs and consequently subdominant specificities are subject to greater immunodomination at these sites. Further, we identify costimulatory molecules as one of the resources that might be limited in LN and therefore drive immunodomination. These data have implications for the interpretation of preclinical vaccinology of vectored vaccines. Beyond these insights this work has ramifications for viral immunology in general, demonstrating clearly the importance of putting responses to any single epitope into the broader context of responses to the whole virus.
Materials and Methods
Viruses and cell lines
The majority of viruses used here were kind gifts: WR (Bernard Moss, NIH); VACV NP-S-GFP [54], [75], WR B7-1&B7-2 (called VACV-CD80&86 here) [72] and VSC-8 (as a TK− control virus for VACV-CD80&86) (Jon Yewdell and Jack Bennink, NIH); VACV-ESminigB [48] (S. Tevethia, Penn State Medical College). VACV-fullgB was made by standard homologous recombination methods using plasmid pSC11 [76] to insert the full coding sequence of HSVgB under the control of the p7.5 promoter into the thymidine kinase gene of VACV WR. All recombinant antigens in viruses used here were expressed by the p7.5 promoter from a disrupted thymidine kinase gene of strain WR. Immortalized cell lines, BHK-21 and BS-C-1, were maintained in Dulbecco's Modified Eagle medium (DMEM, Invitrogen) with glutamine and 10% or 2% fetal bovine serum (FBS). VACVs were grown in BHK-21 and purified by centrifugation through a 36% sucrose cushion, then infectivity was titrated on BS-C-1 cells using standard methods.
Ethics statement
All experiments were done according to Australian NHMRC guidelines contained within the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and under approvals F-BMB-38.8 and A2011-01 from the Australian National University Animal Ethics and Experimentation Committee.
Mice and infections
Specific pathogen-free C57BL/6, B6.SJL and OT-I transgenic mice were obtained from the Animal Resource Centre (Perth, Australia) and APF (Canberra, Australia). Unless stated, mice were infected with 1×106 PFU of VACV by i.p., i.v. or s.c. in 200 µl of PBS or by i.d. [77] in 5 or 10 µl of PBS.
Stimulation with peptides and intracellular cytokine staining
Mice were euthanized 7 days after infection and spleens were taken for analysis of CD8+ T cell responses by intracellular cytokine staining (ICS) as described previously [47]. Briefly, splenocytes were plated at 1×106 cells/well into round-bottom 96-well plates. Synthetic peptides (Table 1) were added to a final concentration of 10−7 M and plates were incubated at 37°C with 5% CO2. After 1 hour, 50 µg/ml brefeldin A (Sigma-Aldrich, St. Louis, MO) was added. Plates were incubated for another 3 hours, then spun at 4°C to remove the medium. Cells were resuspended in 40 µl of 1/150-diluted anti-CD8-PE antibody (clone 53-6.7; BD Biosciences, San Jose, CA). After 30 min incubation on ice, cells were washed, resuspended in 50 µl of 1% paraformaldehyde, and incubated at room temperature for 20 min. After two washes, cells were stained with 40 µl of 1/200-diluted anti-IFN-γ allophycocyanin antibody (clone XMG1.2; BD Biosciences) with 0.25% saponin (Sigma-Aldrich) overnight at 4°C. Cells were washed three times before acquisition using a FACS LSRII (BD Biosciences). Analysis was done in Flowjo software (Tree Star, Ashland, OR). Events were gated for live lymphocytes on forward scatter (FSC) × side scatter (SSC), followed by CD8+ cells × IFN-γ. Backgrounds as determined for samples without peptide were usually in the order of 0.1–0.2% and were subtracted from the values presented for test samples.
Virus titration by plaque assay
Organs harvested from infected mice were homogenized in 1 ml glass homogenizers (Wheaton) then rapidly frozen and thawed 3 times in liquid nitrogen and a 37°C water bath. The homogenized organs were then 10-fold serial diluted in DMEM with 2% FBS before adding to the 6-well plates with BS-C-1 cell monolayer. After 90 min of incubation at 37°C with 5% CO2, the virus inoculates were removed and replaced by 2 ml/well of 0.4% Sodium carboxymethyl cellulose (CMC, Sigma-Aldrich) in DMEM with 2% FBS. Plates were incubated at 37°C with 5% CO2 for 3 days, then crystal violet (Sigma-Aldrich) used to stain. Plaques were counted and virus titers were determined according to the dilution factor.
In vivo T cell activation and CD69 staining
CD8+ T cells were prepared from the spleens and lymph nodes of naive OT-I mice using magnetic bead-based negative selection (Miltenyi), and resuspended in PBS with 2% FBS for i.v. transfer. Each B6.SJL mouse received about 5×106 OT-I CD8+ T cells and rested for >18 hours. 24 hours after infection with 106 PFU of VACV NP-S-GFP by various routes, these infected and naive mice were sacrificed. Single cell suspensions were made from the spleens and lymph nodes, and stained with anti-CD8-PE antibody (clone 53-6.7; BD Biosciences, San Jose, CA), anti-CD45.2-APC antibody (clone 104; BioLegend, San Diego, CA) and anti-CD69-PE-Cy7 (clone H1.2F3; BD Biosciences, San Jose, CA) before acquiring by FACS LSRII (BD Biosciences). The activation of OVA-specific T cells is determined by CD69 expression on CD45.2+CD8+ cells.
In vivo cytotoxicity assay
Target cells for cytotoxicity detection were prepared from naive C57BL/6 mice and split into two populations for peptide-pulsing with B820 and SIINFEKL respectively at 37°C for 1 hour. These two populations were then labeled with different concentration of CFSE (Sigma-Aldrich), 9 µM for B820 (CFSEHi) and 0.9 µM for SIINFEKL (CFSELo), before mixing equal number of cells together for i.v. transfer. A total of 2×107 cells were injected into each mouse which was infected with 1×106 PFU of VACV WR for days as described. After 4 hours, the mice were sacrificed for their spleens and lymph nodes. The cell numbers in two CFSE-positive populations were acquired by FACS LSRII (BD Biosciences) for analysis. The specific lysis was calculated by the following formula: [1−(CFSELo/CFSEHi)naive/(CFSELo/CFSEHi)infected]×100.
Statistical analysis
Unless stated otherwise, statistical comparisons were done using an unpaired t test with Welch's correction because populations had unequal variance. All tests were analyzed with the aid of GraphPad Prism software (GraphPad, La Jolla, CA).
Supporting Information
Time course of cytotoxicity in LN and spleen after i.d. infection. C57BL/6 mice were i.d. injected with 1×106 PFU of VACV WR, and in vivo cytotoxicity in lymphoid organs were determined on designated days post-infection by measuring the specific lysis of B820 peptide-pulsed splenocytes. Graph shows the average ± SEM; data are from more than two experiments and three or more mice for each time point. Statistical significance was determined by unpaired Student's t-test.
(TIF)
Similar immunodominance was observed after infection with VACV NP-S-GFP via various routes. CD8+ T cell responses to 15 VACV epitopes in C57BL/6 mice infected with 1×106 PFU of VACV NP-S-GFP were determined by intracellular staining of IFN-γ. Graph shows the fraction of all measured responses (sum of responses to all 15 VACV peptides) accounted for by B820-specific (IDE) and the sum of SDE-specific CD8+ T cells. Data are from two experiments and total mice for each route were: i.v. 6, i.p. 9 and i.d. 6.
(TIF)
Immunodominance was maintained through the course of infection. CD8+ T cell responses to VACV epitopes B820, A8189, K36 and B254 in C57BL/6 mice injected i.p. with 1×103 PFU of VACV WR were determined as previously described on designated day after infection. Graph shows the mean and SEM of the fraction of the total measured response accounted for by B820–specific CD8+ T cells. Data are from 3 mice per group on each day, except for day 6 (marked with #) where only 1 of 3 mice had detectable responses to any peptide.
(TIF)
No significant suppression of VACV-specific responses by OT-I in the absence of OVA257 expression. After being transferred with designated numbers of OT-I CD8+ T cells and rested for overnight, B6.SJL mice were infected with 1×106 PFU of control virus VSC-8, which does not express OVA257. Seven days later, CD8+ T cell responses to 15 VACV epitopes plus OVA257 were measured by intracellular staining of IFN-γ. Graph shows the average ± SEM of sum of VACV-specific responses in percent of CD8+ cells (left) or total number of CD8+ cells per spleen (right).
(TIF)
Acknowledgments
The authors thank Stewart Smith and Natasha Noel for general laboratory assistance and RSB animal services for maintenance of mice. Drs Yewdell, Bennink and Moss and Prof Tevethia are thanked for provision of viruses.
Funding Statement
This work was funded by NIH grant R01AI067401 and Australian NHMRC Project Grant APP1023141. DCT was supported by an NHMRC CDA (418108) and an Australian ARC Future Fellowship (FT110100310). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Yewdell JW (2006) Confronting Complexity: Real-World Immunodominance in Antiviral CD8+ T Cell Responses. Immunity 25: 533–543. [DOI] [PubMed] [Google Scholar]
- 2. Yewdell JW, Bennink JR (1999) Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annual Review of Immunology 17: 51–88. [DOI] [PubMed] [Google Scholar]
- 3. Bousso P, Levraud JP, Kourilsky P, Abastado JP (1999) The Composition of a Primary T Cell Response Is Largely Determined by the Timing of Recruitment of Individual T Cell Clones. J Exp Med 189: 1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Busch DH, Pamer EG (1998) MHC class I/peptide stability: implications for immunodominance, in vitro proliferation, and diversity of responding CTL. J Immunol 160: 4441–4448. [PubMed] [Google Scholar]
- 5. Chen W, Anton LC, Bennink JR, Yewdell JW (2000) Dissecting the multifactorial causes of immunodominance in class I- restricted T cell responses to viruses. Immunity 12: 83–93. [DOI] [PubMed] [Google Scholar]
- 6. Chen W, Norbury CC, Cho Y, Yewdell JW, Bennink JR (2001) Immunoproteasomes shape immunodominance hierarchies of antiviral CD8(+) T cells at the levels of T cell repertoire and presentation of viral antigens. J Exp Med 193: 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Del Val M, Schlicht HJ, Ruppert T, Reddehase MJ, Koszinowski UH (1991) Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighboring residues in the protein. Cell 66: 1145–1153. [DOI] [PubMed] [Google Scholar]
- 8. Deng Y, Yewdell JW, Eisenlohr LC, Bennink JR (1997) MHC affinity, peptide liberation, T cell repertoire, and immunodominance all contribute to the paucity of MHC class I-restricted peptides recognized by antiviral CTL. J Immunol 158: 1507–1515. [PubMed] [Google Scholar]
- 9. Eisenlohr LC, Yewdell JW, Bennink JR (1992) Flanking sequences influence the presentation of an endogenously synthesized peptide to cytotoxic T lymphocytes. J Exp Med 175: 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kotturi MF, Scott I, Wolfe T, Peters B, Sidney J, et al. (2008) Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J Immunol 181: 2124–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. La Gruta NL, Kedzierska K, Pang K, Webby R, Davenport M, et al. (2006) A virus-specific CD8+ T cell immunodominance hierarchy determined by antigen dose and precursor frequencies. PNAS 103: 994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pang KC, Sanders MT, Monaco JJ, Doherty PC, Turner SJ, et al. (2006) Immunoproteasome subunit deficiencies impact differentially on two immunodominant influenza virus-specific CD8+ T cell responses. J Immunol 177: 7680–7688. [DOI] [PubMed] [Google Scholar]
- 13. Probst HC, Tschannen K, Gallimore A, Martinic M, Basler M, et al. (2003) Immunodominance of an antiviral cytotoxic T cell response is shaped by the kinetics of viral protein expression. J Immunol 171: 5415–5422. [DOI] [PubMed] [Google Scholar]
- 14. Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, et al. (1994) The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol 153: 5586–5592. [PubMed] [Google Scholar]
- 15. van der Most RG, Murali-Krishna K, Lanier JG, Wherry EJ, Puglielli MT, et al. (2003) Changing immunodominance patterns in antiviral CD8 T-cell responses after loss of epitope presentation or chronic antigenic stimulation. Virology 315: 93–102. [DOI] [PubMed] [Google Scholar]
- 16. van der Most RG, Sette A, Oseroff C, Alexander J, Murali-Krishna K, et al. (1996) Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J Immunol 157: 5543–5554. [PubMed] [Google Scholar]
- 17. Weidt G, Utermohlen O, Heukeshoven J, Lehmann-Grube F, Deppert W (1998) Relationship among immunodominance of single CD8+ T cell epitopes, virus load, and kinetics of primary antiviral CTL response. J Immunol 160: 2923–2931. [PubMed] [Google Scholar]
- 18. Badovinac VP, Tvinnereim AR, Harty JT (2000) Regulation of antigen-specific CD8+ T Cell homeostasis by perforin and interferon-gamma. Sci 290: 1354. [DOI] [PubMed] [Google Scholar]
- 19. Choi EY, Christianson GJ, Yoshimura Y, Sproule TJ, Jung NJ, et al. (2002) Immunodominance of H60 is caused by an abnormally high precursor T cell pool directed against its unique minor histocompatibility antigen peptide. Immunity 17: 593–603. [DOI] [PubMed] [Google Scholar]
- 20. Daly K, Nguyen P, Woodland DL, Blackman MA (1995) Immunodominance of major histocompatibility complex class I-restricted influenza virus epitopes can be influenced by the T-cell receptor repertoire. J Virol 69: 7416–7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dzutsev AH, Belyakov IM, Isakov DV, Margulies DH, Berzofsky JA (2007) Avidity of CD8 T cells sharpens immunodominance. Int Immunol 19: 497–507. [DOI] [PubMed] [Google Scholar]
- 22. Flesch IE, Woo WP, Wang Y, Panchanathan V, Wong YC, et al. (2010) Altered CD8+ T cell immunodominance after vaccinia virus infection and the naïve repertoire in inbred and F1 mice. J Immunol 184: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haeryfar SMM, Hickman HD, Irvine KR, Tscharke DC, Bennink JR, et al. (2008) Terminal deoxynucleotidyl transferase establishes and broadens anti-viral CD8+ T cell immunodominance hierarchies. J Immunol 181: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu F, Whitton JL, Slifka MK (2004) The rapidity with which virus-specific CD8+ T cells initiate IFN-γ synthesis increases markedly over the course of infection and correlates with immunodominance. J Immunol 173: 456–462. [DOI] [PubMed] [Google Scholar]
- 25. Obar JJ, Khanna KM, Lefrançois L (2008) Endogenous naive CD8+ T cell crecursor frequency regulates primary and memory responses to infection. Immunity 28: 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perkins DL, Wang YS, Fruman D, Seidman JG, Rimm IJ (1991) Immunodominance is altered in T cell receptor (beta-chain) transgenic mice without the generation of a hole in the repertoire. J Immunol 146: 2960–2964. [PubMed] [Google Scholar]
- 27. Trautmann L, Rimbert M, Echasserieau K, Saulquin X, Neveu B, et al. (2005) Selection of T cell clones expressing high-affinity public TCRs within human cytomegalovirus-specific CD8 T cell responses. J Immunol 175: 6123–6132. [DOI] [PubMed] [Google Scholar]
- 28. Wallace ME, Bryden M, Cose SC, Coles RM, Schumacher TN, et al. (2000) Junctional biases in the naive TCR repertoire control the CTL response to an immunodominant determinant of HSV-1. Immunity 12: 547–556. [DOI] [PubMed] [Google Scholar]
- 29. Haeryfar SMM, DiPaolo RJ, Tscharke DC, Bennink JR, Yewdell JW (2005) Regulatory T cells suppress CD8+ T cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J Immunol 174: 3344–3351. [DOI] [PubMed] [Google Scholar]
- 30. Andreansky SS, Stambas J, Thomas PG, Xie W, Webby RJ, et al. (2005) Consequences of immunodominant epitope deletion for minor influenza virus-specific CD8+ T cell responses. J Virol 79: 4329–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kastenmuller W, Gasteiger G, Gronau JH, Baier R, Ljapoci R, et al. (2007) Cross-competition of CD8+ T cells shapes the immunodominance hierarchy during boost vaccination. J Exp Med 204: 2187–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Webby RJ, Andreansky S, Stambas J, Rehg JE, Webster RG, et al. (2003) Protection and compensation in the influenza virus-specific CD8+ T cell response. PNAS 100: 7235–7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen W, Pang K, Masterman K-A, Kennedy G, Basta S, et al. (2004) Reversal in the immunodominance hierarchy in secondary CD8+ T cell responses to influenza A virus: Roles for cross-presentation and lysis-independent immunodomination. J Immunol 173: 5021–5027. [DOI] [PubMed] [Google Scholar]
- 34. Crowe SR, Turner SJ, Miller SC, Roberts AD, Rappolo RA, et al. (2003) Differential Antigen Presentation Regulates the Changing Patterns of CD8+ T Cell Immunodominance in Primary and Secondary Influenza Virus Infections. J Exp Med 198: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stock AT, Jones CM, Heath WR, Carbone FR (2006) CTL response compensation for the loss of an immunodominant class I-restricted HSV-1 determinant. Immunol Cell Biol 84: 543–550. [DOI] [PubMed] [Google Scholar]
- 36. Rodriguez F, Harkins S, Slifka MK, Whitton JL (2002) Immunodominance in virus-induced CD8+ T-cell responses is dramatically modified by DNA immunization and is regulated by gamma interferon. J Virol 76: 4251–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Y, Flesch IEA, Tscharke DC (2009) Vaccinia virus CD8+ T cell dominance hierarchies cannot be altered by prior immunization with individual peptides. J Virol 83: 9008–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, et al. (2000) T Cells Compete for Access to Antigen-bearing Antigen-presenting Cells. J Exp Med 192: 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Palmowski MJ, Choi EM-L, Hermans IF, Gilbert SC, Chen JL, et al. (2002) Competition between CTL narrows the immune response induced by prime-boost vaccination protocols. J Immunol 168: 4391–4398. [DOI] [PubMed] [Google Scholar]
- 40. Wolpert EZ, Grufman P, Sandberg JK, Tegnesjo A, Karre K (1998) Immunodominance in the CTL response against minor histocompatibility antigens: interference between responding T cells, rather than with presentation of epitopes. J Immunol 161: 4499–4505. [PubMed] [Google Scholar]
- 41. Moutaftsi M, Peters B, Pasquetto V, Tscharke DC, Sidney J, et al. (2006) A consensus epitope prediction approach identifies the breadth of murine TCD8+-cell responses to vaccinia virus. Nat Biotechnol 24: 817–819. [DOI] [PubMed] [Google Scholar]
- 42. Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, et al. (2005) Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med 201: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yuen TJ, Flesch IEA, Hollett NA, Dobson BM, Russell TA, et al. (2010) Analysis of A47, an immunoprevalent protein of vaccinia virus, leads to a reevaluation of the total antiviral CD8+ T cell response. J Virol 84: 10220–10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cornberg M, Sheridan BS, Saccoccio FM, Brehm MA, Selin LK (2006) Protection against vaccinia virus (VV) challenge by CD8 memory T cells resolved by molecular mimicry. J Virol 81: 934–44 JVI.01280-01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moutaftsi M, Tscharke DC, Vaughan K, Koelle DM, Stern L, et al. (2010) Uncovering the interplay between CD8, CD4 and antibody responses to complex pathogens. Future Microbiology 5: 221–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oseroff C, Peters B, Pasquetto V, Moutaftsi M, Sidney J, et al. (2008) Dissociation between epitope hierarchy and immunoprevalence in CD8 responses to vaccinia virus Western Reserve. J Immunol 180: 7193–7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Flesch IEA, Hollett NA, Wong YC, Tscharke DC (2012) Linear fidelity in quantification of anti-viral CD8+ T cells. PLoS ONE 7: e39533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blaney JE Jr, Nobusawa E, Brehm MA, Bonneau RH, Mylin LM, et al. (1998) Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J Virol 72: 9567–9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tscharke DC, Smith GL (1999) A model for vaccinia virus pathogenesis and immunity based on intradermal injection of mouse ear pinnae. J Gen Virol 80: 2751–2755. [DOI] [PubMed] [Google Scholar]
- 50. Fischer MA, Davies ML, Reider IE, Heipertz EL, Epler MR, et al. (2011) CD11b+, Ly6G+ cells produce type I interferon and exhibit tissue protective properties following peripheral virus infection. PLoS Pathog 7: e1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Buller RM, Smith GL, Cremer K, Notkins AL, Moss B (1985) Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. Nat 317: 813–815. [DOI] [PubMed] [Google Scholar]
- 52. Coles RM, Mueller SN, Heath WR, Carbone FR, Brooks AG (2002) Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J Immunol 168: 834–838. [DOI] [PubMed] [Google Scholar]
- 53. Norbury CC, Malide D, Gibbs JS, Bennink JR, Yewdell JW (2002) Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat Immunol 3: 265–271. [DOI] [PubMed] [Google Scholar]
- 54. Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, et al. (2003) Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity 18: 343–354. [DOI] [PubMed] [Google Scholar]
- 55. Jenkins MR, Webby R, Doherty PC, Turner SJ (2006) Addition of a prominent epitope affects influenza A virus-specific CD8+ T cell immunodominance hierarchies when antigen is limiting. J Immunol 177: 2917–2925. [DOI] [PubMed] [Google Scholar]
- 56. Johnson AJ, Njenga MK, Hansen MJ, Kuhns ST, Chen L, et al. (1999) Prevalent Class I-Restricted T-Cell Response to the Theiler's Virus Epitope Db∶VP2121–130 in the Absence of Endogenous CD4 Help, Tumor Necrosis Factor Alpha, Gamma Interferon, Perforin, or Costimulation through CD28. J Virol 73: 3702–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen W, Bennink JR, Morton PA, Yewdell JW (2002) Mice deficient in perforin, CD4+ T Cells, or CD28-mediated signaling maintain the typical immunodominance hierarchies of CD8+ T-cell responses to influenza virus. J Virol 76: 10332–10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Salek-Ardakani S, Arens R, Flynn R, Sette A, Schoenberger SP, et al. (2009) Preferential Use of B7.2 and Not B7.1 in Priming of Vaccinia Virus-Specific CD8 T Cells. J Immunol 182: 2909–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hickman HD, Takeda K, Skon CN, Murray FR, Hensley SE, et al. (2008) Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat Immunol 9: 155–165. [DOI] [PubMed] [Google Scholar]
- 60. Xu RH, Remakus S, Ma X, Roscoe F, Sigal LJ (2010) Direct presentation is sufficient for an efficient anti-viral CD8 T cell response. PLoS Pathog 6: e1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Holst PJ, Ørskov C, Thomsen AR, Christensen JP (2010) Quality of the Transgene-Specific CD8+ T Cell Response Induced by Adenoviral Vector Immunization Is Critically Influenced by Virus Dose and Route of Vaccination. J Immunol 184: 4431–4439. [DOI] [PubMed] [Google Scholar]
- 62. Venturi V, Davenport MP, Swan NG, Doherty PC, Kedzierska K (2011) Consequences of suboptimal priming are apparent for low-avidity T-cell responses. Immunol Cell Biol 90: 216–23. [DOI] [PubMed] [Google Scholar]
- 63. Liu L, Zhong Q, Tian T, Dubin K, Athale SK, et al. (2010) Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med AOP 16: 224–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shen XF, Wong SBJ, Buck CB, Zhang JW, Siliciano RF (2002) Direct priming and cross-priming contribute differentially to the induction of CD8(+) CTL following exposure to vaccinia virus via different routes. J Immunol 169: 4222–4229. [DOI] [PubMed] [Google Scholar]
- 65. Ranasinghe C, Turner SJ, McArthur C, Sutherland DB, Kim J-H, et al. (2007) Mucosal HIV-1 Pox Virus Prime-Boost Immunization Induces High-Avidity CD8+ T Cells with Regime-Dependent Cytokine/Granzyme B Profiles. J Immunol 178: 2370–2379. [DOI] [PubMed] [Google Scholar]
- 66. Kaech SM, Ahmed R (2001) Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nature Immunology 2: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van Stipdonk MJB, Lemmens EE, Schoenberger SP (2001) Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nature Immunology 2: 423–429. [DOI] [PubMed] [Google Scholar]
- 68. Fang M, Sigal LJ (2006) Direct CD28 costimulation is required for CD8+ T cell-mediated resistance to an acute viral disease in a natural host. J Immunol 177: 8027–8036. [DOI] [PubMed] [Google Scholar]
- 69. Fuse S, Zhang W, Usherwood EJ (2008) Control of Memory CD8+ T Cell Differentiation by CD80/CD86-CD28 Costimulation and Restoration by IL-2 during the Recall Response. J Immunol 180: 1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kundig TM, Shahinian A, Kawai K, Mittrucker HW, Sebzda E, et al. (1996) Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity 5: 41–52. [DOI] [PubMed] [Google Scholar]
- 71. Sigal LJ, Reiser H, Rock KL (1998) The role of B7-1 and B7-2 costimulation for the generation of CTL responses in vivo. J Immunol 161: 2740–2745. [PubMed] [Google Scholar]
- 72. Chamberlain RS, Carroll MW, Bronte V, Hwu P, Warren S, et al. (1996) Costimulation enhances the active immunotherapy effect of recombinant anticancer vaccines. Cancer Research 56: 2832–2836. [PMC free article] [PubMed] [Google Scholar]
- 73. Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J (2005) Multiple Costimulatory Modalities Enhance CTL Avidity. J Immunol 174: 5994–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang S, Hodge JW, Grosenbach DW, Schlom J (2005) Vaccines with Enhanced Costimulation Maintain High Avidity Memory CTL. J Immunol 175: 3715–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Norbury CC, Basta S, Donohue KB, Tscharke DC, Princiotta MF, et al. (2004) CD8+ T cell cross-priming via transfer of proteasome substrates. Sci 304: 1318–1321. [DOI] [PubMed] [Google Scholar]
- 76. Chakrabarti S, Brechling K, Moss B (1985) Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol 5: 3403–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin LCW, Smith SA, Tscharke DC (2012) An intradermal model for vaccinia virus pathogenesis in mice. In: Isaacs SN, editor. Vaccinia virus and poxvirology: Methods and protocols. 2nd edition. Totowa, N.J.: Humana Press. pp. 147–159. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time course of cytotoxicity in LN and spleen after i.d. infection. C57BL/6 mice were i.d. injected with 1×106 PFU of VACV WR, and in vivo cytotoxicity in lymphoid organs were determined on designated days post-infection by measuring the specific lysis of B820 peptide-pulsed splenocytes. Graph shows the average ± SEM; data are from more than two experiments and three or more mice for each time point. Statistical significance was determined by unpaired Student's t-test.
(TIF)
Similar immunodominance was observed after infection with VACV NP-S-GFP via various routes. CD8+ T cell responses to 15 VACV epitopes in C57BL/6 mice infected with 1×106 PFU of VACV NP-S-GFP were determined by intracellular staining of IFN-γ. Graph shows the fraction of all measured responses (sum of responses to all 15 VACV peptides) accounted for by B820-specific (IDE) and the sum of SDE-specific CD8+ T cells. Data are from two experiments and total mice for each route were: i.v. 6, i.p. 9 and i.d. 6.
(TIF)
Immunodominance was maintained through the course of infection. CD8+ T cell responses to VACV epitopes B820, A8189, K36 and B254 in C57BL/6 mice injected i.p. with 1×103 PFU of VACV WR were determined as previously described on designated day after infection. Graph shows the mean and SEM of the fraction of the total measured response accounted for by B820–specific CD8+ T cells. Data are from 3 mice per group on each day, except for day 6 (marked with #) where only 1 of 3 mice had detectable responses to any peptide.
(TIF)
No significant suppression of VACV-specific responses by OT-I in the absence of OVA257 expression. After being transferred with designated numbers of OT-I CD8+ T cells and rested for overnight, B6.SJL mice were infected with 1×106 PFU of control virus VSC-8, which does not express OVA257. Seven days later, CD8+ T cell responses to 15 VACV epitopes plus OVA257 were measured by intracellular staining of IFN-γ. Graph shows the average ± SEM of sum of VACV-specific responses in percent of CD8+ cells (left) or total number of CD8+ cells per spleen (right).
(TIF)